Abstract
The molecular mechanism for the anti-inflammatory action of theophylline is currently unknown, but low-dose theophylline is an effective add-on therapy to corticosteroids in controlling asthma. Corticosteroids act, at least in part, by recruitment of histone deacetylases (HDACs) to the site of active inflammatory gene transcription. They thereby inhibit the acetylation of core histones that is necessary for inflammatory gene transcription. We show both in vitro and in vivo that low-dose theophylline enhances HDAC activity in epithelial cells and macrophages. This increased HDAC activity is then available for corticosteroid recruitment and predicts a cooperative interaction between corticosteroids and theophylline. This mechanism occurs at therapeutic concentrations of theophylline and is dissociated from phosphodiesterase inhibition (the mechanism of bronchodilation) or the blockade of adenosine receptors, which are partially responsible for its side effects. Thus we have shown that low-dose theophylline exerts an anti-asthma effect through increasing activation of HDAC which is subsequently recruited by corticosteroids to suppress inflammatory genes.
Keywords: macrophages‖corticosteroids‖histone deacetylation‖granulocyte–macrophage colony-stimulating factor
Asthma is a chronic inflammatory disease of the airways characterized by reduced airway patency, which is regulated by bronchodilators such as β-agonists, and by the infiltration of inflammatory and immune cells, which is treated by corticosteroids (1). Theophylline has been used in the treatment of asthma for over 70 years, but its use has recently declined, as inhaled corticosteroids have become the mainstay of asthma control and inhaled β2-agonists are more effective bronchodilators. Furthermore, side effects, such as nausea and headaches, commonly occur at previously recommended doses of theophylline. Originally, theophylline was used as a bronchodilator and the optimal plasma concentration that gave maximal bronchodilation with the lowest risk of side effects was found to be 10–20 mg/liter (55–110 μM) (2).
There is increasing evidence that theophylline has anti-inflammatory or immunomodulatory actions in asthma (2). Low doses of theophylline, which give a plasma concentration of <5 mg/liter, may achieve control of asthma comparable to a low dose of inhaled corticosteroids in both children and adults (3, 4). In asthmatic patients low-dose theophylline reduces eosinophils and other inflammatory markers (5–7), inhibits the eosinophilia induced by an inhaled allergen (8), and reduces the expression of cytokines, such as interleukin (IL)-5 (9). Long-term treatment with theophylline reduces airway hyperresponsiveness to methacholine challenge (10). In addition, in patients with severe asthma who are withdrawn from theophylline, there is a deterioration of asthma control, despite the fact that patients are maintained on high does of inhaled corticosteroids (11, 12).
Several studies have demonstrated an interaction with corticosteroid therapy and the steroid-sparing effects of theophylline (13). In patients with mild and moderate asthma, the addition of low-dose theophylline gives a greater improvement in asthma control, measured as lung function, symptoms, and rescue β2-agonist use, than that achieved by doubling the dose of inhaled corticosteroid (5, 14, 15).
The molecular mechanisms for the anti-inflammatory action of theophylline are unclear. The bronchodilator action of theophylline can be explained by the inhibition of phosphodiesterases (PDEs) in airway smooth muscle, but this occurs at concentrations of >50 μM (16). In addition, the common side effects of theophylline, nausea and vomiting, are probably because of PDE4 inhibition (13, 17). Another proposed mechanism involves the antagonism of the bronchoconstrictor adenosine, which may also account for some of the serious side effects of theophylline, including cardiac arrhythmias and seizures. There is also evidence for other anti-inflammatory mechanisms that cannot be accounted for by either PDE inhibition or adenosine-receptor antagonism, including the inhibition of nuclear factor κB (NF-κB) (18) and the inhibition of IL-5- and granulocyte-macrophage colony stimulating factor (GM-CSF)-induced eosinophil survival (19, 20).
Acetylation of core histones by coactivator proteins, such as CREB-binding protein (CBP), facilitates transcription (21). We have recently demonstrated that the activation of GM-CSF by IL-1β results from NF-κB activation and increased histone acetyltransferase (HAT) activity, leading to increased inflammatory gene transcription (22). Corticosteroids inhibit the expression of GM-CSF by reversing the activation of HAT through the activated glucocorticoid receptor recruiting corepressor proteins that have histone deacetylase (HDAC) activity (22). HDACs then deacetylate the histones acetylated by NF-κB activation, thereby suppressing inflammatory gene expression. Because theophylline affects gene transcription in low concentrations and appears to interact beneficially with corticosteroids, we studied the effect of theophylline alone, and in combination with dexamethasone, on histone acetylation and deacetylation in vitro by using bronchoalveolar lavage (BAL) macrophages and in bronchial biopsies of asthmatic patients treated with low-dose theophylline.
Materials and Methods
Effect of Theophylline on Clinical Parameters.
We examined the effect of 4 weeks of treatment with low-dose theophylline (Euphylong, 250 mg twice daily) on HDAC activity in 14 mild stable asthmatics by using a double-blind crossover controlled study. Blood concentrations of theophylline were elevated in treated subjects (4.3 ± 0.85 mg/liter) as compared with placebo (<1 mg/liter). The clinical characteristics and effects on eosinophilia have been published elsewhere (5).
