Abstract
Neovascular diseases of the retina include age-related macular degeneration and diabetic retinopathy, and together they comprise the leading causes of adult-onset blindness in developed countries. Current surgical, pharmaceutical, and laser therapies for age-related macular degeneration (AMD) rarely result in improved vision, do not significantly prevent neovascularization (NV), and often result in at least some vision loss. To address this therapeutic gap, we determined the efficacy of recombinant adeno-associated viral (rAAV) serotype-2-mediated expression of pigment epithelium-derived factor (PEDF) or Kringle domains 1–3 of angiostatin (K1K3) in reducing aberrant vessel formation in a mouse model of ischemia-induced retinal NV. Both PEDF and K1K3 are potent inhibitors of NV when injected directly, hence expression of these therapeutic factors from rAAV may provide long-term protection from neovascular eye disease. rAAV vectors expressing the therapeutic gene were injected into one eye of postnatal day 0 (P0) newborn mouse pups. Retinal NV was induced in P7 mice by exposure to elevated oxygen for 5 days followed by room air for another five days. Retinal NV was quantified by the number of vascular-endothelial-cell nuclei above the inner-limiting membrane in P17 eyes. The number of such vascular endothelial cell nuclei in eyes treated with rAAV-PEDF or rAAV-K1K3 was significantly reduced (both P < 0.0000002) compared with control eyes. Ocular protein levels detected by ELISA correlate well with the reduction in NV and confirm that expression of antineovascular agents from rAAV vectors may be a therapeutically useful treatment of retinal or choroidal neovascular disease.
Control of the formation of new blood vessels in the retina is essential to the preservation of vision. Pathologic neovascularization (NV) of the retina is central to several debilitating ocular diseases including proliferative diabetic retinopathy (PDR), age-related macular degeneration (AMD), and retinopathy of prematurity (ROP). Diabetic retinopathy and AMD are the leading causes of blindness in developed countries. Regulation of vascularization in the mature retina involves a balance between endogenous positive growth factors, such as vascular endothelial growth factor (VEGF) (1, 2), and inhibitors of angiogenesis, such as pigment epithelium-derived factor (PEDF) (3). When this balance is upset, pathologic angiogenesis can occur, ultimately leading to a loss of vision.
Several clinical treatment options currently exist for patients presenting with retinal and choroidal NV (CNV). Surgical, laser, and photodynamic therapy (for AMD) techniques have been most commonly used to treat both PDR and AMD (4, 5). Panretinal laser photocoagulation has been used with relative success for a number of years (6, 7). Unfortunately, laser photocoagulation can also damage healthy cells adjacent to or underlying the treated area leading to a significant loss of peripheral and night vision. Laser photocoagulation for CNV secondary to AMD often leads to an immediate significant reduction in visual acuity (8). A more recent development is the use of photodynamic therapy to treat CNV that reduces collateral damage during laser therapy (9, 10–12). Surgical treatments include vitrectomy for the removal of CNV, foveal translocation, and the removal or displacement of subretinal blood for AMD patients and the removal of vitreous hemorrhage and scarring secondary to fibrovascular proliferation in PDR patients (13–15). These surgical interventions carry intrinsic risks to the patient and can create further complications (4, 5). All current treatment options provide solutions that result in some loss of vision and may have only temporary effects. Recurrence of symptoms is common and may ultimately result in loss of vision. Clearly, the need exists for therapies that require minimal surgical manipulation, preserve existing vision, and provide long-term amelioration for any form of NV.
Two of the most potent general inhibitors of neovascularization are Kringle domains 1 through 3 of angiostatin (K1K3) and PEDF. K1K3 is a proteolytic fragment of plasminogen that retains potent angiostatic properties. It is an endogenous regulator of vasculogenesis and, as a naturally occurring peptide, it is not likely to stimulate an immunogenic response (16, 17). Neither plasminogen nor plasmin inhibits endothelial cell proliferation, nor does angiostatin affect coagulation. Although angiostatin is known to inhibit tumor growth in vivo by increasing apoptosis and inhibiting tumor-associated angiogenesis, its precise mechanism of action is unclear. Apoptosis in vitro is induced in endothelial cells by multiple forms of angiostatin (18), and cells have been shown to be arrested at the G2/M transition interface (19). Administration of angiostatin to tumor-bearing mice has not resulted in detectable systemic cytotoxicity; only angiogenic proliferation appears to be inhibited (20–22). Angiostatin, therefore, appears to be an effective and nontoxic inhibitor of NV that is worth evaluating in models of ischemic retinopathy. A recent study has indicated that Kringle 5 of angiostatin may induce PEDF and inhibit VEGF, both in cell culture and in a rat model of ischemic retinopathy (23).
