Abstract
Spiegelmers are high-affinity l-enantiomeric oligonucleotide ligands that display high resistance to enzymatic degradation compared with d-oligonucleotides. The target binding properties of Spiegelmers can be designed by an in vitro-selection process starting from a random pool of oligonucleotides. Applying this method, a Spiegelmer with high affinity (KD = 20 nM) for the peptide hormone, gonadotropin-releasing hormone (GnRH) was isolated. The Spiegelmer acts as an antagonist to GnRH in Chinese hamster ovary cells stably expressing the human GnRH receptor, and its activity is unchanged by linking to 40-kDa polyethylene glycol. In a castrated rat model the Spiegelmer further demonstrated strong GnRH antagonist activity, which is more pronounced and persists longer with the polyethylene glycol-linked derivative. Furthermore, in rabbits the anti-GnRH Spiegelmer was shown to have a very low, possibly negligible immunogenic potential. These studies suggest that Spiegelmers could be of substantial interest in the development of new pharmaceutical approaches against GnRH and other targets.
Keywords: in vitro selection‖mirror-image oligonucleotide‖animal model‖aptamer‖immunogenicity
Spiegelmers are mirror-image, high-affinity oligonucleotide ligands composed of l-ribose or l-2′-deoxyribose units. The chiral inversion results in high stability in plasma compared with natural d-oligonucleotide ligands, aptamers, suggesting that Spiegelmers may display favorable in vivo behavior and present future potential for therapeutic and diagnostic applications (1). Spiegelmers thus offer a promising alternative to aptamers, the limited in vivo stability of which continues to be a major obstacle to clinical development despite extensive efforts to improve the structure of the oligonucleotide backbone (2, 3).
Spiegelmers can fold into distinct three-dimensional structures generating high-affinity ligands that can be selected against defined pharmacological targets. High-affinity Spiegelmers with the desired target-binding properties can be identified by using an adaptation of the SELEX (systematic evolution of ligands by exponential enrichment) procedure (4). Because l nucleic acids are not compatible with SELEX because of the enantio specificity of the enzymes used for amplification, a “mirror-image” SELEX approach is used. The first step is to select an aptamer against the enantiomeric form of the natural target. After trimming to the minimal binding motif, the equivalent l form of the aptamer, the Spiegelmer, then is synthesized, and because of the reciprocal chirality, this Spiegelmer binds with high affinity to the natural target. The basic concept of combining molecular evolution with chiral inversion stemmed from the identification of a d-peptide ligand for the SH3 domain of c-Src by using a phage display approach (5). Mirror-image RNA ligands to adenosine and arginine as well as an enantiomeric DNA specific for vasopressin were identified by mirror-image SELEX and have been described previously (1, 6, 7).
Gonadotropin-releasing hormone (GnRH) is a key peptide hormone in the regulation of mammalian reproduction. It is the trigger signal for the cascade of hormones responsible for controlling the production of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (8). GnRH and its receptor therefore have been identified as therapeutic targets for sex steroid-dependent conditions such as prostate cancer, breast cancer, and endometriosis as well as in assisted-reproduction techniques (9–11).
Apart from immunotherapeutic vaccine approaches, which have yet to demonstrate feasibility, there has been no description of strategies designed to inhibit the GnRH cascade by binding and neutralizing the peptide hormone itself (12). To date, the design of antagonists has been confined to attempts to block the GnRH-binding site of the receptor. Here we report a pharmacological approach to GnRH antagonism that may provide advantages in terms of efficacy and safety. We have isolated a Spiegelmer that specifically binds to GnRH with high affinity and blocks its functional activity in an animal model. We have studied the in vivo effects of modulation of the pharmacokinetic profile and bioavailability. Furthermore, we show that anti-GnRH Spiegelmers have an extremely low capacity to induce a specific immune response in rabbits.
Methods
Identification of Spiegelmer NOX 1255.