Fiberoptic Bronchoscopy and Isolation of BAL Macrophages.
Subjects attended our bronchoscopy suite at 8.30 a.m. after having fasted from midnight and were pretreated with atropine (0.6 mg i.v.) and midazolam (5–10 mg i.v.). Oxygen (3 liters/min) was administered with nasal prongs throughout the procedure and oxygen saturation was monitored with a digital oximeter. While the subject received local anesthesia with lidocaine (4%) to the upper airways and larynx, a fiberoptic bronchoscope (Olympus BF10, Key-Med, Southall, U.K.) was passed through the nasal passages into the trachea. BAL was performed from the right middle lobe by using warmed 0.9% NaCl with four successive aliquots of 60 ml. BAL cells were spun (500 × g; 10 min) and washed twice with Hanks' buffered salt solution (HBSS) (12). Cytospins were prepared and stained with May–Grunwald stain for differential cell counts. Cell viability was assessed by using trypan blue exclusion. In some experiments macrophages were isolated by plastic adhesion and cells (1 × 106) incubated in 24-well plates in the presence of theophylline, dexamethasone, or Salmonella enteritidis lipopolysaccharide (LPS; 100 ng/ml). In addition, we studied BAL macrophages isolated from six normal nonsmoking subjects (28.8 ± 0.9 yr) and six normal smokers (26.3 ± 1.3 yr).
Cell Culture.
A549 cells were grown to 50% confluence in Dulbecco's modified Eagle's medium (DMEM) containing 10% FCS before incubation for 48–72 h in serum-free medium. Cells were stimulated by IL-1β (1 ng/ml) or LPS (3 ng/ml) in the presence of theophylline or dexamethasone.
GM-CSF and IL-8.
GM-CSF and IL-8 were measured by sandwich ELISA (R&D Systems Europe, Abindon, U.K.) according to the manufacturer's instructions.
Direct Histone Extraction.
Histones were extracted from nuclei overnight by using HCl and H2SO4 at 4°C and using a method modified from that as described by Turner (23) and by Yoshida (24). Cells were microcentrifuged for 5 min and the cell pellets extracted with ice-cold lysis buffer (10 mM Tris⋅HCl, pH 6.5/50 mM sodium bisulfite, 1% Triton X-100/10 mM MgCl2/8.6% sucrose/complete protease inhibitor mixture (Roche Molecular Biochemicals) for 20 min at 4°C. The pellet was repeatedly washed in buffer until the supernatant was clear (centrifuged at 7,500 × g, 5 min after each wash) and the nuclear pellet was washed in nuclear wash buffer (10 mM Tris⋅HCl/13 mM EDTA, pH 7.4) and resuspended in 50 μl of 0.2 M HCl and 0.2 M H2SO4 in distilled water. The nuclei were extracted overnight at 4°C and the residue was microcentrifuged for 10 min. The supernatant was mixed with 1 ml of ice-cold acetone and left overnight at −20°C. The sample was microcentrifuged for 10 min, washed with acetone, dried, and diluted in distilled water. Protein concentrations of the histone-containing supernatant were determined by Bradford protein assay kit (Bio-Rad).
Western Blotting.
Immunoprecipitates, whole-cell extractions, or isolated histones were measured by SDS/PAGE and Western blot analysis using ECL (Amersham Pharmacia). Proteins were size-fractionated by SDS/PAGE and transferred to Hybond-ECL membranes. Specific protein bands were detected by ECL according to the manufacturer's instructions.
Histone Acetylation Activity.
Cells were plated at a density of 0.25 × 106 cells/ml and exposed to 0.05 mCi/ml (1 Ci = 37 GBq) of [3H]acetate (Amersham Pharmacia). After incubation for 10 min at 37°C cells were stimulated for 6 h. Histones were isolated and separated by electrophoresis on SDS/16% polyacrylamide gels. Gels were stained with Coomassie brilliant blue, and the core histones (H2A, H2B, H3, and H4) were excised. The radioactivity in extracted core histones was determined by liquid scintillation counting and normalized to protein level.
Histone Deacetylation Activity.
Radiolabeled histones were prepared from A549 cells after incubation with the HDAC inhibitor trichostatin A (TSA), at 100 ng/ml for 6 h, in the presence of 0.1 mCi/ml [3H]acetate. Histones were dried and resuspended in distilled water. Crude HDAC preparations were extracted from total cellular homogenates with Tris-based high-salt buffer (10 mM Tris⋅HCl, pH 8.0/500 mM NaCl/0.25 mM EDTA/10 mM 2-mercaptoethanol) as reported (25). The crude HDAC preparation or immunoprecipitates were resuspended in the Tris-based low-salt buffer (10 mM Tris⋅HCl, pH 8.0/20 mM NaCl/0.25 mM EDTA/10 mM 2-mercaptoethanol), and incubated with 3H-labeled histone for 30 min at 30°C before the reaction was stopped by the addition of 1 M HCl/0.4 M acetic acid. Released 3H-labeled acetic acid was extracted by ethyl acetate, and the radioactivity of the supernatant was determined by liquid scintillation counting. In some experiments the pH and substrate concentrations were altered. Experiments were also conducted using a commercially available fluorescent HDAC assay kit (Fluor de Lys, BioMol, Exeter, U.K.) according to the manufacturer's instructions. The results were essentially identical to those obtained with the radioactive assay.