PEDF, purified from human retinal pigment epithelial cultures as a factor that induces neuronal differentiation of cultured retinoblastoma cells (24, 25), has been recently shown to regulate normal angiogenesis in the eye (3). PEDF is found both intracellularly and extracellularly in the fetus and early adult eye, but is lost at the onset of senescence (26, 27). It is down-regulated by hypoxia and induced in the retina as a result of hyperoxia, is a very potent inhibitor of corneal NV, and prevents endothelial cell migration toward a wide variety of angiogenic inducers (3). PEDF, therefore, appears to be a major angiogenic regulator of the retinal vasculature and is an excellent candidate gene for therapy against ocular NV. As an intraocularly injected protein, PEDF delays the loss of photoreceptors in the rd mouse (28), implying that it may also possess neurotrophic activity in the retina and that the extracellular protein can effectively disperse throughout the retina. We hypothesize here that when expressed in a secretable form as a virally vectored gene, either PEDF or K1K3 may be relatively independent of the retinal cell type supporting expression and may be effective in limiting retinal NV.
To determine whether PEDF, K1K3, or both are potentially useful for therapeutic control of retinal NV, we examined the effect of expression of the angiostatic factors PEDF and K1K3 in a mouse model of ischemic retinopathy. We chose recombinant adeno-associated virus (rAAV) serotype-2 to deliver the therapeutic genes because issues regarding attainment of high vector titers have been resolved (29) and rAAV preparations free of contaminating replication-competent rAAV are now routine (29, 30). rAAV-mediated gene delivery results in long-term expression in a wide variety of tissues, including various cell types in the retina (31) and optic nerve (32). Finally, rAAV vectors have shown very little in vivo toxicity in a variety of tissues (33).
Materials and Methods
Animals.
All animals were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 mice were obtained from the Jackson Laboratory. Breeding pairs of mice were housed in the University of Florida Health Science Center Animal Resources facilities. Females were examined daily for signs of pregnancy and isolated in individual cages for confirmation. Timed-pregnant dams were also occasionally obtained from the same vendor. Animals were euthanized by an overdose of ketamine/xylazine mixture given s.c. (10 μg/g body weight ketamine⋅HCl, 2 μg/g body weight xylazine in an appropriate volume of 0.9% NaCl).
Vector Design, Packaging, and Delivery.
The rAAV-vector cassette consists of a selected promoter upstream of a simian virus 40 early splice donor/splice-acceptor site, the expressed gene, and a simian virus 40 polyadenylation sequence. PEDF cDNA was a gift of D. Zack (Johns Hopkins University, Baltimore) and K1K3 cDNA was a gift of P. Meneses (Weill Medical College of Cornell University, New York). K1K3 has an IgK leader-peptide secretory sequence upstream of the expressed gene. At its carboxyl terminus, K1K3 also has a myc epitope for ELISA detection. The entire expression cassette containing either cDNA is flanked by adeno-associated virus2 terminal repeats required for viral packaging. Viral vectors were packaged and purified as described (29, 30). Briefly, the vector cassette is transfected into human 293 cells along with a helper plasmid containing adeno-associated virus and adenovirus helper functions. The cells are harvested in PBS with EDTA, pelleted, and resuspended in a low-salt buffer, and lysed by freeze–thaw. The virus is purified on an iodixinol gradient followed by heparin sulfate affinity column chromatography. rAAV vector is eluted from the heparin-agarose matrix with 1 M NaCl and concentrated. Initial viral titer is calculated by quantitative competitive PCR and a final titer is determined by infectious center assay.
To determine the optimum promoter to drive expression of the therapeutic gene, we designed five promoter constructs. Each promoter regulates expression in a subset of cells in the retina. Chicken β-actin (CBA) is a ubiquitous strong promoter composed of a cytomegalovirus (CMV) immediate-early enhancer (381 bp) and a CBA promoter-exon1-intron1 element (1,352 bp). cis-Retinaldehyde-binding protein (CRALBP) promoter is a retinal-pigment-epithelium (RPE)-specific promoter (2,265 bp) when administered subretinally in a rAAV vector (A. Timmers and W.W.H., unpublished data). Mouse rod opsin (MOPS) is a photoreceptor-specific promoter (31) (472 bp); platelet-derived growth factor (PDGF) is an endothelial promoter (1,600 bp) that regulates expression in retinal ganglion cells when delivered intravitreally in rAAV vectors (A. Timmers and W.W.H., unpublished data); and the CMV promoter (620 bp), like CBA, is also relatively ubiquitous in the retina but expresses at a lower levels than CBA (34). We inserted each of these promoters upstream of the therapeutic genes, PEDF or K1K3. For the purpose of determining in vivo expression levels, each vector was injected into the subretinal or intravitreal space of adult mice. Approximately 1010 particles (2 × 108 infectious units) in a volume of 1 μl of therapeutic vector was injected into the right eye either subretinally or intravitreally. The contralateral eye was injected with the same volume of PBS. Mouse pups were injected intraocularly with 0.5 μl on postnatal day (P)0 with one of the experimental vectors in the right eye and either no injection or PBS in the contralateral eye.
ELISA.
