Abstract
Six experimentally naive rhesus monkeys produced 0.01 mg/kg/infusion cocaine by lever pressing under a tandem fixed-ratio 1 differential-reinforcement-of-other-behavior schedule. One lever press initiated an unsignaled 15- or 30-s delay culminating in cocaine delivery. Each press made during the delay reset the delay interval. With two exceptions, responding was acquired and maintained at higher rates than responding on a second (inoperative) lever. For the exceptions, a cancellation contingency was arranged in which each formerly inoperative-lever response reset the tandem schedule. This manipulation reduced presses on the inoperative lever. Subsequently, the consequences of responding on the two levers were reversed, and the monkeys again responded at higher rates on the operative lever. As a comparison, 3 additional experimentally naive monkeys received response-independent cocaine deliveries. Although lever pressing was observed, it extinguished and was subsequently reestablished under the tandem schedule. The results suggest that although response-reinforcer contiguity is not required for cocaine to acquire reinforcing functions, a response-reinforcer relation appears necessary.
Keywords: cocaine self-administration, delayed reinforcement, acquisition, lever press, rhesus monkey
Lattal and Gleeson (1990) demonstrated that a new response could be acquired and maintained in food-deprived rats and pigeons when, in the absence of shaping or previous training, the response produced food after unsignaled resetting delays of up to 30 s. Because each response made during the delay reset the delay to reinforcement, Lattal and Gleeson concluded that response-reinforcer contiguity is not necessary for learning to occur. Subsequent studies have extended the generality of this finding to different species and reinforcers (Lattal & Metzger, 1994) and different response topographies (Critchfield & Lattal, 1993). In addition, factors have been identified that affect the rate of acquisition, such as delay length (e.g., Lattal & Gleeson, 1990), food deprivation (Lattal & Williams, 1997), history of response-independent reinforcement (Snycerski, Laraway, Huitema, & Poling, 2004), and drug actions (Byrne, LeSage, & Poling, 1997; LeSage, Byrne, & Poling, 1996).
A few studies have investigated delayed reinforcement within the framework of drug self-administration (Beardsley & Balster, 1993; Gollub & Yanagita, 1974; Stretch, Gerber, & Lane, 1976). For example, Stretch et al. established cocaine self-administration on a fixed-ratio (FR) 1 schedule in squirrel monkeys and then imposed unsignaled delays of 5, 25, 50, and 100 s between the operant response and the cocaine infusion. Those delays reset if a second response followed completion of the FR 1 before the lapse in the delay parameter. Response rates were found to be a linear decreasing function of delay, with the longest delays maintaining the lowest response rates.
The acquisition of drug self-administration with delayed reinforcement has not been demonstrated. Acquisition of drug self-administration differs from acquisition of food-maintained responding in several interesting ways. First, operations (e.g., food deprivation) are not required to establish the drug as a reinforcer. Second, preliminary exposure to the reinforcer (e.g., magazine training) is not necessary. In these respects, acquisition of drug self-administration resembles response acquisition by Siamese fighting fish (Betta splendens) with visual reinforcement (Lattal & Metzger, 1994).
Several studies have demonstrated that onset of action is an important determinant of the reinforcing functions of drugs (Balster & Schuster, 1973; Panlilio, Goldberg, Gilman, Jufer, Cone, & Schindler, 1998; Winger, Hursh, Casey, & Woods, 2002). In typical self-administration procedures, the time between the reinforced response and the drug effect is determined largely by drug onset of action. Investigating drug self-administration with delayed reinforcement is one way to vary the temporal relation between response and reinforcer independently of onset of action, allowing for a better characterization of the reinforcing functions of a drug. To date, delays have been imposed on previously established baselines of responding, and it is possible that responding may be maintained in part by history and by response-generated feedback stimuli that function as immediate conditioned reinforcers due to their previous association with the drug (for a discussion of response-generated feedback, see Dinsmoor, 2001, p. 314). The acquisition period provides a context that is relatively free of these complications.
In this experiment, rhesus monkeys, naive with respect to lever pressing and cocaine self-administration, were employed. Completion of an FR 1 initiated an unsignaled 15- or 30-s delay to reinforcement, culminating in an infusion of cocaine (0.01 mg/kg/infusion). Lever presses made during the delay reset the delay interval. Technically, this arrangement is referred to as a tandem FR 1 differential-reinforcement-of-other-behavior (DRO) 15- or 30-s schedule. We used a two-lever procedure in which responses on an inoperative lever served as a comparison against which to assess response acquisition on a second, operative lever. Three additional experimentally naive monkeys also were employed as comparisons to assess if response-independent cocaine presentations alone—and not the operant contingency—generated responding.