Chromatin Immunoprecipitation Assay.
A-549 cells were pretreated for 30 min with dexamethasone or theophylline before stimulation with IL-1β (1 ng/ml) for 4 h. Protein–DNA complexes were fixed by formaldehyde (1% final concentration) and treated as described (26). Cells were resuspended in 200 μl of SDS lysis buffer (50 mM Tris, pH 8.1/1% SDS/5 mM EDTA/complete proteinase inhibitor mixture) and subjected to three cycles of sonication on ice with 10-s pulses. Sonicated samples were centrifuged to spin down cell debris and the soluble chromatin solution was immunoprecipitated by using an anti-acetylated histone H4 antibody (Upstate Biotechnology, Buckingham, U.K.) as described (27). Protein-bound immunoprecipitated DNA was washed with LiCl wash buffer and 10 mM Tris/1 mM EDTA, pH 8.0, TE buffer, and immune complexes were eluted by adding elution buffer (1% SDS/0.1 M NaHCO3). The elution was treated successively for 4 h at 65°C in 200 mM NaCl/1% SDS to reverse crosslinks and incubated for 1 h at 45°C with 70 μg/ml proteinase K (Sigma). DNA was extracted with phenol/chloroform, precipitated with ethanol/0.3 M NaHCOOH/20 μg glycogen, and resuspended in 50 μl of TE. Quantitative PCR was performed with 10 μl of DNA sample and 30 cycles (94°C, 45 s; 61°C, 45 s; 72°C, 45 s. Primer pairs of GM-CSF were as follows: forward 5′-CTGACCACCTAGGGAAAAGGC-3′; reverse 5′-CAGCCACATCCT CCTCCAGAGAAC-3′. PCR products were resolved by electrophoresis through a 3% agarose gel and visualized with ethidium bromide.
Statistics.
Results are expressed as mean ± standard error of the mean (SEM). A multiple comparison was made between the mean of the control and the mean from each individual treatment group in the in vitro study by Dunnett's test, and a comparison between the mean of placebo and that of theophylline treatment in clinical study by paired t test using SAS/STAT software (SAS Institute, Cary, NC). All statistical testing was performed by using a two-sided 5% level of significance. The concentrations of dexamethasone or TSA producing 50% inhibition (IC50) were calculated from concentration–response curves by linear regression.
Results
Effect of Theophylline on HDAC Expression and Activity in Vivo.
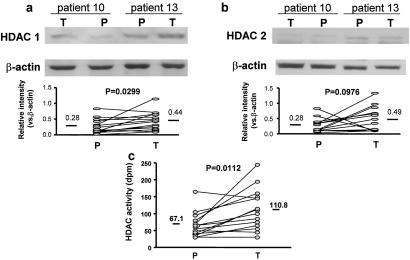
HDAC1 and HDAC2 were predominantly localized to the epithelium in bronchial biopsies and their distribution was not altered by theophylline treatment (data not shown). Western blot analysis demonstrated a significant increase in HDAC1 (0.28 ± 0.06 vs. 0.44 ± 0.07, P = 0.0299), but not HDAC2 (0.28 ± 0.06 vs. 0.49 ± 0.10, P = 0.1) expression, after theophylline treatment (Fig. 1 a and b). In addition, there was a significant increase in total HDAC activity in biopsies from subjects treated with theophylline (67 ± 9 vs. 111 ± 17 dpm/mg of protein, P = 0.0112) (Fig. 1c).
Figure 1.
Effect of theophylline on HDAC expression and activity in vivo. (a and b Upper) Western blot analysis of HDAC1 (a) and HDAC2 (b) expression in bronchial biopsies from mild asthmatic subjects treated with low-dose theophylline (T) or placebo (P) (20). (a and b Lower) Graphical expression of the effect of low-dose theophylline (T) and placebo (P) on HDAC1 and HDAC2 expression relative to β-actin. (c) Effect of low-dose theophylline (T) and placebo (P) on HDAC activity in bronchial biopsies. Mean values are given by bars. n = 14 for HDAC1, HDAC2, and HDAC activity assays.
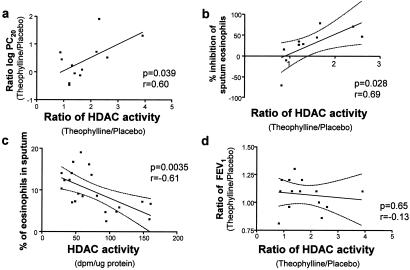
There was a significant correlation between the induction of HDAC activity by low-dose theophylline and improvements in PC20 methacholine (r = 0.60, P = 0.039, Fig. 2a) and the inhibition of sputum eosinophils (r = 0.69, P = 0.028, Fig. 2b). In addition, there was a significant negative correlation between HDAC activity and sputum eosinophilia (r = −0.61, P = 0.0035, Fig. 2c). However, there was no correlation between the induction of HDAC activity by low-dose theophylline and the change in FEV1 (r = 0.13, P = 0.65, Fig. 2d).