Eyes from age-matched animals were enucleated and quickly frozen in 100 μl of PBS, pH 7.4/0.05% PMSF, and manually homogenized on ice by using a ground-glass tissue homogenizer, followed by three freeze–thaw cycles on liquid nitrogen and wet ice. The homogenate was centrifuged in a refrigerated desktop centrifuge at 5,000 × g for 2 min to pellet the insoluble material. The resulting whole-eye extract was loaded into sample wells for detection by ELISA. We determined the ocular levels of PEDF protein by an indirect sandwich ELISA procedure by using a biotin-conjugated final antibody and horseradish peroxidase (HRP)-conjugated avidin for detection. Rabbit anti-PEDF (gift from P. Campochiaro, Johns Hopkins University, Baltimore) was coated on 96-well Immulon flat-bottom microtiter plate (Dynex Technologies, Middlesex, U.K.) in 0.1 M NaHCO3 overnight at 4°C. The wells were blocked with 10% FBS in PBS, pH 7.4, for 2 h at 37°C. We then loaded PEDF protein standards and eye-extract samples as 100-μl aliquots into the wells and incubated the plate overnight at 4°C. Detection consisted of a secondary mouse polyclonal anti-PEDF (gift of P. Hargrave, University of Florida, Gainesville) followed by a biotin-conjugated rat anti-mouse IgG (ICN) and HRP-conjugated avidin (PharMingen). Each step of the detection was conducted with plate agitation at room temperature for 1–2 h and the plate was washed five times between steps. TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase substrate system (Kirkegaard & Perry Laboratories) was pipetted into all wells and allowed to reach fully developed color, usually for 30 min, before stopping the reaction with 1 M H3PO4. The plates were read by absorbance at 450 nm in an automated microplate reader. A similar method was used to determine K1K3 levels. Rat anti-K1K3 (Enzyme Research Laboratories, South Bend, IN) was coated onto plates, and K1K3 samples were loaded as described. We then used a biotinylated secondary antibody to the myc epitope (Invitrogen) on the expressed K1K3 to detect bound protein. HRP-conjugated avidin followed by TMB peroxidase substrate completed the detection step as described above.
Hyperoxia Treatment.
Mouse pups, with their nursing dam, were placed in a chamber at 73% oxygen at P7 and maintained in this environment for 5 days until P12. At this time the pups and nursing dam were returned to normal room air and maintained for another 5 days. At P17, the pups were euthanized as described above and their eyes were enucleated and fixed for embedding and sectioning. Representative pups from each group were anesthetized and perfused through the left ventricle with 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4) containing 5 mg/ml FITC-dextran for visualizing the retinal vasculature (see below).
Quantitative and Qualitative Assessment of Retinal NV.
Both eyes of each P17 pup were enucleated and fixed for paraffin embedding and serially sectioned at 5 μm thickness as described by Smith et al. (35). Representative sections (every 30th section) through the full eyecup were stained with hematoxylin and eosin to visualize cell nuclei. Trained investigators masked to the identity of each section counted all endothelial cell nuclei above the internal limiting membrane in all representative sections through each eye. Vascular cell nuclei were considered to be associated with NV if they were on the vitreous side of the internal limiting membrane. Data were analyzed by a paired t test with vector-treated and contralateral uninjected eyes serving as determinants. For qualitative assessment of retinal NV both eyes of each perfused P17 pup were enucleated and the retina was dissected and flat-mounted as described by D'Amato et al. (36). Flatmounted retinas were photographed by fluorescence microscopy using a Zeiss Axioplan2 microscope, Zeiss Plan-Fluar 10× lens, Sony DXC-970MD camera with tile field imaging, and mcid software (Imaging Research, St. Catherine's, ON, Canada). At least three eyes from each treatment group were examined in this way.
Results
Optimizing Ocular Expression from the Vector Constructs.
To determine the optimal promoter construct for use in antiangiogenic experimental therapies, we initially assayed the amount of ocular protein in adult mice expressed from each of five vector constructs containing different promoters. To a first approximation the level of PEDF or K1K3 protein expression in the eye should correlate with how well that vector performed in reducing aberrant NV. We chose a set of promoters that restrict the expression of genes to different specific subsets of retinal cells. Each vector-promoter construct was tested by either intravitreal or subretinal vector inoculation. We measured ocular protein expression levels by ELISA 6 weeks after vector injection. Only the nature of the promoter regulating the PEDF or K1K3 or the site of intraocular injection was changed. ELISA has advantages over other methods to examine the protein expression in the eye. PCR-based methods of determining promoter efficiency reflect only the amount of mRNA made and may not accurately reflect mature protein levels. ELISA provides a direct quantification that is more precise than Western blotting. The results are summarized in Table 1 and indicate that for both PEDF and K1K3 the CBA-hybrid promoter produces the most consistently robust ELISA measurements of protein expression. Interestingly, subretinal or intravitreal routes of vector delivery generated approximately equivalent levels of ocular protein. For purposes of maximizing protein expression in the retina, we therefore conclude that rAAV-CBA-PEDF and rAAV-CBA-K1K3 vectors are best suited for therapeutic evaluation in a retinal NV setting.