METHOD
Subjects
Nine rhesus monkeys (Macaca mulatta), naive with respect to cocaine administration and lever pressing, served as subjects. Monkeys lived in the experimental chambers and were fed 10 to 15 Purina® monkey chow biscuits twice daily to maintain their adult body weights. Daily fresh fruit and other treats supplemented this diet. Water was continuously available. In accordance with institutional animal care and use requirements, environmental enrichment toys also were provided on a regular rotating basis.
Table 1 shows the sex, age, weight, and experimental history of each monkey. With the exception of Monkey 3511, all monkeys were female. Six monkeys were experimentally naive. Three monkeys (3503, 3511, and 4643) previously had been used in an ethanol self-administration study in which spout licking resulted in ethanol delivery (both oral and intravenous routes of administration were investigated). Experimental histories for these monkeys were remote (greater than 1 year), and the intelligence panel and response manipulandum used in the current experiment differed from that previously employed.
Table 1. Subject characteristics, experimental histories, and initial group assignment.
| Monkey | Sex | Age (year) | Weight (kg) | Experimental history | Group |
| 1412 | F | 7 | 7.1 | None | FR 1 DRO 15 s |
| 1418 | F | 8 | 9.6 | None | FR 1 DRO 15 s |
| 3511 | M | 9 | 10.7 | Ethanol SA | FR 1 DRO 15 s |
| 3503 | F | 8 | 9.8 | Ethanol SA | FR 1 DRO 30 s |
| 3953 | F | 9 | 7.6 | None | FR 1 DRO 30 s |
| 4643 | F | 12 | 10.3 | Ethanol SA | FR 1 DRO 30 s |
| 1411 | F | 12 | 5.7 | None | VT |
| 1419 | F | 11 | 6.4 | None | VT |
| 6593 | F | 12 | 5.6 | None | VT |
Note. SA = self-administration. See text for details regarding experimental histories.
Apparatus
Monkeys were permanently housed in stainless steel cages (83.3 cm long by 76.2 cm wide by 91.4 cm deep). The front, top, and bottom of the cage were made of barred stainless steel, and a pan was located below the floor to collect waste. Located on the wall to the left of the barred front door was an intelligence panel 20 cm in length and 15.4 cm in height, approximately 10 cm from the front and 19 cm from the bottom of the cage. Across the top of the stimulus panel, 1.5 cm apart, were three circular openings, 2.5 cm in diameter, covered with translucent plastic and capable of being illuminated from behind with 5-W colored bulbs. The two side lights could be illuminated red and the center light green. Centered below the right and left stimulus lights were response levers (Model 121-07, BRS-LVE) capable of being operated by 10 to 15 g (0.10 to 0.15 N) of force. Experimental control was provided by an IBM® PS/2 computer located in an adjoining room and programmed with Med-PC® (Med-Associates, Georgia, VT) software.
Each monkey wore a Teflon mesh jacket (Lomir, Quebec, Canada) connected to a flexible stainless-steel spring tether attached to the rear of the cage. Monkeys previously had been implanted with indwelling intravenous catheters in an internal or external jugular, or femoral vein, under ketamine (10 mg/kg, IM) and xylazine (2 mg/kg, IM) anesthesia. Catheters were run subcutaneously from the site of implantation to an exit site in the middle of the back. Tubing was then fed through the steel spring tether and passed to the outside rear of the cage, where it was connected to a stock solution of 0.01 mg/kg/ml cocaine and additional infusion lines that passed through the rollers of an infusion pump. Operation of the infusion pump delivered 1-ml solution over 5 s.
Procedure
Two sessions were conducted each day, one in the morning (10:00 a.m.) and one in the afternoon (4:00 p.m.). Sessions lasted 120 min or until 100 cocaine infusions were delivered, whichever came first. The onset of the session was signaled by the illumination of the red stimulus light above the lever on which presses produced cocaine (the operative lever). The stimulus light over the second, inoperative lever was not illuminated. Cocaine infusions were accompanied by the offset of the red stimulus light and the onset of the center green stimulus light. Infusions were 5 s in duration. At the end of an infusion, the green light was turned off and the side red light was illuminated. The offset of all lights signaled the end of the session.
Table 1 also shows initial group assignments. Six monkeys were exposed to a tandem FR 1 DRO 15- or 30-s schedule (3 monkeys per group). In addition, 3 monkeys were exposed to response-independent cocaine deliveries according to a variable-time (VT) schedule. Specific details regarding the tandem and VT schedules are presented below.