Figure 2.
Correlation between theophylline actions on HDAC activity and clinical parameters. (a and b) Correlation between changes in HDAC activity induced by theophylline and theophylline-induced changes in PC20 (concentration that provokes a 20% change in FEV) for methacholine (a) and inhibition of sputum eosinophils (b). (c) Correlation between HDAC activity and sputum eosinophils in normal and asthmatic subjects. (d) No correlation between FEV1 (forced expiratory volume in 1 s) and theophylline-induced HDAC activity. Dotted lines represent 95% confidence limits.
Effect of Theophylline on HDAC Activity in BAL Macrophages.
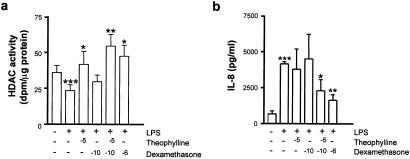
We next examined whether low-dose theophylline could also have an effect on HDAC activity in a clinically relevant cell, such as macrophages that are involved in asthmatic inflammation. Alveolar macrophages were incubated for 12 h in the presence of LPS (10 ng/ml) and increasing concentrations of theophylline and dexamethasone. LPS significantly reduced whole-cell HDAC activity (Fig. 3a) and theophylline caused a concentration-dependent increase in LPS-suppressed HDAC activity that was maximal at 10−5 M and was returned back to control levels at 10−3 M (data not shown). Dexamethasone also enhanced HDAC activity in a concentration-dependent manner with a maximal effect at 10−6 M (Fig. 3a). Neither the PDE4 inhibitor rolipram (10 μM) nor the nonspecific PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX) (500 μM) had any effect on HDAC activity in these cells (data not shown).
Figure 3.
Effect of theophylline on HDAC activity. BAL macrophages were incubated for 12 h with LPS after treatment with theophylline for 10 min or dexamethasone for 30 min. HDAC activity (a) and IL-8 secretion (b) were measured in normal nonsmoking subjects as described in Materials and Methods. Results are expressed as mean ± SEM (n = 5; *, P < 0.05; **, P < 0.01 vs. LPS control; ***, P < 0.05 vs. unstimulated cells.
Interaction with Corticosteroids.
We have previously shown that a major component of glucocorticoid actions in the suppression of inflammatory cytokine production is through the recruitment of HDAC activity to the activated transcriptional complex (22). Because theophylline enhances HDAC activity directly we have examined whether theophylline enhances glucocorticoid activity in vitro in a manner similar to that seen clinically (14, 15). In alveolar macrophages from nonsmokers we found that theophylline (10−5 M) significantly enhanced HDAC activity in vitro, whereas a low concentration of dexamethasone (10−10 M) had no effect (Fig. 3a). Combined treatment with dexamethasone (10−10 M) and theophylline (10−5 M) markedly enhanced the effect seen with theophylline alone and this effect was similar that seen with 10−6 M dexamethasone (Fig. 3a). These results correlated with functional repression of LPS-induced IL-8 release by combined theophylline and dexamethasone treatment (Fig. 3b). Dexamethasone (10−10 M) failed to suppress LPS-induced IL-8 production. Low concentrations of theophylline alone had no effect on LPS-induced IL-8 release, presumably because the increased HDAC activity is not targeted to the activated transcriptional complex. In contrast, combined dexamethasone (10−10 M) and theophylline (10−5 M) caused a 50% reduction in LPS-induced IL-8 release similar to levels seen with the highest concentration of dexamethasone tested (10−6 M).
Effect of Theophylline on HDAC Activity in A549 Cells.
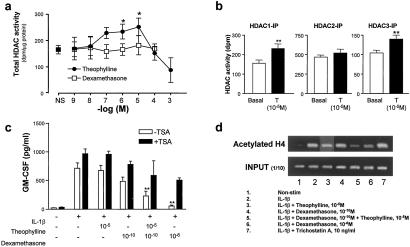
To determine whether the effect of theophylline was specific to macrophages or if it was more universal we used the human lung epithelium-like A549 cell line. We examined the direct effect of theophylline and dexamethasone on nuclear extracts containing HDAC activity in vitro, as described (22). Theophylline gave a concentration-dependent increase in HDAC activity that reached a maximum at 10−5 M, whereas at higher concentrations (10−4 to 10−3 M) theophylline inhibited HDAC activity (Fig. 4a). A similar effect was seen with another methyl xanthine, enprofylline (data not shown). In contrast, dexamethasone had no direct effect on HDAC activity (Fig. 4a). IBMX (10−11 to 10−3 M) also had no effect on HDAC activity (data not shown). This suggests that theophylline enhances HDAC activity by modulating enzyme function directly, whereas dexamethasone increases HDAC activity indirectly through an increase in HDAC expression. This effect of theophylline on HDAC activity was not specific to all HDAC isoforms. Theophylline (10−5 M) enhanced immunoprecipitated HDAC1 and HDAC3 activity but had little effect on HDAC2 activity (Fig. 4b).
Figure 4.