Table 1.
Ocular K1K3 or PEDF levels for various viral vector constructs
| Vector construct | PEDF, ng per retina | K1K3, ng per retina |
|---|---|---|
| CBA | 20–70 | 6–60 |
| CRALBP | 3–4 | <1 |
| MOPS | 1–2 | <1 |
| PDGF | 2–3 | 3–4 |
| CMV | <1 | <1 |
| CBA (neonatal) | 1–8 | 2–3 |
Expression of K1K3 or PEDF was determined by ELISA from whole eye homogenates. rAAV vectors with the CBA promoter produced the highest levels of K1K3 or PEDF expression from either intravitreal or subretinal inoculations. There was no significant difference between intravitreal and subretinal injection for either K1K3 or PEDF. Neonatal expression levels measured between P2 and P17 for the CBA promoter for both K1K3 and PEDF are lower than the adult but still above the estimated therapeutic threshold (see Discussion). MOPS, mouse rod opsin; CRALBP, cis-Retinaldehyde-binding protein; CMV, cytomegalovirus.
Vector Behavior in the ROP Mouse Model.
To functionally test whether PEDF or K1K3 vectors reduced NV in vivo, we chose to examine their performance in a mouse model of ischemic retinopathy. Retinal NV is induced in a modification of a described protocol (35, 36). However, before analysis of any therapeutic effects, we needed to know what levels of each agent we could produce in the eyes of P1 to P17 neonatal mice because our initial survey of intraocular levels of passenger-gene expression was in adult mice with a mature retina. P1 neonatal mice were injected intraocularly with rAAV-CBA-PEDF or rAAV-CBA-K1K3 as described above. Levels of PEDF and K1K3 expression were followed by ELISA after injection. Expression of PEDF was detectable as early as P2 and persisted at levels well above contralateral uninjected eyes to the last time point at P17. The PEDF levels measured in the neonatal mice (1–8 ng per eye) were not as high as those measured in adult mouse eyes (20–70 ng per eye), however; they were 4- to 16-fold above levels in the untreated contralateral eyes. K1K3 levels measured in injected eyes of neonatal mice were 2–3 ng per eye. Quantitative differences between PEDF and K1K3 levels achievable in adults vs. neonates are likely related to size, developmental differences, and the time after injection.
Assessment of Antineovascular Effects.
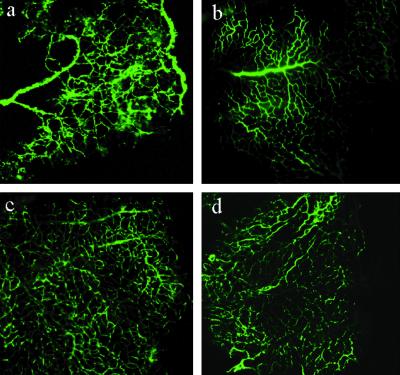
To qualitatively assess retinal NV after vector treatment we viewed the retinal vasculature in flat-mounted FITC-dextran-perfused whole retinas. Whole mounts were imaged using tile-field-mapping fluorescent microscopy, which allows the whole retina to be examined at high resolution. Such visual assessment of full retinal vascular beds provides a useful initial comparison between treatment groups. The patterns of NV observed in the FITC-dextran-perfused retinas were consistent with what has been reported in this model (refs. 35–37; Fig. 1). Retinas of neonatal mice exposed to hyperoxia exhibit increased tortuosity of radial vessels accompanied by increased perfusion of peripheral vessels and absence or reduction of perfusion in central vessels (Fig. 1a). For comparison, a whole mount from the normoxic age-matched animal shows the normal pattern of retinal vasculature (Fig. 1b). The uninjected, hyperoxia-exposed retina in Fig. 1a can be directly contrasted with the contralateral PEDF- (Fig. 1c) or K1K3- (Fig. 1d) vector-treated retinas. PEDF- or K1K3-vector-treated retinas showed a decrease in the number of neovascular tufts with a relatively uniform perfusion pattern over the full retina, and the overall vasculature pattern appeared much more like that in the normal animal (Fig. 1b). However, these sorts of images do not lend themselves readily to a quantitative analysis of NV, and are subject to several preparation artifacts (38). Therefore, an independent and more quantifiable additional analysis of the treatment groups was performed.
Figure 1.
Qualitative determination of neovascularization from composite tile-field-mapped ×10 photomicrographs of whole-mounts of retinas from 17-day mouse pups. The pups were perfused with FITC-dextran in 4% formaldehyde to visualize the retinal vasculature. Each panel shows one quadrant of the retinal whole-mount oriented with the central retina on the left and the peripheral on the right. (a) An untreated control pup exposed to 5 days of hyperoxia followed by 5 days at room oxygen exhibited the expected abnormal retinal vasculature. Note the increased peripheral perfusion, the dilated, tortuous radial vessels, and the largely avascular central retina. (b) A normoxic age-matched animal shows the normal pattern of retinal vasculature. ROP animals injected with either PEDF vector (c) or K1K3 vector (d) exhibited a vasculature much closer to that seen in a normal age-matched animal, with uniform perfusion over the retina and fewer areas of apparent vascular leakage.