Tandem Schedule: Acquisition
During acquisition, a tandem FR 1 DRO 15- or 30-s schedule was employed in which one lever press on the right (operative) lever initiated an unsignaled delay interval culminating in a 5-s infusion of cocaine (0.01 mg/kg/inf). Operative-lever presses made during the delay reset the delay interval. Presses on the left (inoperative) lever were recorded, but had no programmed consequences. The DRO (delay) value was 15 s for Monkeys 1412, 1418, and 3511, and 30 s for Monkeys 3503, 3953, and 4643. The acquisition phase lasted a minimum of 10 sessions. For most monkeys the acquisition phase was terminated when response rates on the operative lever were higher than response rates on the inoperative lever for several consecutive sessions (assessed visually). Responding was not maintained with a 30-s delay for Monkey 3953; as a result we reduced the delay to 15 s. This procedure established responding; however, operative- and inoperative-lever response rates were undifferentiated for both this monkey and another (3511). These monkeys were then exposed to a cancellation procedure described below.
Tandem Schedule: Cancellation Procedure
A cancellation contingency developed by Wilkenfield, Nickel, Blakely, and Poling (1992) was arranged on the formerly inoperative (left) lever. If a left-lever press was made during the delay interval, the tandem schedule was reset. That is, the monkey was required to emit another right (operative) lever press and then abstain from lever pressing for 15 s to produce a cocaine infusion. Operative-lever presses made during the delay continued to reset the delay interval, but did not reset the response requirement. For Monkey 3511, the delay was subsequently decreased to 7.5 s and the cancellation procedure remained in effect.
Tandem Schedule: Contingency Reversal
Once differential responding (operative > inoperative) was observed, the consequences of responding were reversed. Only the stimulus light above the left lever was illuminated. Responses on the left lever produced cocaine infusions and the center-green stimulus light according to the tandem schedule. Responses on the right lever had no effect for Monkeys 1412, 1418, 3503, and 4643, and reset the tandem schedule for Monkeys 3511 and 3953. This condition lasted a minimum of 10 sessions and until responding on the operative lever was maintained at rates higher than those on the inoperative (or cancellation) lever.
Response-Independent Cocaine Delivery
Monkeys 1411, 1419, and 6593 initially were exposed to response-independent cocaine infusions (0.01 mg/kg/inf). The onset of the session was signaled by the illumination of the red stimulus light above the right lever. Cocaine infusions were accompanied by the offset of the right red stimulus light and the onset of the center green stimulus light. Infusions were 5 s in duration. At the end of an infusion, the green light was turned off and the right red light was illuminated.
Cocaine was delivered according to a VT schedule that was constructed from the distribution of interinfusion intervals obtained during the last three sessions of acquisition from the monkey with the highest obtained reinforcement rate, and largest range of reinforcement rates, over this period (Monkey 1412). At the onset of each session, an interval from the distribution was sampled at random. When the interval elapsed, cocaine was infused. At the end of the infusion, another interval was selected randomly and without replacement. Lever presses had no effect on cocaine infusions. Sessions lasted 120 min or until 100 infusions were delivered, whichever came first. Monkeys were exposed to this schedule for a minimum of 10 sessions, and until response rates on both the right and left levers were less than 0.3 responses per minute for three consecutive sessions. Subsequently, these monkeys were exposed to a tandem FR 1 DRO 15-s schedule as described above. In a final condition, the delay was reduced to 7.5 s. Monkey 1411 developed health problems (unrelated to the ongoing experiment) in this condition and was removed from the study.
RESULTS
Tandem Schedule
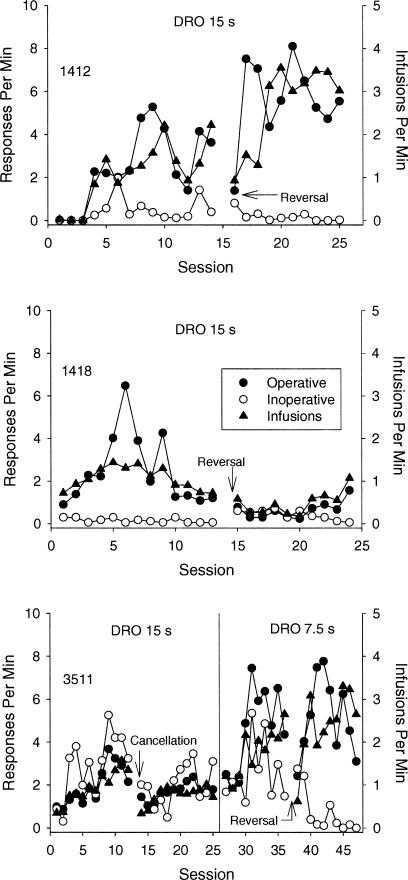
Figure 1 shows results from those monkeys initially exposed to the tandem FR 1 DRO 15-s schedule. All 3 monkeys acquired the lever-press response. Monkeys 1418 and 1412 responded almost exclusively on the operative lever beginning on the first and fourth session, respectively. When the consequences of responding were reversed across levers, operative-lever response rates were reliably higher than inoperative-lever response rates by the second (Monkey 1412) and eighth (Monkey 1418) session.