A chemical single-stranded DNA [ssDNA, 5′-d(CCAAGCTTGCATGCCTGCAG-N60-GGTACCGAGCTCGAATTCCC)-3′] library was amplified by PCR. To generate ssDNA after amplification, the 5′ primer was modified with a C18 spacer and a T20 tail (13, 14). DNA strands were purified and separated by PAGE. After denaturing, ≈1015 different 32P-labeled ssDNA molecules were incubated with d-GnRH-derivatized Sepharose 6B (62.5 μM, after the third round 11.3 μM). Washing steps were done in binding buffer (20 mM Tris⋅HCl, pH 7.4/137 mM NaCl/5 mM KCl/2 mM CaCl2/1 mM MgCl2/0.005% Triton X-100). The first two rounds were performed at room temperature, and the subsequent 10 rounds at 37°C. From the third selection round, pools were preincubated with underivatized Sepharose to remove potential matrix binders. The ratio of ssDNA/D-GnRH was reduced from 1:40 to 1:1 during the first 10 selection rounds. Elution was performed with a 10-fold excess of d-GnRH in binding buffer, and in later rounds a 5-fold excess was used. The eluted material was extracted with phenol/chloroform, ethanol-precipitated, and amplified for the next selection round. The enriched pool was cloned and sequenced. The best binder was truncated to a 67-mer. A KD of 20 nM was determined at room temperature by equilibrium dialysis and the surface plasmon resonance technique (BIAcore 2000, BIAcore, Uppsala).
Synthesis of Spiegelmers NOX 1255 and NOX 1257.
The Spiegelmers NOX 1255 (5′-CCAAGCTTGCGTAAGCAGTCTCCTCTCAGGGGAGGTTGGGCGGTGCGTAAGCACCGGTTTGCAGGGG-3′) and NOX 1257 were synthesized on an Oligopilot II DNA synthesizer (Amersham Pharmacia) in a 780-μmol batch on 1,000-Å controlled pored glass by using phosphoramidite chemistry.
The product was cleaved from the solid support and deprotected by using 33% ammonia at 65°C for 8 h. The crude product was dried, redissolved in 10 mM NaOH, purified by ion-exchange HPLC (Waters), and desalted on Sephadex G10 (Amersham Pharmacia).
For NOX 1257 the last coupling step was done with a 6-(monomethoxytritylamino)-hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite. After deprotection the monomethoxytrityl group was cleaved by using 0.4% trifluoroacetic acid at room temperature for 30 min. The trifluoroacetic acid was removed from the 5′-aminohexyl Spiegelmer by coevaporation with ethanol and subsequent precipitation.
PEGylation of Spiegelmer NOX 1257.
The 5′-aminohexyl Spiegelmer (7.5 μmol) in 0.3 M NaHCO3, pH 8.5/dimethylformamide 60:40 (vol/vol) (125 ml) was heated to 37°C before the N-hydroxysuccinimide-activated ester of branched 40,000-Da polyethylene glycol (PEG, Shearwater Polymers, Huntsville, AL; 2 equivalents, 180 μmol) was added stepwise. The reaction was monitored by PAGE. The crude product was separated from the excess of PEG by ion-exchange chromatography and subsequently from unreacted Spiegelmer by reverse-phase HPLC. The final product was desalted on Sephadex G10 and lyophilized.
Functional Assay in Cell Culture.
Chinese hamster ovary cells expressing the human GnRH receptor were grown overnight at 37°C. Dye loading was performed after removal of the medium (Ham's F-12 with 19.2 μM Fluo-III/0.04% pluronic acid/4.8 mM probenecid/19 mM Hepes, 100 μl per well) followed by a 1-h incubation at 37°C. The plate was washed three times with Hanks' solution (138 mM NaCl/6 mM KCl/1 mM MgSO4/2 mM CaCl2/1 mM Na2HPO4/5.5 mM glucose/20 mM Hepes, pH 7.5) containing 5 mM probenecid and 0.2% BSA. Spiegelmers NOX 1255 and NOX 1257 were incubated in a concentration range between 10−6 and 3 × 10−10 M with 2 × 10−9 M GnRH or buserelin for 20 min before adding the mixture to Chinese hamster ovary cells. The Ca2+ release was detected in a fluorescent-imaging plate reader (Molecular Devices).
Animal Model.