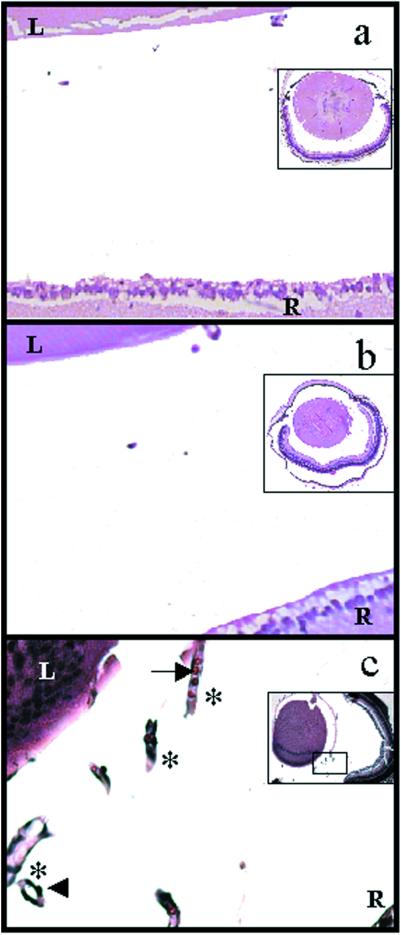
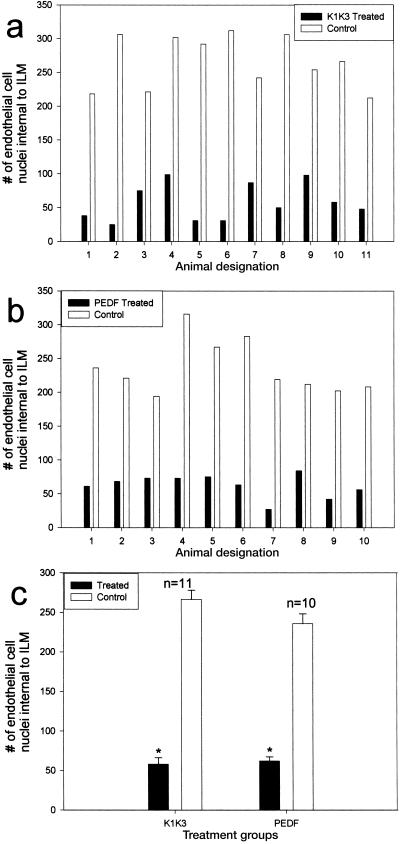
To gain better insight into the efficacy of treatment with rAAV-CBA-PEDF or rAAV-CBA-K1K3, direct enumeration of endothelial cells in the retinal vasculature was assessed as described (35). We enucleated and fixed both vector-treated and control eyes from P17 pups for paraffin-embedded sectioning. Representative sections spanning the entire retina provide a reliable method for quantitatively assessing the total level of retinal NV in each eye. Individuals masked as to the identity of the treatment groups quantified NV by enumerating all endothelial cell nuclei found in the vitreous space above the inner limiting membrane (ILM). Comparisons were made between one eye of each animal injected with therapeutic vector and the contralateral uninjected eye serving as an internal control (Fig. 2). In tissue sections, uninjected control eyes typically showed abundant longitudinal and transverse aberrant microvessels in the vitreous space above the ILM (Fig. 2c). Eyes injected with K1K3 or PEDF vector had a dramatically reduced number of aberrant vessels (Fig. 2 a and b, respectively). Vehicle-injected eyes and eyes injected with rAAV-CBA-GFP did not significantly differ from uninjected eyes in this assay (data not shown). Control eyes injected with rAAV-CBA-GFP demonstrate that the therapeutic effect is because of the expressed passenger genes and not because of the vector itself. Treatment with either rAAV-CBA-PEDF or rAAV-CBA-K1K3 significantly reduced the neovascular response (both P < 0.0000002) when compared with the paired uninjected control eye (Fig. 3). Average endothelial cell counts in PEDF-treated eyes were reduced by 74% compared with paired controls and 78% compared with paired controls for K1K3-treated eyes.
Figure 2.
Photomicrographs showing a portion of transverse sections of whole eyes from hyperoxia-treated mouse pups. Tile-field-mapped photomicrographs from representative whole eyes are inset in each panel. The arrowhead indicates a representative vascular endothelial cell nucleus and the arrow indicates a red blood cell within a longitudinal section of an abnormal microvessel (indicated by asterisks) that has penetrated the ILM. The vitreal space into which new blood vessels have penetrated is defined anteriorly by the lens (L) and posteriorly by the retina (R). Assessment of neovascularization requires quantitation of endothelial cell nuclei occurring on the vitreous side of the ILM (see Fig. 3). Eyes injected with rAAV-CBA-K1K3 had fewer endothelial cell nuclei in representative sections (a) than uninjected control eyes (c). Similarly, rAAV-CBA-PEDF treatment resulted in a reduced number of endothelial cell nuclei (b) compared with uninjected control eyes (c).