Fig. 1. Shown are session-by-session operative-lever (filled circles) and inoperative-lever (open circles) response rates, and reinforcement rates (triangles) for the monkeys initially exposed to the tandem FR 1 DRO 15-s schedule.
For Monkey 3511, the DRO value was reduced to 7.5 s. For this monkey, the point at which the cancellation contingency was implemented is indicated. All subsequent sessions employed the cancellation contingency. For all monkeys, the point at which the contingencies were reversed across levers also is indicated.
For Monkey 3511, responding on both levers increased during the acquisition period. The introduction of the cancellation contingency decreased overall responding, but response rates on the two levers were undifferentiated. When the delay was reduced to 7.5 s, responding increased, and operative-lever responding was maintained at higher rates than responding on the cancellation lever. When the contingencies were reversed, operative-lever responding was maintained and responding on the cancellation lever extinguished.
For all monkeys, the obtained reinforcement rate was substantially lower than the maximal possible rate of reinforcement (approximately four infusions per minute). The low overall reinforcement rates were a joint function of responding during the delay interval (which reset the delay) and occasional postreinforcement pausing.
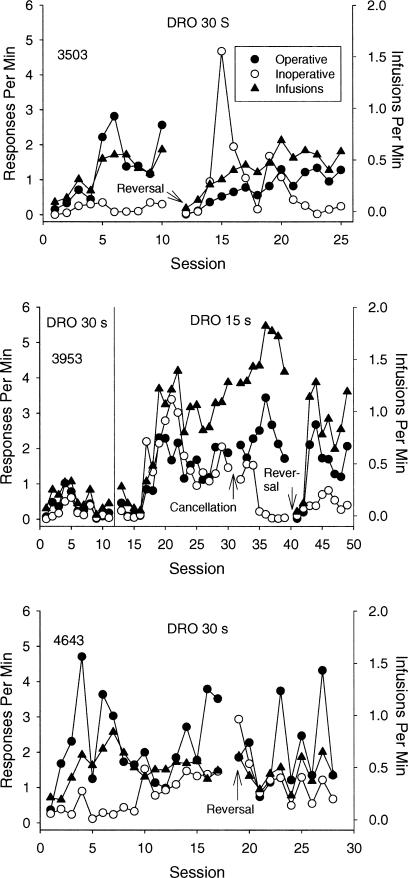
Figure 2 shows results from those monkeys exposed initially to the tandem FR 1 DRO 30-s schedule. Monkeys 4643 and 3503 acquired the response, with operative-lever responding reliably maintained at higher rates than inoperative-lever responding beginning on the second and fifth sessions, respectively. Inoperative-lever responding remained at near-zero levels for Monkey 3503 but increased across the acquisition period for Monkey 4643. In the lever-reversal condition, operative-lever responding was maintained at higher rates than inoperative-lever responding beginning on the 5th (Monkey 4643) and 10th (Monkey 3503) sessions.
Fig. 2. Session-by-session results for monkeys initially exposed to the tandem FR 1 DRO 30-s schedule.
For Monkey 3953, the DRO value was reduced to 15 s. See Figure 1 for more details.
For Monkey 3953, responding initially occurred at low rates but subsequently decreased to near-zero levels. Responding was reestablished when the delay was reduced to 15 s, but operative- and inoperative-lever response rates were undifferentiated. The implementation of the cancellation procedure reduced responding on the cancellation lever to near-zero levels within four sessions, and responding occurred almost exclusively on the operative lever beginning on the third session of exposure to the reversed contingencies.
Similar to the monkeys exposed to the 15-s delay, the obtained reinforcement rate for monkeys exposed to the 30-s delay was substantially lower than the maximal possible rate of reinforcement (approximately two infusions per minute).
Response-Independent Cocaine Delivery
The obtained interinfusion intervals from the last three sessions of acquisition for Monkey 1412 were used to construct a VT schedule for monkeys initially exposed to response-independent cocaine delivery. The VT schedule was composed of 298 intervals, ranging from 16.2 s to 2008.6 s. The distribution of these intervals was positively skewed: The mean interfusion interval was 65.7 s, and the median interinfusion interval was 37 s. This skewness was the result of occasional breaks in responding and was characteristic of the performance of all monkeys exposed to the tandem schedule.