Theophylline synergizes with dexamethasone through the induction of HDAC activity in A549 cells. (a) Direct effect of theophylline and dexamethasone on HDAC activity in A549 cells. Nuclear proteins containing HDAC activity were isolated from untreated cells and incubated with 3H-labeled histones for 45 min in the presence of theophylline or dexamethasone. (b) Effect of theophylline (T, 10−5 M) on immunoprecipitated HDAC1, HDAC2, and HDAC3 in A549 cells. Nuclear protein was extracted and immunoprecipitated with anti-HDAC1, anti-HDAC2, and anti-HDAC3 antibody. The immunoprecipitates were stimulated with theophylline for 30 min. HDAC assays were performed and the effect of theophylline on each HDAC was examined. Results are expressed as mean ± SEM (n = 3–5; **, P < 0.01). (c) GM-CSF release into the culture medium of IL-1β-stimulated cells in the presence of theophylline, dexamethasone, or combined treatment was determined by ELISA after 24 h. In additional experiments the effect of pretreatment with TSA (10 ng/ml) on these actions was examined. Results are expressed as mean ± SEM (n = 3–5; *, P < 0.05; **, P < 0.01). (d) Theophylline and dexamethasone in combination inhibit IL-1β-stimulated association of acetylated histone 4 with the GM-CSF promoter. A549 cells pretreated with theophylline (10−5 M) or dexamethasone (10−10 M or 10−6 M) for 30 min were incubated with IL-1β (1 ng/ml) for 4 h. Proteins and DNA were crosslinked by formaldehyde treatment, and chromatin pellets were extracted. After sonication, acetylated histone H4 was immunoprecipitated and the associated DNA was amplified by PCR. Results are representative of three independent experiments.
Functionally, both theophylline (10−5 M) and dexamethasone (10−10 M) alone failed to cause significant suppression of IL-1β-induced GM-CSF release, whereas combined dexamethasone (10−10 M) and theophylline (10−5 M) caused a significant 70% reduction in IL-1β-induced GM-CSF release (229 ± 84 vs. 714 ± 94 pg/ml).
To confirm that the effects of theophylline were mediated through HDAC activity we investigated the effect of the HDAC inhibitor TSA (10 ng/ml) on repression of IL-1β-stimulated GM-CSF release. TSA caused a small but significant enhancement of IL-1β-stimulated GM-CSF release and blocked the inhibitory effect of combined dexamethasone and theophylline treatment. Dexamethasone (10−6 M) alone caused a 96% inhibition of IL-1β-stimulated GM-CSF release, which was inhibited by 48% with TSA (506 ± 40 vs. 969 ± 84 pg/ml, Fig. 4c), thus confirming a role for HDACs in dexamethasone-mediated gene repression.
Theophylline in Combination with Dexamethasone Reduces Histone H4 Acetylation at the GM-CSF Promoter.
To be functionally relevant this reduced acetylation must occur at the correct promoter sites. By using chromatin immunoprecipitation assays, we showed that histone H4 acetylation was involved in IL-1β-stimulated GM-CSF promoter activation. Both theophylline (10−5 M) and dexamethasone (10−10 M) had no effect on the IL-1β-stimulated increase of GM-CSF promoter associated with acetylated H4 (Fig. 4d). In contrast, the combination of theophylline (10−5 M) and dexamethasone (10−10 M) markedly reduced the amount of IL-1β-stimulated acetylated histone H4 associated with the GM-CSF promoter to a level similar to that seen with 10−6 M dexamethasone. As described (22) the HDAC inhibitor TSA (10 ng/ml) increased the amount of acetylated lysine residues stimulated by IL-1β (Fig. 4d, lane 7).
Theophylline Does Not Act Through PDE4 or Adenosine Receptors.
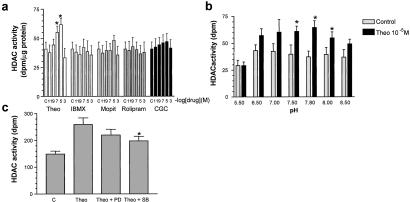
It has been postulated that the effects of theophylline are mediated through the inhibition of PDE4 or by means of the antagonism of adenosine receptors. We therefore examined the effect of a PDE4 inhibitor (rolipram, 10−11 to 10−3 M), a PDE3 inhibitor [Motapizone (Byk-Gulden, Konstanz, Germany), 10−11 to 10−3 M], and an adenosine receptor antagonist (CGS 15943, 10−11 to 10−3 M), on HDAC activity (Fig. 5a). Neither these drugs nor the nonselective PDE inhibitor IBMX (10−1 to 10−3 M) had any direct effect on HDAC activity (Fig. 5a), indicating that this is a previously undocumented molecular action of theophylline. It is also one of the few effects that has been reported at therapeutic drug concentrations. Neither IBMX, rolipram, nor motapizone (all at 10−5 M) had any direct effect on HAT activity (data not shown).
Figure 5.