Male Wistar Shoe rats (Tierzucht Schoenwalde, Schoenwalde, Germany) weighing 250–300 g were used. The study was done with five animals per group. Test compounds were administered 8 days after castration. For experiment 1, NOX 1255 was dissolved in PBS, pH 7.4, at 25 mg/ml. Cetrorelix was dissolved in 5% mannitol/H2O at 100 μg/ml. NOX 1255 was administered s.c. at a dose of 100 mg/kg and Cetrorelix at a dose of 100 μg/kg. The negative control and intact rats received PBS s.c. Blood samples were taken before castration (day 1) and 0, 0.5, 1.5, 3, 6, and 24 h after administration. The LH concentration was measured by RIA (Schering).
For experiment 2, the study was done with seven animals per group. The Spiegelmer NOX 1257 was dissolved in PBS, pH 7.4, at 64 mg/ml. Cetrorelix was dissolved as described above. The Spiegelmer was administered i.v. at a dose of 150 mg/kg and Cetrorelix at a dose of 100 μg/kg. The negative control and the intact rats received PBS i.v. Blood samples were taken before castration (day 1) and 0, 1, 3, 6, 8, and 24 h after administration. The LH concentration was determined as described above. Statistical analyses were performed mainly by using ANOVA on ranks (Dunn's method, P < 0.05).
Immunogenicity Study with NOX 1255 and NOX 1257.
For both Spiegelmers the study was performed in three parallel arms by using five Zimmermann rabbits (BioGenes GmbH, Berlin) each according to a standard immunization protocol over 14 weeks. The immunization schedules included five s.c. administrations within 6 weeks (days 0, 7, 14, 28, and 35) and two additional monthly administrations on days 63 and 91. The rabbits were immunized at day 0 with 400 μg of NOX 1255 or NOX 1257, respectively, and all following immunizations were performed with 200 μg of Spiegelmer per animal. Blood samples were taken before each administration. NOX 1255 and NOX 1257 were dissolved in PBS, pH 7.4, at 1 mg/ml, and the rabbits were immunized with either Spiegelmer or Spiegelmer in combination with adjuvant (lipopolysaccharides of green-blue-alga). A positive control group was immunized with Spiegelmer conjugated to cationic BSA (cBSA) in combination with adjuvant. Serum samples were analyzed by ELISA.
Results
Generation of Spiegelmers Against GnRH.
Spiegelmers against GnRH that display dose-dependent inhibition of the hormone-signaling pathway in a cell-based assay have been generated (14). By using increased stringency in the selection protocol, the Spiegelmer NOX 1255 with improved GnRH-binding properties was identified. The binding constant (KD) of NOX 1255 was determined to be 20 nM by using equilibrium dialysis and the surface plasmon resonance technique (data not shown).
By positron emission tomography imaging studies in primates it was shown recently that a Spiegelmer similar to NOX 1255, administered intravenously, is excreted in the urine with an elimination half-life of less than 1 h. Because GnRH is released over time in a pulsatile manner, a sustained level of Spiegelmer will be required for effective therapy. In an effort to maximize the pharmacological response of NOX 1255 by increasing its bioavailability, the Spiegelmer was modified by linking a 40-kDa PEG moiety to the 5′ end, generating NOX 1257 (15, 16).
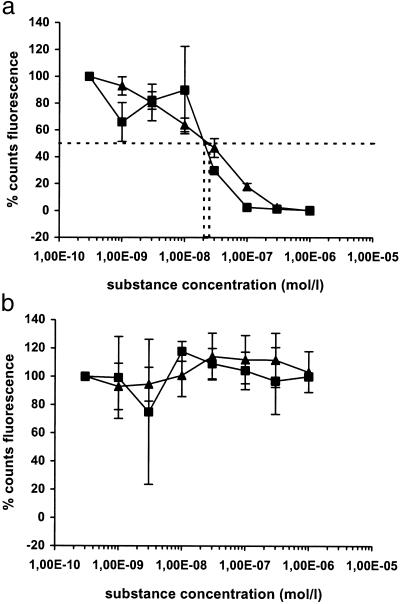
To assess the ability of the Spiegelmers NOX 1255 and NOX 1257 to inhibit the cellular GnRH response, the intracellular Ca2+ release triggered by receptor-binding in a Chinese hamster ovary cell line expressing the human GnRH receptor (17) was analyzed. Both Spiegelmers effectively inhibited the GnRH response with an IC50 in the range of 20 nM, indicating that PEGylation did not influence the binding properties of the Spiegelmer (Fig. 1a). No inhibition was observed when Buserelin, a synthetic decapeptide GnRH-receptor agonist structurally related to GnRH, was used for stimulation of the cells, confirming the target specificity of the Spiegelmers (Fig. 1b; ref. 18).