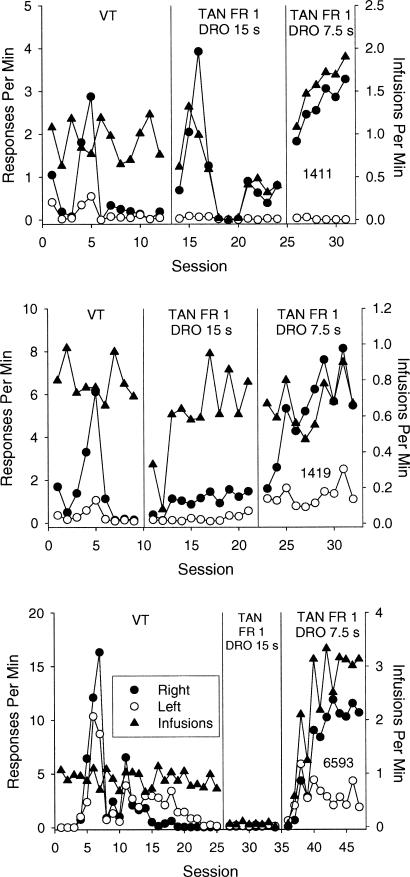
Figure 3 shows the obtained response and reinforcement rates from monkeys initially exposed to the VT schedule of cocaine delivery. This procedure was partially successful at generating reinforcement rates comparable to those obtained in the terminal sessions of the acquisition condition for the tandem FR 1 DRO 15-s group. Averaged across monkeys and over the last three sessions of VT exposure, the mean obtained reinforcement rate was 0.9 infusions per minute. The mean obtained reinforcement rate over the last three sessions of exposure to the tandem FR 1 DRO 15-s schedule was 1.2 infusions per minute. By comparison, the mean reinforcement rate obtained over the last three sessions of exposure to the tandem FR 1 DRO 30-s schedule was 0.3 infusions per minute.
Fig. 3. Shown are session-by-session response rates on the right (filled circles) and left (open circles) levers, and reinforcement rates (triangles) for the monkeys exposed to the VT schedule prior to the tandem schedule.
The right lever was operative under the tandem schedules. (Note the different scales for the y axes.)
All 3 monkeys exposed to the VT schedule responded. For Monkey 1411, response rates on the right lever increased through the fifth session, and then immediately decreased to near-zero levels. Responding usually did not occur on the left lever. A similar pattern of responding was observed for Monkey 1419. For Monkey 6593, response rates on both the right and left levers increased over the first six sessions. Responding on the right lever peaked at approximately 17 responses per minute, which was the highest response rate observed in this study. Thereafter, responding on both levers decreased slowly. Sessions were conducted until response rates on both levers decreased to less than 0.3 responses per minute for three consecutive sessions. We chose to conduct this condition until responding extinguished to establish a baseline from which to assess response reacquisition after noncontingent cocaine presentations alone no longer maintained responding. Overall, the monkeys required 12 (Monkey 1411), 9 (Monkey 1419), and 25 (Monkey 6593) sessions of VT exposure to meet this criterion.
Next, these monkeys were exposed to a tandem FR 1 DRO 15-s schedule. Responding on the right (operative) lever was reestablished in Monkeys 1411 and 1419. Lever pressing was not reestablished in Monkey 6593. Subsequently, the DRO value was reduced to 7.5 s for all monkeys. Lever pressing was reestablished in Monkey 6593. For all monkeys, response and reinforcement rates increased, with operative (right lever) responding greater than inoperative (left lever) responding.
Assessment of Group Differences
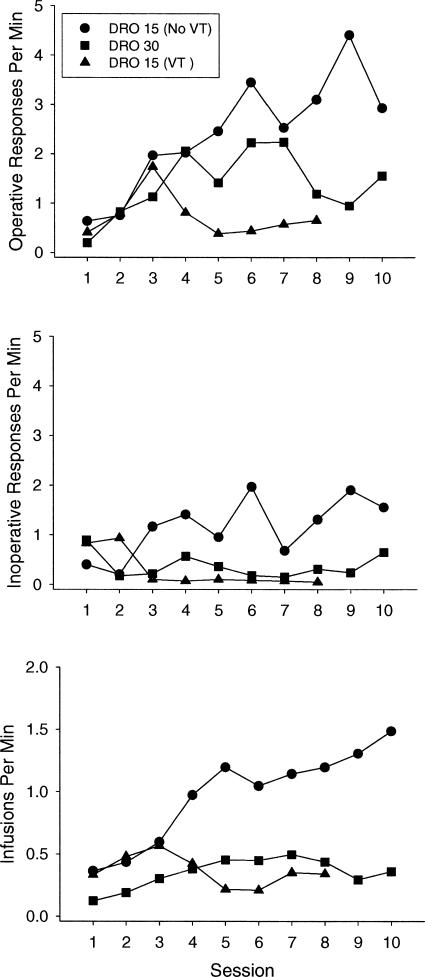
We assessed group differences in session-by-session performance among the monkeys exposed to the tandem FR 1 DRO 15-s (without a VT history), DRO 30-s, and DRO 15-s (with a VT history) schedules. Figure 4 shows mean operative-lever response rates (top panel), inoperative-lever response rates (middle panel), and reinforcement rates (bottom panel) for each group across the first eight (VT history) and 10 sessions of initial exposure to the tandem schedule. We were only able to calculate mean response and reinforcement rates across the first eight sessions for the VT-history group because Monkey 6593 received only eight sessions under the tandem FR 1 DRO 15-s schedule.