Theophylline effects on HDAC activity are not mediated through inhibition of PDEs in A549 cells. (a) Direct effect of theophylline (Theo, 10−11 to 10−3 M), the nonspecific PDE inhibitor IBMX (10−11 to 10−3 μM), the PDE3 inhibitor motapizone (10−11 to 10−3 M), the PDE4 inhibitor rolipram (10−11 to 10−3 M), and the adenosine receptor antagonist CGS-15943 (CGS) (10−11 to 10−3 M) on HDAC activity. Nuclear proteins containing HDAC activity were isolated from untreated cells and incubated with 3H-labeled histones for 45 min. Results are expressed as mean ± SEM (n = 3–5; *, P < 0.05). (b) Direct effect of pH on theophylline (Theo, 10−5 M) induction of HDAC activity. Nuclear proteins containing HDAC activity were isolated from untreated cells and incubated with 3H-labeled histones for 45 min in differing assay pH conditions. Results are expressed as mean ± SEM (n = 3; *, P < 0.05). (c) Direct effect of inhibition of p38 MAPK (SB203580) and MEK (PD098059) on theophylline (Theo, 10−5 M) induced HDAC activity. Results are expressed as mean ± SEM (n = 3; *, P < 0.05).
Because theophylline may be acting allosterically to alter HDAC activity and since allosteric activators work best away from maximal assay conditions, we examined the effect of pH on theophylline-induced HDAC activity. Altering the pH of the assay changed both the basal HDAC activity and the ability of theophylline to induce HDAC activity from a 40% increase at pH 8.0 to a 75% increase at pH 7.8 (Fig. 5b). Because HDACs are phosphoproteins whose activity is altered on phosphorylation status (28) we also examined the effect of mitogen-activated protein kinase (MAPK) inhibition on theophylline actions. The treatment of HDAC extracts with alkaline phosphatase reduced HDAC activity (521 ± 53 vs. 897 ± 64 dpm/μg of protein), confirming a role for phosphorylation in HDAC activity. The inhibition of p38 MAPK by SB203580 partially inhibited theophylline-induced HDAC activity (198 ± 17 vs. 259 ± 25 dpm), whereas the inhibition of MEK by PD098059 had no effect (220 ± 21 vs. 259 ± 25 dpm) (Fig. 5c). This finding suggests that theophylline may modulate HDAC activity, at least in part, by stimulating p38 MAPK-modulated HDAC phosphorylation.
Discussion
Low concentrations of theophylline had a marked effect on HDAC activity in BAL macrophages and in a human epithelial cell line (A549). Theophylline at concentrations of 10−6 to 10−5 M had a significant stimulatory effect on whole-cell HDAC activity even after the LPS-induced repression of HDAC activity. This effect was because of a direct induction of HDAC enzymatic activity, rather than an induction of HDAC protein or gene expression, although prolonged in vivo treatment with theophylline for 4 weeks may also result in increased HDAC expression. After this period, HDAC expression may play a role in theophylline actions. However, previous studies (14, 15) indicate that the beneficial effects of theophylline in combination with inhaled steroids occur within 1 week of treatment. This is too early to be accounted for by theophylline induction of HDAC expression in these patients. Interestingly, the improvement in lung function continues between 4 and 6 weeks, which may reflect the additional effect of theophylline on HDAC expression seen in this study. The effects of combined theophylline and dexamethasone treatment in cells suggest that this direct effect of theophylline on HDAC activity is predominant, at least during the short-term treatment, when rapid increases in lung function are seen. Steroids, which also induce HDAC expression over the long term, take up to 8 weeks clinically to achieve a comparable effect seen with the combination therapy over 1 week (14, 15).
The anti-inflammatory effects of theophylline have previously been ascribed to PDE4 inhibition and adenosine receptor antagonism, although these effects occur at higher concentrations of theophylline than are needed for anti-inflammatory actions (2). We demonstrated that neither PDE inhibition nor adenosine receptor antagonism mimicked the effects of theophylline on HDAC activation. This result suggests that we are describing a molecular mechanism of the action of theophylline. In contrast, glucocorticoids at high concentrations increased total cell HDAC activity through means of the induction of HDAC protein and gene expression. This action of theophylline on HDAC activity is selective, targeting HDACs 1 and 3 preferentially, and shows the previously undocumented existence of a selective activator of HDAC activity.
Several studies have shown that low doses of theophylline have an anti-inflammatory or immunomodulatory effect in vivo (3, 4, 6–12, 29). In our current study we did not find any significant effect of theophylline on lung function or airway hyperresponsiveness (5). However, we found a significant decrease in BAL and airway eosinophils, along with an induction of HDAC activity in bronchial biopsies and BAL macrophages. Eosinophil infiltration is characteristic of asthmatic airway inflammation, and eosinophil survival in the airways depends on GM-CSF secretion from epithelial cells (30). The modest anti-inflammatory effects seen in our patients may be because the subjects had mild asthma, allowing little room for improvement, and the fact that these patients were not treated with inhaled corticosteroids. The level of endogenous cortisol may be sufficient in these subjects to target activated HDAC to the site of inflammatory gene expression.
The molecular mechanisms for the anti-inflammatory action of theophylline are currently unclear. PDE inhibition in airway smooth muscle can explain the bronchodilator action of theophylline (16). However, this action occurs at doses too high to be relevant in our study (>10−4 M). However, many of the side effects of theophylline, including nausea and headaches, can be ascribed to PDE inhibition, suggesting that if the mechanism of the anti-asthma effect were identified it might be possible to develop safer drugs in the future.