Figure 3.
Enumeration of endothelial cell nuclei above the ILM in sections of whole eyes from hyperoxia-treated P17 mouse pups. These quantitative results agree with the qualitative assessment (see Fig. 1), that vector-mediated expression of PEDF (a) or K1K3 (b) reduces the level of oxygen-induced NV. (c) Statistical analysis of the paired eyes shows a significant difference between control and treated eyes for both PEDF and K1K3 (P < 0.0000002).
Discussion
Effective, long-lasting treatment of retinal neovascular disorders, including diabetic retinopathy, remains one of the greatest challenges in ophthalmology today. The number of individuals suffering from diabetes has increased worldwide in recent years and is projected to continue to rise (39). PDR is a common complication in diabetic patients. PDR shares a pathophysiology with our model of ischemic retinopathy because the initial ischemic insult and the subsequent pathologic outgrowth of new vessels from the retinal vasculature occur in both PDR and ROP and ultimately lead to blindness in either disease. Retinal NV can also occur secondarily to CNV in AMD (40, 41).
Although the minimum effective intraocular dose of PEDF or K1K3 for controlling ischemic retinopathy is unknown, previous studies following the administration of PEDF protein in the same model we employ here showed the therapeutic threshold to be ≈5–11 μg/day (42) when administered systemically. If we make the simplest and most conservative assumptions, that PEDF is stable and partitioned into the eye from the systemic vasculature based simply on the volume of the eye vs. the volume of the whole animal, the concentration of ocular PEDF needed to inhibit retinal NV is estimated to be 1–2 ng per eye. This conservative calculation does not consider any pharmacokinetic stability parameters or the existence of the blood–retinal barrier, both of which would tend to reduce further the amount of PEDF reaching the eye from the circulation. It is therefore possible that the true intraocular therapeutic threshold for PEDF is much lower than this rough estimate. We demonstrated here that rAAV-vector-expressed PEDF produces intraocular concentrations of 20–70 ng in the adult mouse and 1–8 ng in the neonatal mouse. This is at or considerably higher than our estimated therapeutic threshold. Similar calculations based on another study in which one dose of K1K3 angiostatin administered systemically was able to effectively reduce retinal NV in the ROP mouse (43) yielded an estimated maximum intraocular therapeutic threshold of 1.6 ng per eye for K1K3. Our rAAV-vector expressing K1K3 angiostatin produces levels of 6–60 ng per eye in the adult or 2–3 ng per eye in the neonate, again, above our estimated maximum threshold. Therefore, it appears that we can achieve and maintain intraocular levels of either PEDF or K1K3 angiostatin in neonatal and adult mice sufficient to expect significant reduction of retinal NV.
Administration of rAAV vectors expressing cDNAs for antiangiogenic proteins can therefore be as effective as the administration of the corresponding proteins systemically, but without the requirement for repeated injections. In addition, use of rAAV vectors offers several potentially important advantages over the systemic administration of antiangiogenics. Local production and secretion of PEDF or K1K3 through vector gene delivery is likely to restrict any antineovascular activity to an area specific to the pathological angiogenesis in the subject. Proteins produced in the posterior ocular compartment are not likely to interfere with the normal angiogenic processes necessary for wound healing or tissue repair elsewhere in the body, and perhaps not even in the anterior segment of the same eye, although this remains to be tested. Further, rAAV vectors have demonstrated long-term, sustained high-level expression in the retina (32), and we observe nanogram levels in rat eyes for at least 21 months (B.J.R. and W.W.H., unpublished data), indicating that a single injection of rAAV bearing a therapeutic gene could provide durable inhibition of NV. This could obviate the need for repeated injections of antiangiogenic compounds systemically or intraocularly.
For the purposes of maximizing protein expression in the retina, we found that rAAV-CBA-PEDF and rAAV-CBA-K1K3 vectors produced the highest levels of ocular protein. The lower levels of expression seen for vectors with MOPS, CRALBP, and PDGF promoters may be due to the different cell specificity of these promoters compared with CBA, or, in the case of CMV, to a generally lower ability to support transcription in the same set of retinal cell types. When subretinally injected, CBA supports expression well in both retinal-pigment-epithelium (RPE) cells and photoreceptors (44). In contrast, the MOPS promoter is largely rod-photoreceptor-specific (31) and yields much lower intraocular expression, thus suggesting that the key source of secreted PEDF or K1K3 from vectors injected subretinally is the RPE cell. When injected into the vitreous, CBA-containing rAAV vectors express very well predominantly in retinal ganglion cells (45). Since there was no significant difference in the antineovascular effectiveness of subretinally or intravitreally administered PEDF or K1K3 vectors with the CBA promoter, it appears that the potentially less traumatic intravitreal route of vector delivery may be favored.