Fig. 4. Mean operative-lever response rates (top panel), inoperative-lever response rates (middle panel), and reinforcement rates (bottom panel), as a function of group across the first 10 sessions of initial exposure to the tandem schedule.
Circles represent the DRO 15-s group, squares represent the DRO 30-s group, and triangles represent the group exposed initially to the VT schedule. Because Monkey 6593 received only eight sessions of exposure to the tandem FR 1 DRO 15-s schedule, session-by-session means were calculated for only the first eight sessions for the VT group.
A series of two-way mixed analyses of variance (ANOVA) were conducted with group as the fixed factor and session as the repeated measure. We first examined the effects of delay (15 vs. 30 s), excluding the group initially exposed to the VT schedule. With respect to operative-lever responding, the main effect of session was significant, F(9, 36) = 3.92, p < .01. Operative-lever responding increased across sessions. The main effects of delay and the delay x session interaction were not significant. Although monkeys exposed to the 15-s delay tended to have higher response rates than those exposed to the 30-s delay, there was considerable within-group variability.
With respect to inoperative-lever responding, the main effects of delay, session, and the delay x session interaction were not significant. Inoperative-lever responding usually did not increase across sessions. The elevation in inoperative-lever responding for the 15-s group shown in Figure 4 is almost entirely due to the results from Monkey 3511. With respect to the obtained reinforcement rates, monkeys exposed to the 15-s delay had higher infusion rates than monkeys exposed to the 30-s delay, F(1, 4) = 22.64, p < .01. In addition, reinforcement rates increased across sessions, F(9, 36) = 4.20, p < .001. The delay x session interaction was not significant.
We also examined performance on the tandem FR 1 DRO 15-s schedule as a function of VT history (VT vs. No VT). With respect to operative-lever responding, the history x session interaction approached statistical significance, F(7, 28) = 2.28, p = .057. Operative-lever responding increased across sessions for monkeys without a VT history, but remained low for monkeys with a VT history. The main effect of history also was not significant, F(1, 4) = 6.04, p = .069. However, if the performance of Monkey 6593 (that did not respond) is extrapolated and the analysis is based on 10 rather than eight sessions, the main effect of history and the history x session interaction reaches statistical significance at p < .05.
With respect to inoperative-lever responding, the main effects of history and session, as well as the history x session interaction, were not significant. Finally, with respect to obtained reinforcement rates, the main effects of history and session were not significant, but there was a significant history x session interaction, F(7, 28) = 2.80, p < .05. Infusion rates for monkeys without a VT history increased across sessions, whereas infusion rates for monkeys with a VT history did not.
DISCUSSION
The results suggest that cocaine self-administration in rhesus monkeys can be acquired and maintained with unsignaled resetting delays to reinforcement of up to 30 s. One problem with the current study is that monkeys exposed to response-independent cocaine deliveries initially responded at comparable (or even higher) rates than monkeys exposed to the tandem schedule. Previous studies investigating response acquisition with delayed reinforcement typically have not observed much responding by comparison subjects exposed to response-independent reinforcer deliveries (Dickinson, Watt, & Griffiths, 1992; LeSage et al., 1996; Sutphin, Byrne, & Poling, 1998). Complicating matters, we constructed the VT schedule from interinfusion intervals obtained from the final three sessions of exposure to the tandem schedule rather than employing a yoking procedure in which the session-by-session interinfusion intervals were equated.
Given these results, it could be argued that the responding observed on the tandem schedule does not reflect sensitivity to the operant. Although responding during the early sessions of acquisition should not be used as evidence for sensitivity to the operant, we contend that by the end of the acquisition period, and in subsequent conditions, responding appeared sensitive to the prevailing contingencies. We base this contention on the following observations: First, responding on the VT schedule extinguished, whereas responding on the tandem schedule, once established, did not. Once responding had extinguished on the VT schedule, it was reacquired under the tandem schedule. In addition, operative-lever responding generally increased across the initial exposure to the tandem schedule, whereas inoperative-lever responding did not, and responding shifted from one to the other lever in accordance with the contingency reversal.
Of particular interest is identifying the variables responsible for the responding observed under the VT schedule. Presumably, these same variables are responsible for responding early in acquisition under the tandem schedule. One possibility is that this initial responding was the result of observational learning. Subiaul, Cantlon, Holloway, and Terrace (2004) reported that the acquisition of novel response sequences was facilitated when rhesus monkeys could observe another “expert” monkey perform the sequence, relative to learning by trial and error. In this experiment, all monkeys in the colony could view other monkeys lever pressing, although the prevailing reinforcement contingencies varied widely throughout the colony.