More recently it has been shown that low concentrations of theophylline were able to inhibit the activation of NF-κB and reduce the expression of inflammatory genes in a manner similar to corticosteroids (18). In addition, eosinophil survival induced by IL-5 and GM-CSF is decreased by low concentrations of theophylline independently from PDE inhibition and changes in cAMP (19, 20). Our proposed mechanism of action of theophylline may account for these effects of theophylline on eosinophil survival through the inhibition of IL-5 and GM-CSF gene transcription.
The effects of theophylline are relatively small (up to 75% increase), but, by using a chromosome immunoprecipitation assay we can see that this drug can markedly reduce histone H4 acetylation at the GM-CSF promoter when targeted by dexamethasone. Waterborg (31) has shown that even in the resting state histones are acetylated and that small differences in the number of acetylated histones result in biophysical changes. Histone acetylation is now thought to be an important area of regulation whereby only small alterations in the number of acetylated lysines can rapidly switch a gene from an inactive to an active state and vice versa.
HDACs are phosphoproteins whose activity is modified according to their phosphorylation status (28). We have shown that the effect of theophylline is mediated, at least in part, by p38 MAPK. Using a protein motif sequence scanning program (scanprosite within Expasy, http://www.expasy.ch) we could find consensus phosphorylation sites within HDAC1, HDAC2, and HDCA3. However, a p38 MAPK docking site was detected only within HDAC3, and a potential docking site was found within HDAC1 but not within HDAC2. In addition, we found that the presence of tyrosine phosphorylation sites also differentiates between HDAC2, HDAC1, and HDCA3. Changing the assay conditions enhanced the effect of theophylline on HDAC activity, suggesting a possible allosteric action. It is unclear exactly how theophylline increases HDAC activity, whether it is allosteric or not, but it probably involves a phosphorylation event unrelated to protein kinase A (PKA).
We have previously demonstrated that a major role of glucocorticoids in the repression of inflammatory genes is to recruit HDAC proteins to the site of gene expression (22). These data suggest that theophylline should enhance glucocorticoid actions by enabling glucocorticoids to recruit HDACs with increased activity. We have shown in the present study that low concentrations of theophylline and low concentrations of dexamethasone can increase the repression of inflammatory cytokine release in both macrophages and epithelial cells. The importance of HDACs in this process was confirmed by the ability of the HDAC inhibitor TSA to completely block the combined action of low concentrations of theophylline and dexamethasone on IL-1β-stimulated GM-CSF release. This result suggests that the enhanced HDAC activity seen in the theophylline-treated patients would enable low doses of glucocorticoids to have enhanced efficacy in controlling airway inflammation. These studies suggest a mechanism to explain the in vivo beneficial interaction between low-dose theophylline and corticosteroids in asthmatic subjects (13, 14).
In summary, we have shown that both in vitro and in vivo theophylline induced a direct activation of HDAC activity. In vitro experiments indicated that this enhanced HDAC activity induced by theophylline was capable of synergizing with glucocorticoids on increasing total cell HDAC activity, inhibiting GM-CSF release, and modulating histone H4 acetylation at the GM-CSF promoter. This result suggests that the molecular mechanism behind the synergistic effect of theophylline on glucocorticoid actions in vivo is related to increased HDAC activity being recruited by glucocorticoid receptor to suppress inflammatory genes. These data may also explain why theophylline alone is not a very effective anti-inflammatory agent. In the absence of glucocorticoids the activated HDAC is not targeted to the site of inflammatory gene transcription. These studies suggest that there is potential to develop novel therapeutic agents that increase HDAC activity resulting in improved anti-inflammatory actions. These agents would act as steroid add-on therapies enhancing the transrepression/transactivation ratio of steroids and thus possessing a reduced side effect profile.
Acknowledgments
We thank the Clinical Research Committee (Royal Brompton Hospital, London), Byk-Gulden (Konstanz, Germany), Sankyo (Tokyo), and Glaxo-SmithKline (Stevenage, U.K.) for financial support.