Figure 1.
Functional activity of Spiegelmers NOX 1255 and NOX 1257 in cell culture. Chinese hamster ovary cells expressing the human GnRH receptor are stimulated with GnRH (a) and Buserelin (b) after preincubation with increasing concentrations of Spiegelmer NOX 1255 (■) and NOX 1257 (▴). The results show the percentage of fluorescence signal normalized to the signal obtained with the lowest Spiegelmer concentration (e.g., 3,00E-10 = 3.00 × 10−10). The experiments were done in triplicate, and the value shown is the average.
Pharmacological Activity of Spiegelmers Against GnRH.
The Spiegelmers then were studied in a widely used model of GnRH regulation in which the serum LH concentration in castrated rats represents the efficacy parameter (19). GnRH controls LH release, which in turn regulates testosterone production in males. The testosterone level then provides a negative feedback signal to the hypothalamus, completing the regulation cycle of sex-hormone production. In castrated rats the lack of testosterone production leads to an increase in the frequency of GnRH pulses resulting in elevated LH levels. Neutralization of the GnRH activity leads to the return of the LH-plasma concentration to the level in intact animals.
Rats were orchidectomized 8 days before administration of the test substances to induce the equilibration of the LH concentration at an elevated level. A group of intact rats provided the baseline level of LH. The non-PEGylated Spiegelmer was expected to have a faster renal clearance and a shorter half-life, and therefore, to maximize the effect, s.c. injection as the route of administration was chosen. Because of its high molecular weight and anticipated superior plasma half-life, the PEGylated NOX 1257 was administered i.v. PEGylation tripled the molecular weight of the Spiegelmer. Because of solubility limitations, it was not possible to administer the same amount of the core ligand as for the study with NOX 1255. The maximal dose of NOX 1257 that could be administered corresponds to approximately half the dose of NOX 1255. The GnRH-receptor antagonist Cetrorelix served as positive control (20).
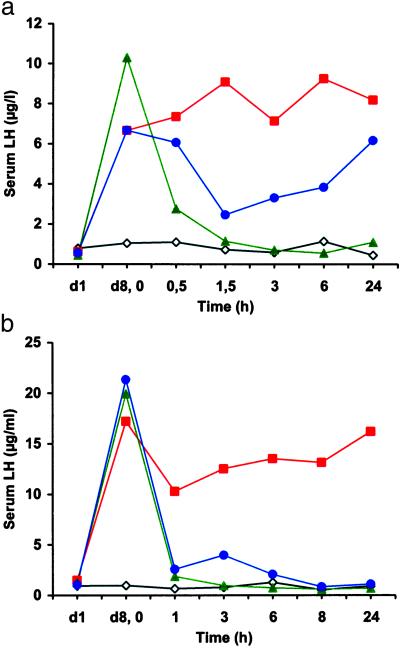
There was a distinct pharmacological effect with NOX 1255 with a maximum suppression of the LH level 1.5 h after administration. After 6 hours the effect leveled off (Fig. 2a). With NOX 1257, serum LH concentrations decreased to baseline, with sustained suppression during the entire observation period (Fig. 2b). The pharmacological effect of the hormone scavenger NOX 1257 was comparable to that of the receptor antagonist Cetrorelix in that a similar effect was obtained at a molar dose 30-fold higher. These results were confirmed in a study of longer duration. After 48 h, the LH levels in the NOX 1257 and Cetrorelix groups returned to the level of the negative control (data not shown). With a 10-fold lower dose of NOX 1257 the response was reduced and transient, similar to the profile for NOX 1255 presented in Fig. 2a (data not shown). These data provide clear evidence of the pharmacological activity of Spiegelmers.