For optimizing the safety of ocular gene therapy for NV diseases it may be important to limit the expression of a therapeutic protein even more specifically, either to just a single retinal cell type or to a more defined topologic area. By altering the promoter used to drive PEDF or K1K3 expression it may be possible to fine-tune therapeutic gene expression to a pharmacologically significant but highly localized cellular pattern. The CBA promoter drives expression in multiple cell types, whereas a more specific promoter could be used to target expression selectively. Alternatively, delivering the vector specifically to the intravitreal or subretinal space in a larger human eye may also define the localization of expression. Advanced stages of AMD are characterized by a NV of the chorocapillaris within or adjacent to the macula where treatment might be most effective if the therapeutic vector is administered subretinally near potentially active CNV regions. This type of subretinal administration effectively limits the lateral spread of vector-mediated expression (44), whereas vitreal administration may allow less constrained vector diffusion to a wider and less controlled retinal area. Full testing of these ideas will require development of an animal model with a retinal area closer in size to that in humans than that of the mouse.
In summary, we have demonstrated that rAAV vectors incorporating a CBA promoter are capable of producing sustained therapeutic levels of PEDF and K1K3 in the mouse eye. Intraocular injection of rAAV-CBA-PEDF or rAAV-CBA-K1K3 significantly reduced the level of retinal NV in a mouse model of ischemic retinopathy. Other studies have recently demonstrated effective gene therapy approaches for controlling ocular angiogenesis. Expression of PEDF from either rAAV (45) or adenovirus (46) vectors was effective in reducing CNV in rodent models. Adenoviral vectors expressing endostatin (47) or plasminogen-activator inhibitor-1 (48) were also effective in inhibiting retinal NV. Furthered by our present report, the generality of efficient and well-targeted gene-based approaches for treating neovascular diseases of the eye coupled with the potential of rAAV vectors for persistently delivering antiangiogenic proteins to the retina is becoming apparent.
Acknowledgments
We acknowledge the expert technical assistance of V. Chiodo, V. McCord, T. Vaught, N. Cortez, J. Alexander, and A. Timmers. This work was supported by National Eye Institute Grants EY11123, T32 EY07043, NS36302, and EY13729, the Macular Vision Research Foundation, Research to Prevent Blindness, Juvenile Diabetes Research Foundation, and The Foundation Fighting Blindness.
Abbreviations
- NV
neovascularization
- CNV
choroidal NV
- AMD
age-related macular degeneration
- rAAV
recombinant adeno-associated virus
- CBA
chicken β-actin
- PEDF
pigment epithelium-derived factor
- K1K3
Kringle domains 1–3 of angiostatin
- PDR
proliferative diabetic retinopathy
- ROP
retinopathy of prematurity
- Pn
postnatal day n
- ILM
inner limiting membrane
References
- 1.Ferrara N. Recent Prog Horm Res. 2000;55:15–35. [PubMed] [Google Scholar]
- 2.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 3.Dawson D W, Volpert O V, Gillis P, Crawford S E, Xu H, Benedict W, Bouck N P. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 4.Spranger J, Pfeiffer A F. Exp Clin Endocrinol Diabetes. 2002;109:S438–S450. doi: 10.1055/s-2001-18601. [DOI] [PubMed] [Google Scholar]
- 5.Votruba M, Gregor Z. Eye. 2001;15:424–429. doi: 10.1038/eye.2001.147. [DOI] [PubMed] [Google Scholar]
- 6.Le D, Murphy R P. Semin Ophthalmol. 1994;9:2–9. doi: 10.3109/08820539409060252. [DOI] [PubMed] [Google Scholar]
- 7.Boulanger A, Liu S, Henningsgaard A A, Yu S, Redmond T M. J Biol Chem. 2000;275:31274–31282. doi: 10.1074/jbc.M003441200. [DOI] [PubMed] [Google Scholar]
- 8.Lamkin J C, Singerman L J. Semin Ophthalmol. 1994;9:10–22. doi: 10.3109/08820539409060253. [DOI] [PubMed] [Google Scholar]
- 9.Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, Povelka M, Frohner U, Kruger A, Hilgers R D, et al. Am J Ophthalmol. 2002;133:215–225. doi: 10.1016/s0002-9394(01)01373-3. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Erfurth U, Hasan T. Surv Ophthalmol. 2000;45:195–214. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 11.Margherio R R, Margherio A R, DeSantis M E. Retina. 2000;20:325–330. doi: 10.1097/00006982-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Smiddy W E, Flynn H W., Jr Surv Ophthalmol. 1999;43:491–507. doi: 10.1016/s0039-6257(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 13.Dogru M, Nakamura M, Inoue M, Yamamoto M. Jpn J Ophthalmol. 1999;43:217–224. doi: 10.1016/s0021-5155(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 14.Ladd B S, Solomon S D, Bressler N M, Bressler S B. Am J Ophthalmol. 2001;132:659–667. doi: 10.1016/s0002-9394(01)01198-9. [DOI] [PubMed] [Google Scholar]