Stimulus novelty and cocaine-induced increases in activity also may have contributed to the initial responding. These processes, however, cannot account for the entire set of results. For example, if responding was maintained initially by stimulus novelty, then it might be expected to extinguish with continued exposure to the stimuli. If responding was reflective merely of an increase in activity, then differences in operative- and inoperative-lever responding would not have been observed.
A recent study from our laboratory suggests another possible account. We informally observed most monkeys touching or licking the stimulus lights within the first experimental session. This light-directed behavior results in an orientation in front of the operant panel, increasing the probability of contact with the levers. Reilly, Berndt, Woods, and Winger (2005) attempted to isolate the variables controlling this light-directed behavior by manipulating the correlation between cocaine delivery and the illumination of the stimulus lights. Reilly et al. argued that stimulus novelty might be responsible for the initial light-directed behavior. Thereafter, light-directed behavior may be maintained by both adventitious reinforcement and possible Pavlovian eliciting functions of the stimuli. From a Pavlovian account, cocaine is the unconditioned stimulus (US) and the stimulus lights are conditioned stimuli (CS). The conditioned response (CR) consists of light-directed behavior such as mouthing or touching the stimulus lights. Reilly et al. noted that it is still unclear why the CR is structurally different than the unconditioned response (UR) to cocaine. In our experience, rhesus monkeys do not, in the absence of stimulus lights, exhibit mouthing or touching responses to cocaine infusions alone.
A unique feature of our procedure is that we illuminated the red light over the operative lever, but did not illuminate the light over the inoperative lever. Previous experiments investigating response acquisition with operative and inoperative manipulanda have not employed differential discriminative stimuli (e.g., Wilkenfield et al., 1992). As this was an initial attempt to demonstrate acquisition of cocaine self-administration with delayed reinforcement, we chose to employ differential stimuli to increase the probability that the operative response would be acquired. Similarly, Lattal and Gleeson (1990) employed a key extension in some conditions to increase the probability that pigeons' pecks would be captured, fostering contact with the contingency. A drawback of our procedure is that it is difficult to ascertain if higher response rates on the operative lever reflect sensitivity to the operant contingency or simply are the result of the eliciting or discriminative functions of the stimulus light. Our monkeys did approach, touch, and mouth the stimulus over the operative lever, and this may have biased responding by making it more likely that lever presses would occur on the operative lever. Moreover, if the red light was functioning as a discriminative stimulus, the results from the contingency-reversal condition could be due entirely to stimulus control.
An interpretation based entirely on stimulus control is problematic. Several monkeys initially responded on the inoperative lever at rates equal to or greater than rates on the operative lever. In addition, for some monkeys it took a number of sessions for responding to shift following the contingency reversal. Nevertheless, the use of differential stimuli may have fostered contact with the operant contingency. A replication of the current experiment using nondifferential stimuli associated with the operative and inoperative levers would provide a more stringent test of sensitivity to delayed reinforcement.
After responding extinguished on the VT schedule, response reacquisition on the tandem schedule was retarded. These results are similar to ones obtained by Snycerski et al. (2004) who investigated the effects of VT food delivery on subsequent acquisition with delayed reinforcement. Snycerski et al. found that if the response levers were present in the chamber during VT food delivery, then subsequent acquisition was retarded. If the levers were not present in the chamber during VT food delivery, then acquisition occurred at the same rate as for subjects that did not receive a VT history. In addition, two studies have demonstrated retardation of an autoshaped keypeck in chickens and pigeons as a function of extended exposure to noncontingent food delivery (Downing & Neuringer, 1976; Engberg, Hansen, Welker, & Thomas, 1972).
Onset of action has been shown to be an important determinant of drug reinforcing effects (Balster & Schuster, 1973; Panlilio et al., 1998; Winger et al., 2002). The results from this experiment demonstrate that cocaine functioned as an effective reinforcer even though its onset of action was temporally removed from the operant response. It would be interesting to compare the reinforcing functions of a drug with a rapid onset of action available after a delay with a drug with a slower onset of action available immediately. Studying drug self-administration during acquisition eliminates any effects of prior exposure to the drug. Ultimately, the effects of delayed reinforcement on drug self-administration may shed light on applied issues, such as how some drugs with relatively slow onsets of action acquire reinforcing functions, and how drug-procuring behavior can exist at considerable strength despite being temporally removed from drug ingestion.
Acknowledgments
This research was supported by NIH/NIDA Grant Numbers DA01961, DA00254, and F007190. We would like to thank our research technicians, Erich Dosier, Sean Gallagher, Amy Hall, and Debbie Huntzinger, who carried out daily laboratory operations. We also would like to thank Tiffany Bass for determining the experimental history of monkeys prior to their arrival in our facility, Gary Duma for help in manuscript preparation, and Adam Doughty, Tammy Wade-Galuska, and Gail Winger for providing helpful comments on an earlier draft of this manuscript.