Figure 2.
Time course of the LH level in serum of castrated rats after administration of the test substances. (a) s.c. administration of the unmodified Spiegelmer NOX 1255 (●, blue). (b) i.v. administration of PEGylated Spiegelmer NOX 1257 (●, blue). The control groups received Cetrorelix (▴, green) and vehicle (■, red). A group of intact animals receiving vehicle (⋄, black) provided the baseline level of LH. The blood samples were analyzed by RIA. Each data point represents the average value of all animals per group. d1 represents the LH concentration before castration. The suppression observed in the experiment shown in a was not statistically significant; however, the suppression found in the experiment shown in b showed a clear statistical significance. One animal that received NOX 1257 showed a delayed response, causing the slight increase of the curve at time point 3 h.
Immunogenicity Study with Spiegelmers NOX 1255 and NOX 1257.
A major safety concern in the development of novel therapeutic approaches using high molecular weight compounds is the induction of an immune response, leading to the formation of neutralizing antibodies or other adverse effects (21). In a pilot study the question of the degree of immunogenicity of Spiegelmers was addressed.
A standard immunization protocol was used that entailed five administrations of NOX 1255 over 6 weeks followed by two additional monthly boosts. Before each boost a blood sample was taken for antibody titer determination by ELISA. In three parallel groups, rabbits received NOX 1255 (group 1), NOX 1255 with adjuvant (group 2), or NOX 1255 conjugated to cBSA with adjuvant (group 3). The time course of the antibody titer development was monitored over a period of 14 weeks.
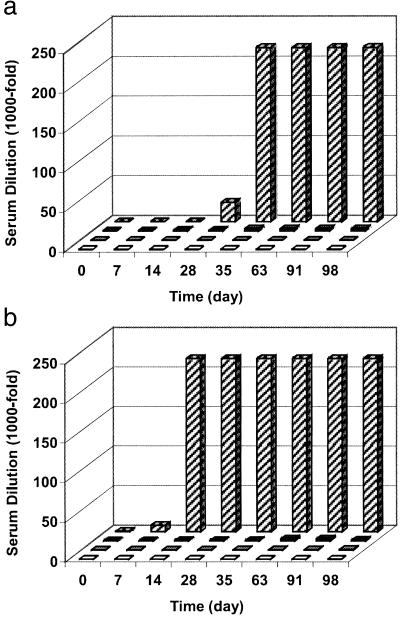
No Spiegelmer-specific antibodies in the sera from groups 1 or 2 could be detected (Fig. 3a). In group 3 very low serum titers were observed rising to a range of only 1:1,000–1:3,000. To compare these results to the immune response triggered by a potent antigen, the cBSA-specific antibody titer in the group inoculated with the conjugate was determined also. Elevated titers persisted at a level of >1:200,000, indicating a strong response to the cBSA moiety. In comparison, the titers of Spiegelmer-specific antibodies represent a weak response to the antigen. The same results were obtained in a similar study with NOX 1257 (Fig. 3b). These results suggest that these Spiegelmers have only weak, if any, antigenic potential.
Figure 3.
Time course of Spiegelmer-specific antibody titer in the serum of rabbits after repeated administration of NOX 1255 (a) and NOX 1257 (b). The groups received either Spiegelmer (□), Spiegelmer in combination with adjuvant (□), and Spiegelmer conjugated to cBSA in combination with adjuvant (■) compared with cBSA-specific antibody titer in the serum of animals from the last group ( ). Blood samples for the analysis with ELISA were drawn before each boost.
Discussion
A number of approaches, notably combinatorial chemistry in conjunction with high-throughput screening and antibody technology, have been developed over recent years with the aim of identifying novel products for pharmaceutical applications. There remains, however, a need for new technologies to provide high-quality candidates with high affinity and specificity for pharmacological targets. The initial data presented here suggest that Spiegelmers are effective in vivo and have a low immunogenic potential. These qualities imply that Spiegelmers could be of substantial interest for a variety of pharmaceutical applications.