Abbreviations
- HDAC
histone deacetylase
- PDE
phosphodiesterase
- IL
interleukin
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HAT
histone acetyltransferase
- BAL
bronchoalveolar lavage
- LPS
lipopolysaccharide
- TSA
trichostatin A
- IBMX
3-isobutyl-1-methylxanthine
- MAPK
mitogen-activated protein kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Barnes P J, Chung K F, Page C P. Pharmacol Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- 2.Barnes P J, Pauwels R A. Eur Respir J. 1994;7:579–591. doi: 10.1183/09031936.94.07030579. [DOI] [PubMed] [Google Scholar]
- 3.Tinkelman D G, Reed C E, Nelson H S, Offord K P. Pediatrics. 1993;92:64–77. [PubMed] [Google Scholar]
- 4.Reed C E, Offord K P, Nelson H S, Li J T, Tinkelman D G. J Allergy Clin Immunol. 1998;101:14–23. doi: 10.1016/s0091-6749(98)70187-3. [DOI] [PubMed] [Google Scholar]
- 5.Lim S, Tomita K, Carramori G, Jatakanon A, Oliver B, Keller A, Adcock I, Chung K F, Barnes P J. Am J Respir Crit Care Med. 2001;164:273–276. doi: 10.1164/ajrccm.164.2.2006043. [DOI] [PubMed] [Google Scholar]
- 6.Ward A J, McKenniff M, Evans J M, Page C P, Costello J F. Am Rev Respir Dis. 1993;147:518–523. doi: 10.1164/ajrccm/147.3.518. [DOI] [PubMed] [Google Scholar]
- 7.Jaffar Z H, Sullivan P, Page C, Costello J. Eur Respir J. 1996;9:456–462. doi: 10.1183/09031936.96.09030456. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Lancet. 1994;343:1006–1008. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 9.Finnerty J P, Lee C, Wilson S, Madden J, Djukanovic R, Holgate S T. Eur Respir J. 1996;9:1672–1677. doi: 10.1183/09031936.96.09081672. [DOI] [PubMed] [Google Scholar]
- 10.Page C P, Cotter T, Kilfeather S, Sullivan P, Spina D, Costello J F. Eur Respir J. 1998;12:24–29. doi: 10.1183/09031936.98.12010024. [DOI] [PubMed] [Google Scholar]
- 11.Brenner M, Berkowitz R, Marshall N, Strunk R C. Clin Allergy. 1988;18:143–150. doi: 10.1111/j.1365-2222.1988.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 12.Kidney J, Dominguez M, Taylor P M, Rose M, Chung K F, Barnes P J. Am J Respir Crit Care Med. 1995;151:1907–1914. doi: 10.1164/ajrccm.151.6.7767539. [DOI] [PubMed] [Google Scholar]
- 13.Markham A, Faulds D. Drugs. 1998;56:1081–1091. doi: 10.2165/00003495-199856060-00018. [DOI] [PubMed] [Google Scholar]
- 14.Evans D J, Taylor D A, Zetterstrom O, Chung K F, O'Connor B J, Barnes P J. N Engl J Med. 1997;337:1412–1418. doi: 10.1056/NEJM199711133372002. [DOI] [PubMed] [Google Scholar]
- 15.Ukena D, Harnest U, Sakalauskas R, Magyar P, Vetter N, Steffen H, Leichtl S, Rathgeb F, Keller A, Steinijans V W. Eur Respir J. 1997;10:2754–2760. doi: 10.1183/09031936.97.10122754. [DOI] [PubMed] [Google Scholar]
- 16.Rabe K F, Magnussen H, Dent G. Eur Respir J. 1995;8:637–642. [PubMed] [Google Scholar]
- 17.Rabe K F, Dent G. Clin Exp Allergy. 1998;28, Suppl. 3:35–41. [PubMed] [Google Scholar]
- 18.Tomita K, Chikumi H, Tokuyasu H, Yajima H, Hitsuda Y, Matsumoto Y, Sasaki T. Naunyn-Schmiedebergs Arch Pharmakol. 1999;359:249–255. doi: 10.1007/pl00005349. [DOI] [PubMed] [Google Scholar]
- 19.Ohta K, Sawamoto S, Nakajima M, Kubota S, Tanaka Y, Miyasaka T, Nagai A, Hirai K, Mano K, Miyashita H. Clin Exp Allergy. 1996;26, Suppl. 2:10–15. doi: 10.1111/j.1365-2222.1996.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Yasui K, Hu B, Nakazawa T, Agematsu K, Komiyama A. J Clin Invest. 1997;100:1677–1684. doi: 10.1172/JCI119692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imhof A, Wolffe A P. Curr Biol. 1998;8:R422–R424. doi: 10.1016/s0960-9822(98)70268-4. [DOI] [PubMed] [Google Scholar]
- 22.Ito K, Barnes P J, Adcock I M. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner B M, Fellows G. Eur J Biochem. 1989;179:131–139. doi: 10.1111/j.1432-1033.1989.tb14530.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida M, Kijima M, Akita M, Beppu T. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 25.Kolle D, Brosch G, Lechner T, Lusser A, Loidl P. Methods. 1998;15:323–331. doi: 10.1006/meth.1998.0636. [DOI] [PubMed] [Google Scholar]
- 26.Hecht A, Grunstein M. Methods Enzymol. 1999;304:399–414. doi: 10.1016/s0076-6879(99)04024-0. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Jazrawi E, Cosio B, Barnes P J, Adcock I M. J Biol Chem. 2001;276:30208–30215. doi: 10.1074/jbc.M103604200. [DOI] [PubMed] [Google Scholar]
- 28.Pflum M K, Tong J K, Lane W S, Schreiber S L. J Biol Chem. 2001;276:47733–47741. doi: 10.1074/jbc.M105590200. [DOI] [PubMed] [Google Scholar]
- 29.Shute J K, Tenor H, Church M K, Holgate S T. Clin Exp Allergy. 1998;28, Suppl. 3:47–52. [PubMed] [Google Scholar]
- 30.Giembycz M A, Lindsay M A. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 31.Waterborg J H. J Biol Chem. 2000;275:13007–13011. doi: 10.1074/jbc.275.17.13007. [DOI] [PubMed] [Google Scholar]