REFERENCES
- Balster R.L, Schuster C.R. Fixed-interval schedule of cocaine reinforcement: Effect of dose and infusion duration. Journal of the Experimental Analysis of Behavior. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley P.M, Balster R.L. The effects of delay of reinforcement and dose on the self-administration of cocaine and procaine in rhesus monkeys. Drug & Alcohol Dependence. 1993;34:37–43. doi: 10.1016/0376-8716(93)90044-q. [DOI] [PubMed] [Google Scholar]
- Byrne T, LeSage M.G, Poling A. Effects of chlorpromazine on rats' acquisition of lever-press responding with immediate and delayed reinforcement. Pharmacology, Biochemistry & Behavior. 1997;58:31–35. doi: 10.1016/s0091-3057(96)00454-6. [DOI] [PubMed] [Google Scholar]
- Critchfield T.S, Lattal K.A. Acquisition of a spatially defined operant with delayed reinforcement. Journal of the Experimental Analysis of Behavior. 1993;59:373–387. doi: 10.1901/jeab.1993.59-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Watt A, Griffiths W.J.H. Free-operant acquisition with delayed reinforcement. The Quarterly Journal of Experimental Psychology. 1992;45B:241–258. [Google Scholar]
- Dinsmoor J.A. Stimuli inevitably generated by behavior that avoids electric shock are inherently reinforcing. Journal of the Experimental Analysis of Behavior. 2001;75:311–333. doi: 10.1901/jeab.2001.75-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing K, Neuringer A. Autoshaping as a function of prior food presentations. Journal of the Experimental Analysis of Behavior. 1976;26:463–469. doi: 10.1901/jeab.1976.26-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg L.A, Hansen G, Welker R.L, Thomas D.R. Acquisition of key-pecking via autoshaping as a function of prior experience: “Learned laziness”? Science. 1972, December 8;178:1002–1004. doi: 10.1126/science.178.4064.1002. [DOI] [PubMed] [Google Scholar]
- Gollub L, Yanagita T. Delayed cocaine reinforcement on lever-pressing in rhesus monkeys. Bulletin of Psychonomic Science. 1974;4:263. [Google Scholar]
- Lattal K.A, Gleeson S. Response acquisition with delayed reinforcement. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:27–39. [PubMed] [Google Scholar]
- Lattal K.A, Metzger B. Response acquisition by Siamese fighting fish (Betta splendens) with delayed visual reinforcement. Journal of the Experimental Analysis of Behavior. 1994;61:35–44. doi: 10.1901/jeab.1994.61-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal K.A, Williams A.M. Body weight and response acquisition with delayed reinforcement. Journal of the Experimental Analysis of Behavior. 1997;67:131–143. doi: 10.1901/jeab.1997.67-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage M.G, Byrne T, Poling A. Effects of d-amphetamine on response acquisition with immediate and delayed reinforcement. Journal of the Experimental Analysis of Behavior. 1996;66:349–367. doi: 10.1901/jeab.1996.66-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio L.V, Goldberg S.R, Gilman J.P, Jufer R, Cone E.J, Schindler C.W. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Reilly M.P, Berndt S.I, Woods J.H, Winger G. Drug sign tracking in rhesus monkeys. 2005 Manuscript submitted for publication. [Google Scholar]
- Snycerski S, Laraway S, Huitema B.E, Poling A. The effects of behavioral history on response acquisition with immediate and delayed reinforcement. Journal of the Experimental Analysis of Behavior. 2004;81:51–64. doi: 10.1901/jeab.2004.81-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretch R, Gerber G.J, Lane E. Cocaine self-injection behaviour under schedules of delayed reinforcement in monkeys. Canadian Journal of Physiology and Pharmacology. 1976;54:632–638. doi: 10.1139/y76-089. [DOI] [PubMed] [Google Scholar]
- Subiaul F, Cantlon J.F, Holloway R.L, Terrace H.S. Cognitive imitation in rhesus macaques. Science. 2004, July 16;305:407–410. doi: 10.1126/science.1099136. [DOI] [PubMed] [Google Scholar]
- Sutphin G, Byrne T, Poling A. Response acquisition with delayed reinforcement: A comparison of two-lever procedures. Journal of the Experimental Analysis of Behavior. 1998;69:17–28. doi: 10.1901/jeab.1998.69-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkenfield J, Nickel M, Blakely E, Poling A. Acquisition of lever-press responding in rats with delayed reinforcement: A comparison of three procedures. Journal of the Experimental Analysis of Behavior. 1992;58:431–443. doi: 10.1901/jeab.1992.58-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Hursh S.R, Casey K.I, Woods J.H. Relative reinforcing effects of three NMDA antagonists with different onsets of action. Journal of Pharmacology and Experimental Therapeutics. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]