A number of GnRH agonists and antagonists effecting the suppression of gonadotropin production and the reduction of sex-steroid levels have been synthesized and investigated for therapeutic use (18, 20). The continuous application of agonists results in a desensitization of the gonadotropic cells and down-regulation of pituitary receptors. However, there is a transient initial response to agonists that leads to stimulation of the release of gonadotropins and sex steroids, causing a temporary exacerbation of the disease. This mechanism stresses the need to identify antagonists with an immediate beneficial pharmacological response after a single administration (20). An alternative pharmacological approach to blocking the GnRH receptor is to neutralize GnRH directly through affinity binding. Apart from efforts to develop anti-GnRH vaccination, a comparable approach targeting the peptide hormone has not been described (11). The major difficulties in developing an active immunotherapy has been the diversity of individual immune responses and the late onset of action (12, 22).
GnRH presents a convenient target for Spiegelmer technology. First, the decapeptide GnRH can be prepared conveniently by chemical synthesis. Second, GnRH is highly conserved throughout mammalian species, and therefore Spiegelmers against human GnRH can be studied in animal models. Third, it is secreted into the blood stream and therefore is readily accessible by systemic administration.
First the mechanism of action of the Spiegelmers in cell culture was verified. A dose-dependent inhibition of GnRH function and a correlation between the biological activity represented by the IC50 and the binding constant of the Spiegelmer–GnRH complex was observed. In addition, the Spiegelmers showed high target specificity, demonstrated by a lack of inhibition of the response to Buserelin, a peptide analog of GnRH with a closely related structure. These results confirm that the biological effect is mediated by direct GnRH binding as was described recently (14). Furthermore, these data demonstrate that the PEG modification at the 5′ end of NOX 1257 has no influence on the biological activity and specificity of the core ligand.
The animal studies suggested that the effective NOX 1255 plasma concentration could not be maintained long enough to effectuate the complete and sustained blockade of GnRH signaling. However, with the PEGylated NOX 1257, a pharmacological response comparable to Cetrorelix in terms of activity and duration of effect was obtained. In conjunction with the data from the cell assays, it can be concluded that this effect is caused by reduced clearance. In addition, the sustained duration of the effect provides strong evidence for the enhanced in vivo stability of Spiegelmers compared with aptamers (15).
One of the major concerns of taking high molecular weight compounds into clinical trials is their immunogenic potential. It is mandatory to know whether antibodies specific to the active compound will neutralize the activity or alter the pharmacokinetic properties of the compound and whether immune reactions may compromise the safety of the treatment (21). The immunogenic potential of NOX 1255 and NOX 1257 were investigated in a rabbit model. The rabbit was chosen as a model species to obtain enough serum for further characterization of the antibodies if elevated Spiegelmer-specific titers were to be found. A trace antibody response to the Spiegelmers was observed only after conjugation to cBSA. The Spiegelmers alone were incapable of inducing a response even after repeated administration. These results suggest that Spiegelmers are not recognized by the immune system, at least in terms of antibody responses (23, 24), which indicates that Spiegelmers could be administered repeatedly over long time periods without antibody response or related adverse effects.
In conclusion, we have shown that anti-GnRH Spiegelmers have a potent pharmacological effect in vivo, suggesting that this approach could be envisaged for the generation of effective and safe drug candidates. We anticipate that these compounds will have future applications in a variety of indications.
Acknowledgments
The excellent technical assistance of G. Borowicz, B. Jahnke, P. Kuhlmann, and K. Ratajczak (all from Schering AG) is gratefully acknowledged. We thank T. Reissmann (ASTA MEDICA AG, Frankfurt/Main, Germany) for providing Cetrorelix and E. Ottow and W. D. Schleuning (both from Schering AG) for valuable discussions. We thank Dr. M. Courtney for many helpful discussions and critical revision of the manuscript. This work was supported by Grant Biotech 01/99 from the Bundesministerium für Bildung und Forschung.
