Full text
PDF
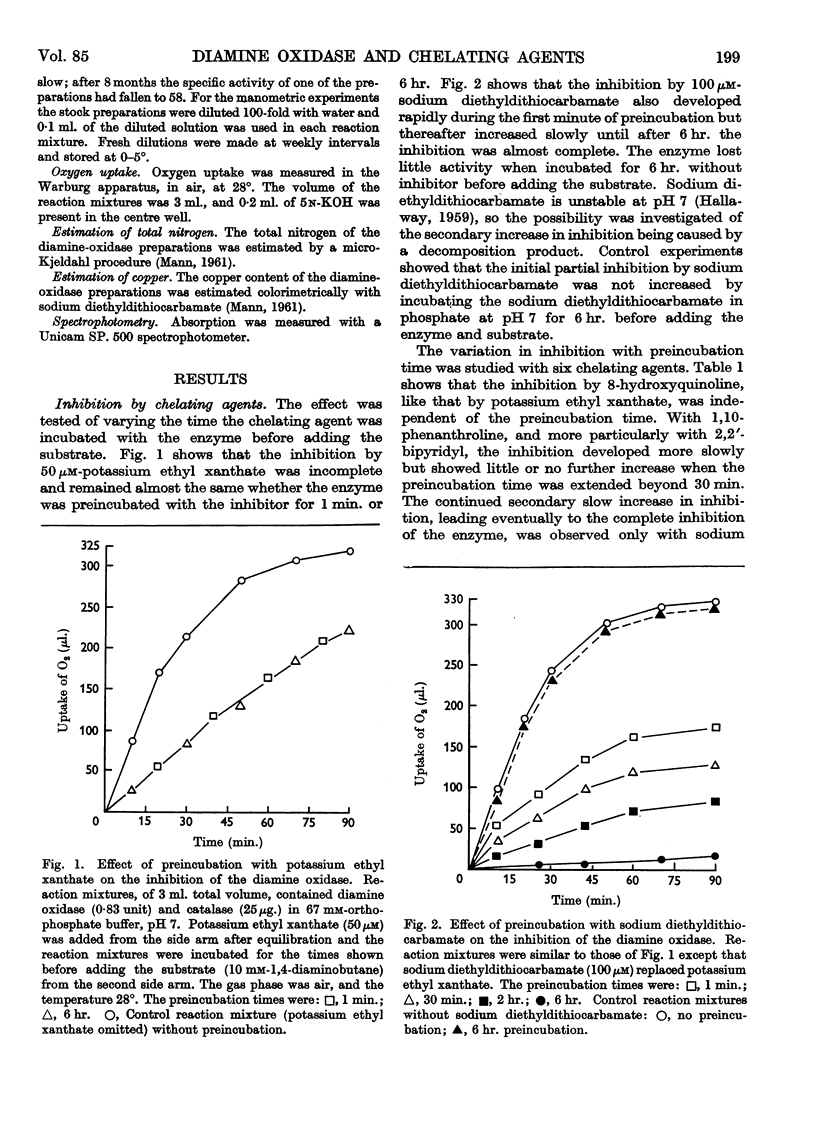
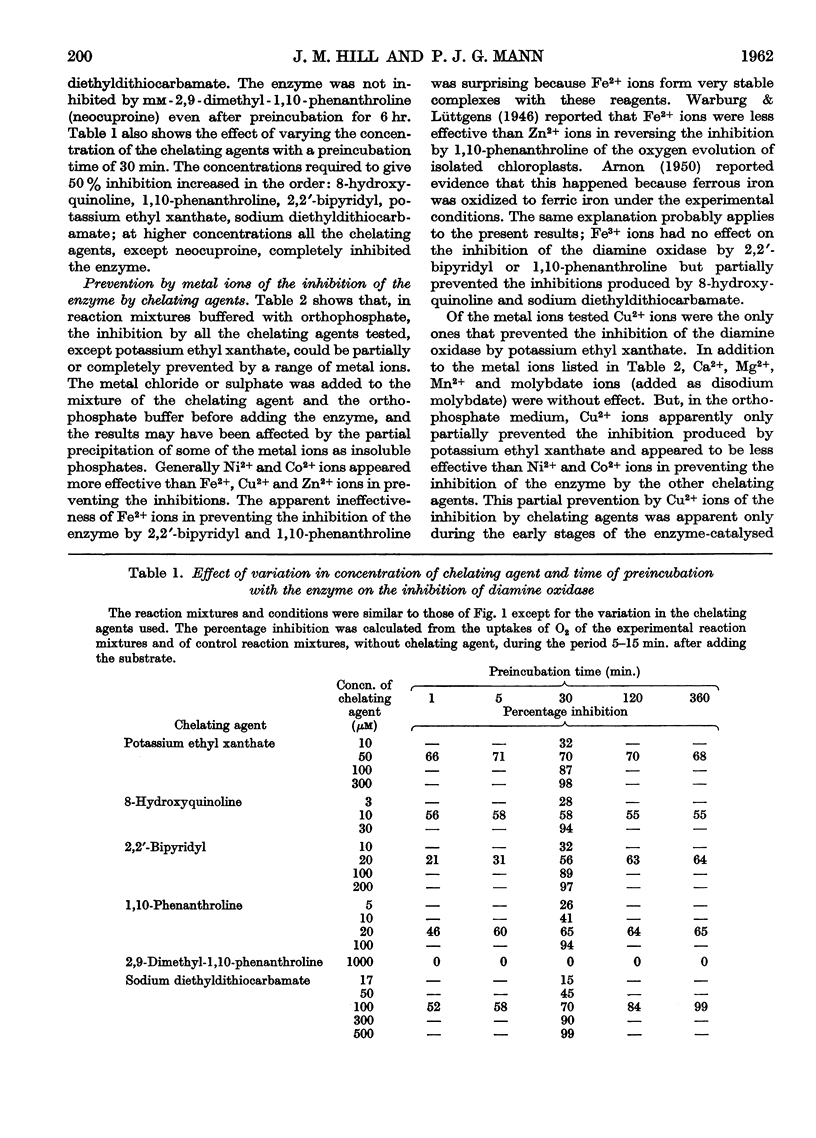

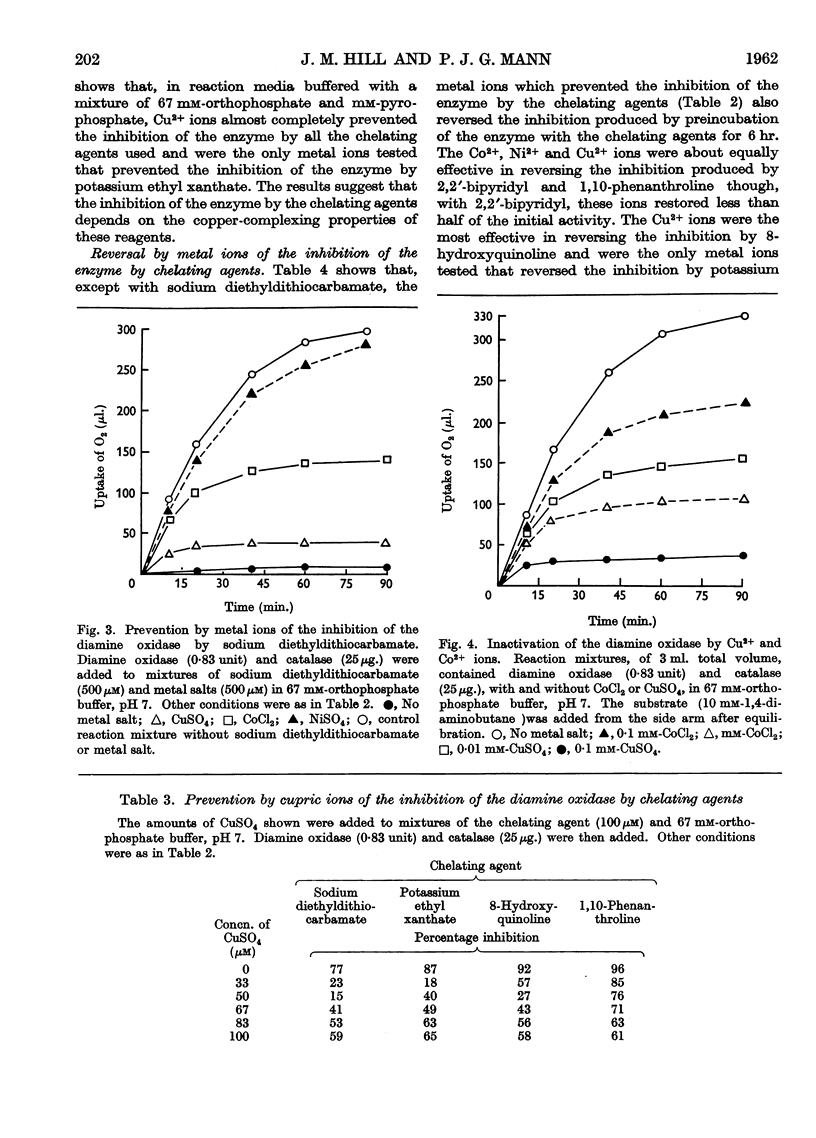
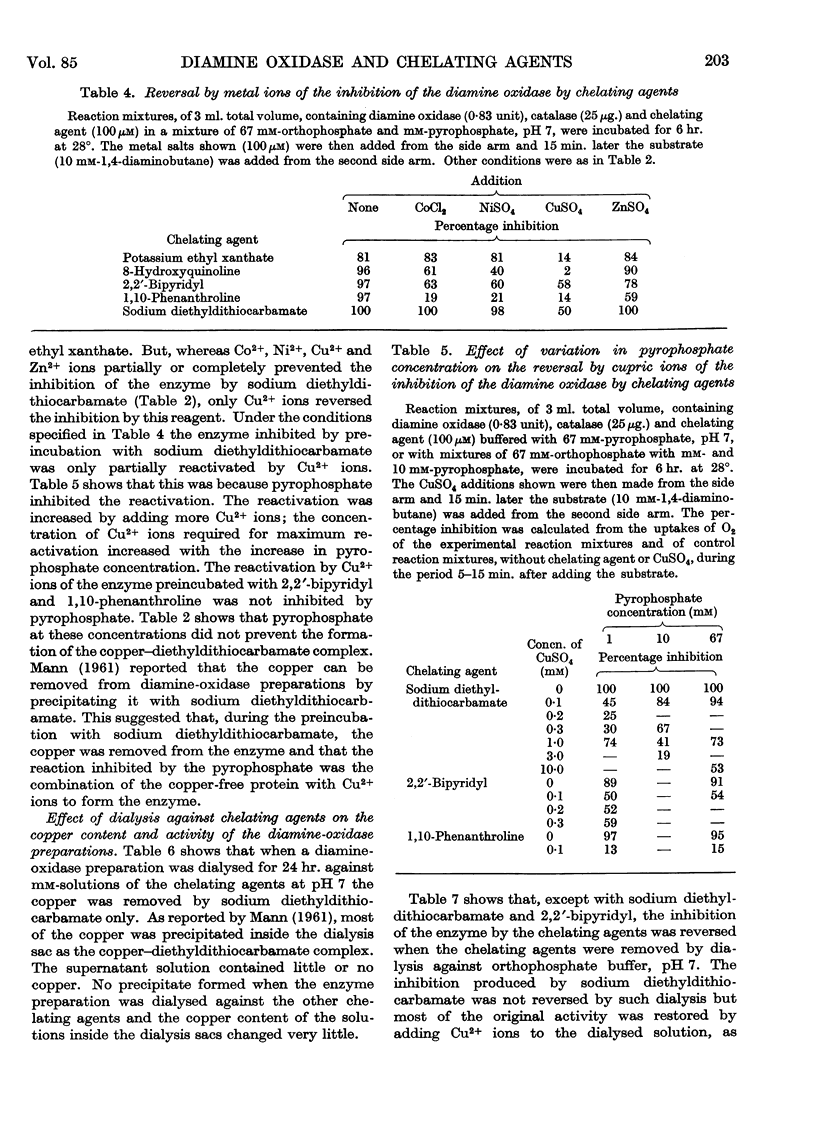



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A., Gledhill W. S. The choice of a chelating agent for inactivating trace metals: I. A survey of commercially available chelating agents. Biochem J. 1947;41(4):529–533. [PMC free article] [PubMed] [Google Scholar]
- BENHAMOU N., MAGEE R. J., DAWSON C. R. The effect of cupric ion on the reaction inactivation of ascorbic acid oxidase. Arch Biochem Biophys. 1959 Mar;81(1):135–145. doi: 10.1016/0003-9861(59)90184-5. [DOI] [PubMed] [Google Scholar]
- CLARKE A. J., MANN P. J. Plant enzyme reactions leading to the formation of heterocyclic compounds. 3. Plant amine oxidase and the formation of pyrrolidine and piperidine alkaloids. Biochem J. 1959 Apr;71(4):596–609. doi: 10.1042/bj0710596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE A. J., MANN P. J. The oxidation of tryptamine to 