- 15.Luke C, Aisenbrey S, Luke M, Marzella G, Bartz-Schmidt K U, Walter P. Br J Ophthalmol. 2001;85:928–932. doi: 10.1136/bjo.85.8.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch M, Schackert G, Black P M. J Neurooncol. 2000;50:173–180. doi: 10.1023/a:1006453428013. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y. Int J Biochem Cell Biol. 2001;33:357–369. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 18.Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota S J, Borlat F, Sim B K, Wu Z, Grau G E, Shing Y, et al. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- 19.Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Proc Natl Acad Sci USA. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 21.O'Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, O'Reilly M S, Folkman J, Shing Y. Biochem Biophys Res Commun. 1997;236:651–654. doi: 10.1006/bbrc.1997.7032. [DOI] [PubMed] [Google Scholar]
- 23.Gao G, Li Y, Gee S, Dudley A, Fant J, Crosson C, Ma J X. J Biol Chem. 2002;277:9492–9497. doi: 10.1074/jbc.M108004200. [DOI] [PubMed] [Google Scholar]
- 24.Tombran-Tink J, Chader G G, Johnson L V. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 25.Steele F R, Chader G J, Johnson L V, Tombran-Tink J. Proc Natl Acad Sci USA. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becerra S P. Adv Exp Med Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 27.Araki T, Taniwaki T, Becerra S P, Chader G J, Schwartz J P. J Neurosci Res. 1998;53:7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Cayouette M, Smith S B, Becerra S P, Gravel C. Neurobiol Dis. 1999;6:523–532. doi: 10.1006/nbdi.1999.0263. [DOI] [PubMed] [Google Scholar]
- 29.Hauswirth W W, Lewin A S, Zolotukhin S, Muzyczka N. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 30.Zolotukhin S, Byrne B J, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski R J, Muzyczka N. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 31.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy J, Qi X, Muzyczka N, Hauswirth W W. Arch Ophthalmol. 1999;117:929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 33.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, et al. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett J, Duan D, Engelhardt J F, Maguire A M. Invest Ophthalmol Visual Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 35.Smith L E, Wesolowski E, McLellan A, Kostyk S K, D'Amato R, Sullivan R, D'Amore P A. Invest Ophthalmol Visual Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 36.D'Amato R, Wesolowski E, Smith L E. Microvasc Res. 1993;46:135–142. doi: 10.1006/mvre.1993.1042. [DOI] [PubMed] [Google Scholar]
- 37.Mino R P, Spoerri P E, Caballero S, Player D, Belardinelli L, Biaggioni I, Grant M B. Invest Ophthalmol Visual Sci. 2001;42:3320–3324. [PubMed] [Google Scholar]
- 38.Penn J S, Henry M M. Microvasc Res. 1996;51:126–130. doi: 10.1006/mvre.1996.0014. [DOI] [PubMed] [Google Scholar]
- 39.Mokdad A H, Ford E S, Bowman B A, Nelson D E, Engelgau M M, Vinicor F, Marks J S. Diabetes Care. 2001;24:412. doi: 10.2337/diacare.24.2.412. (lett.). [DOI] [PubMed] [Google Scholar]
- 40.Lip P L, Blann A D, Hope-Ross M, Gibson J M, Lip G Y. Ophthalmology. 2001;108:705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 41.Yannuzzi L A, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, Freund K B, Sorenson J, Orlock D, Borodoker N. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Stellmach V, Crawford S E, Zhou W, Bouck N. Proc Natl Acad Sci USA. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meneses P I, Hajjar K A, Berns K I, Duvoisin R M. Gene Ther. 2001;8:646–648. doi: 10.1038/sj.gt.3301423. [DOI] [PubMed] [Google Scholar]
- 44.Acland G M, Aguirre G D, Ray J, Zhang Q, Aleman T S, Cideciyan A V, Pearce-Kelling S E, Anand V, Zeng Y, Maguire A M, et al. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 45. Mori, K., Gehlbach, P., Yamamoto, S., Duh, E., Zack, D. J., Berns, K. I., Raisler, B. J., Hauswirth, W. W. & Campochiaro, P. A. (2002) Invest. Ophthalmol. Visual Sci., in press. [PubMed]
- 46.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang H S, Zack D J, Ettyreddy D, et al. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 47.Mori K, Ando A, Gehlbach P, Nesbitt D, Takahashi K, Goldsteen D, Penn M, Chen C T, Mori K, Melia M, et al. Am J Pathol. 2001;159:313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambert V, Munaut C, Noel A, Frankenne F, Bajou K, Gerard R, Carmeliet P, Defresne M P, Foidart J M, Rakic J M, et al. FASEB J. 2001;6:1021–1027. doi: 10.1096/fj.00-0393com. [DOI] [PubMed] [Google Scholar]