Abbreviations
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- PEG
polyethylene glycol
- cBSA
cationic BSA
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Klussmann S, Nolte A, Bald R, Erdmann V A, Fürste J P. Nat Biotechnol. 1996;14:1112–1115. doi: 10.1038/nbt0996-1112. [DOI] [PubMed] [Google Scholar]
- 2.Bacher J M, Ellington A D. Drug Discov Today. 1998;3:265–273. [Google Scholar]
- 3.Tucker C E, Chen L S, Judkins M B, Farmer J A, Gill S C, Drolet D W. J Chromatogr B Biomed Sci Appl. 1999;732:203–212. doi: 10.1016/s0378-4347(99)00285-6. [DOI] [PubMed] [Google Scholar]
- 4.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher T N, Mayr L M, Minor D L, Jr, Milhollen M A, Burgess M W, Kim P S. Science. 1996;271:1854–1857. doi: 10.1126/science.271.5257.1854. [DOI] [PubMed] [Google Scholar]
- 6.Nolte A, Klussmann S, Bald R, Erdmann V A, Fürste J P. Nat Biotechnol. 1996;14:1116–1119. doi: 10.1038/nbt0996-1116. [DOI] [PubMed] [Google Scholar]
- 7.Williams K P, Liu X H, Schumacher T N, Lin H Y, Ausiello D A, Kim P S, Bartel D P. Proc Natl Acad Sci USA. 1997;94:11285–11290. doi: 10.1073/pnas.94.21.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conn P M, Crowley W F., Jr Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 9.Schally A V. Peptides (Tarrytown, NY) 1999;20:1247–1262. doi: 10.1016/s0196-9781(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 10.Kettel L M, Hummel W P. Obstet Gynecol Clin North Am. 1997;24:361–373. doi: 10.1016/s0889-8545(05)70309-0. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig M, Felberbaum R E, Küpker W, Diedrich K. Reprod Med Rev. 2000;8:41–56. [Google Scholar]
- 12.Talwar G P. Immunol Rev. 1999;171:173–192. doi: 10.1111/j.1600-065x.1999.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams K P, Bartel D P. Nucleic Acids Res. 1995;23:4220–4221. doi: 10.1093/nar/23.20.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leva S, Lichte A, Burmeister J, Muhn P, Jahnke B, Fesser D, Erfurth J, Burgstaller P, Klussmann S. Chem Biol. 2002;9:351–359. doi: 10.1016/s1074-5521(02)00111-4. [DOI] [PubMed] [Google Scholar]
- 15.Watson S R, Chang Y F, O'Connell D, Weigand L, Ringquist S, Parma D H. Antisense Nucleic Acid Drug Dev. 2000;10:63–75. doi: 10.1089/oli.1.2000.10.63. [DOI] [PubMed] [Google Scholar]
- 16.Reddy K R. Ann Pharmacother. 2000;34:915–923. doi: 10.1345/aph.10054. [DOI] [PubMed] [Google Scholar]
- 17.Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. J Biol Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- 18.Brogden R N, Buckley M M, Ward A. Drugs. 1990;39:399–437. doi: 10.2165/00003495-199039030-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez V D, Pickle R L, Lin W W. J Steroid Biochem Mol Biol. 1991;40:143–154. doi: 10.1016/0960-0760(91)90177-7. [DOI] [PubMed] [Google Scholar]
- 20.Reissmann T, Schally A V, Bouchard P, Riethmüller H, Engel J. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 21.Wierda D, Smith H W, Zwickl C M. Toxicology. 2001;158:71–74. doi: 10.1016/s0300-483x(00)00410-8. [DOI] [PubMed] [Google Scholar]
- 22.Simms M S, Scholfield D P, Jacobs E, Michaeli D, Broome P, Humphreys J E, Bishop M C. Br J Cancer. 2000;83:443–446. doi: 10.1054/bjoc.2000.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein J, Horejsi V. Immunology. Oxford: Blackwell Scientific; 1997. pp. 394–397. [Google Scholar]
- 24.Levin A A. Biochim Biophys Acta. 1999;1489:69–84. doi: 10.1016/s0167-4781(99)00140-2. [DOI] [PubMed] [Google Scholar]