3-indolylacetaldehyde by plant amine oxidase. Biochem J. 1957 Apr;65(4):763–774. doi: 10.1042/bj0650763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases: stability constants and enzymatic characteristics. J Biol Chem. 1961 Aug;236:2244–2249. [PubMed] [Google Scholar]
- FELSENFELD G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960 Apr;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- FOUTS J. R., BLANKSMA L. A., CARBON J. A., ZELLER E. A. Amine oxidases. XIII. Monoamines as substrates of diamine oxidase. J Biol Chem. 1957 Apr;225(2):1025–1031. [PubMed] [Google Scholar]
- FRIEDEN E. Cupric ion inhibition of ascorbic acid oxidase. Biochim Biophys Acta. 1952 Dec;9(6):696–697. doi: 10.1016/0006-3002(52)90232-1. [DOI] [PubMed] [Google Scholar]
- GORKIN V. Z. [On certain properties of monoaminoxidase in liver and brain mitochondria in rats]. Biokhimiia. 1959 Sep-Oct;24:826–832. [PubMed] [Google Scholar]
- HALLAWAY M. The stability of sodium diethyldithiocarbamate in biochemical experiments. Biochim Biophys Acta. 1959 Dec;36:538–540. doi: 10.1016/0006-3002(59)90199-4. [DOI] [PubMed] [Google Scholar]
- HARTUNG G., WERLE E. Zur Kenntnis der Diaminoxydase mit besonderer Berücksichtigung des Enzymes aus Rotklee. Biochem Z. 1956;328(3):228–238. [PubMed] [Google Scholar]
- KENTEN R. H., MANN P. J. G. The oxidation of amines by extracts of pea seedlings. Biochem J. 1952 Jan;50(3):360–369. doi: 10.1042/bj0500360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN P. J. Further purification and properties of the amine oxidase of pea seedlings. Biochem J. 1961 Jun;79:623–631. doi: 10.1042/bj0790623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN P. J. Purification and properties of the amine oxidase of pea seedlings. Biochem J. 1955 Apr;59(4):609–620. doi: 10.1042/bj0590609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN P. J., SMITHIES W. R. Plant enzyme reactions leading to the formation of heterocyclic compounds. 1. The formation of unsaturated pyrrolidine and piperidine compounds. Biochem J. 1955 Sep;61(1):89–100. doi: 10.1042/bj0610089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN P. J., SMITHIES W. R. Plant enzyme reactions leading to the formation of heterocyclic compounds. 2. The formation of indole. Biochem J. 1955 Sep;61(1):101–105. doi: 10.1042/bj0610101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASON A., EVANS H. J. Triphosphopyridine nucleotide-nitrate reductase in Neurospora. J Biol Chem. 1953 Jun;202(2):655–673. [PubMed] [Google Scholar]
- TOKUYAMA K., DAWSON C. R. On the reaction inactivation of ascorbic acid oxidase. Biochim Biophys Acta. 1962 Jan 29;56:427–439. doi: 10.1016/0006-3002(62)90594-2. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., WILLIAMS R. J., COLEMAN J. E. Nitrogen and sulphur at the active centre of carboxypeptidase A. Nature. 1961 May 13;190:633–634. doi: 10.1038/190633a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. J. Binding of zinc in carboxypeptidase. Nature. 1960 Oct 22;188:322–322. doi: 10.1038/188322a0. [DOI] [PubMed] [Google Scholar]


