Abstract
Treatment with isolated allogeneic mesenchymal cells has the potential to enhance the therapeutic effects of conventional bone marrow transplantation in patients with genetic disorders affecting mesenchymal tissues, including bone, cartilage, and muscle. To demonstrate the feasibility of mesenchymal cell therapy and to gain insight into the transplant biology of these cells, we used gene-marked, donor marrow-derived mesenchymal cells to treat six children who had undergone standard bone marrow transplantation for severe osteogenesis imperfecta. Each child received two infusions of the allogeneic cells. Five of six patients showed engraftment in one or more sites, including bone, skin, and marrow stroma, and had an acceleration of growth velocity during the first 6 mo postinfusion. This improvement ranged from 60% to 94% (median, 70%) of the predicted median values for age- and sex-matched unaffected children, compared with 0% to 40% (median, 20%) over the 6 mo immediately preceding the infusions. There was no clinically significant toxicity except for an urticarial rash in one patient just after the second infusion. Failure to detect engraftment of cells expressing the neomycin phosphotransferase marker gene suggested the potential for immune attack against therapeutic cells expressing a foreign protein. Thus, allogeneic mesenchymal cells offer feasible posttransplantation therapy for osteogenesis imperfecta and likely other disorders originating in mesenchymal precursors.
Marrow stromal cells (MSCs) are bone marrow-derived mesenchymal progenitors that can serve as long-term precursors for the regeneration of a variety of nonhematopoietic tissues, including bone, cartilage, muscle, and possibly neural elements (1–8). Preclinical studies have suggested that unmanipulated bone marrow contains mixtures of mesenchymal progenitors, some possessing an unrestricted potential for mesenchymal differentiation with others showing commitment to one or perhaps two lineages (9–14). This observation, together with recent advances in the isolation, expansion, and characterization of human MSCs, has raised the possibility of improved cell-based therapy for genetic disorders of mesenchymal tissues. However, the engraftment capacity of isolated allogeneic MSCs in patients and their ability to produce objective clinical benefits remain unknown.
Osteogenesis imperfecta (OI) is a genetic disorder of mesenchymal cells characterized by defective type I collagen, the major structural protein in bone. Patients with severe OI have numerous painful fractures, progressive skeletal deformities, and retarded bone growth, resulting in short stature (15–17). There is no cure for OI, and only one class of drugs, the bisphosphonates, has shown therapeutic potential (18–20). We previously demonstrated the feasibility of allogeneic bone marrow transplantation (BMT) for children with severe OI (21). In that study, functional marrow-derived mesenchymal cells engrafted and contributed to the formation of new dense bone. This improvement was associated with accelerated linear growth and increases in total body bone mineral content over 18–36 mo of clinical follow-up (22). However, with increasing time posttransplantation, growth rates slowed and eventually reached a plateau while bone mineral content continued to increase. We hypothesized that additional therapy using isolated MSCs without marrow ablative treatment would safely boost responses seen after transplantation of unmanipulated bone marrow, providing a model for future clinical trials of MSC-based therapies. The results reported here indicate that isolated populations of donor MSCs can engraft after transplantation, differentiate to osteoblasts as well as skin fibroblasts, and produce clinical benefits attributable to the engraftment of functional mesenchymal precursors.
Methods
Patients.
Six children with OI were enrolled (with parental informed consent) in a clinical study approved by the Institutional Review Board of St. Jude Children's Research Hospital, the U.S. Food and Drug Administration, and the Recombinant DNA Advisory Committee of the National Institutes of Health. Each patient had been enrolled in an earlier clinical trial evaluating allogeneic BMT for children with severe OI (22).
Vectors.
Both retroviral vector supernatants were prepared at the Vector Production Facility of Indiana University (Indianapolis, IN) by using PG13 producer cell lines. Supernatant and producer cells were certified according to current Good Manufacturing Practice (cGMP) regulations. LNc8 is a clone of the LN vector encoding the neomycin phosphotransferase gene (neoR), whose expression is driven by the retroviral long terminal repeat (LTR; ref. 23). G1PLII, developed by Dunbar and colleagues (24), encodes nonexpressing β-galactosidase (β-gal) and neoR sequences that bear ATG → CTG mutations.
Isolation, Expansion and Retroviral Transduction of MSCs.
Fifty milliliters of bone marrow were harvested from the patients' original marrow donors. The mononuclear cell fraction was cultured in standard medium [DMEM (Bio Whittaker) supplemented with 2 mM l-glutamine, 45 μg/ml gentamicin, and 10% heat-inactivated FBS (Bio Whittaker)]. After 3 days, the medium was replaced, leaving MSCs adhering to the plastic surface of the culture dish. The cells were grown to about 80% confluence and then divided into two fractions with 1 × 106 cells per flask placed in T162 flasks. After 1 day in culture, the MSCs were transduced for 6 h on 3 consecutive days with either LNc8 or G1PLII retroviral supernatant diluted 1:1 with medium containing 4 μg/ml protamine sulfate.
One cell fraction, designated minimally cultured cells, was harvested and infused into the patient. The other was expanded over a total of three passages, harvested, and infused into the patient as the expanded cell fraction. At the time of harvest, an aliquot of cells from each fraction was assayed for sterility, lymphohematopoietic cell (CD3+, CD14+, and CD45+) contamination, transduction efficiency, and potential for osteogenic differentiation potential in vitro.
MSC Infusions.
Each patient received two 10- to 15-min infusions of MSCs 8 to 21 days apart. Target doses for the first and second infusions were 1 × 106 cells/kg of body weight and 5 × 106 cells/kg, respectively. The actual dose varied according to the number of available cells after ex vivo expansion (Table 1). Each cell preparation was suspended in 10 ml of normal saline with 5% human serum albumin. The patients received diphenhydramine, hydrocortisone, and acetaminophen before each MSC infusion. All patients were evaluated for toxicity (physical examination, complete blood count, clinical chemistry panel, urine analysis, and chest radiograph) before, and 24 h after, each infusion. They were monitored at the bedside during, and for 6 h after, each infusion.
Table 1.
Characteristics of the patients and cell dose
| Patient | Age* | Sex | Length† | Months from BMT‡ | Actual cell dose§ |
|---|---|---|---|---|---|
| 1 | 3 y 5 mo | M | 71 cm | 28 | 1.0 × 106 cells/kg |
| (8 m) | 5.0 × 106 cells/kg | ||||
| 2 | 4 y 9 mo | M | 82 cm | 25 | 1.0 × 106 cells/kg |
| (17.5 m) | 4.37 × 106 cells/kg | ||||
| 3 | 2 y 10 mo | M | 64 cm | 18 | 1.0 × 106 cells/kg |
| (4 m) | 1.0 × 106 cells/kg | ||||
| 4 | 3 y 9 mo | F | 83.5 cm | 18 | 1.0 × 106 cells/kg |
| (20.5 m) | 2.85 × 106 cells/kg | ||||
| 5 | 3 y 5 mo | F | 73.5 cm | 34 | 1.0 × 106 cells/kg |
| (11.5 m) | 5.0 × 106 cells/kg | ||||
| 6 | 3 y 11 mo | M | 74.5 cm | 23 | 1.0 × 106 cells/kg |
| (10.5 m) | 5.0 × 106 cells/kg |
In years (y) and months (mo).
Numbers in parentheses are median ages (in mo) for attaining indicated lengths in the general population (26).
Interval from BMT to the first infusion of isolated MSCs.
The actual dose of minimally cultured cells is given first, followed by the dose of expanded cells.
Transduction Efficiency Assay.
DNA was isolated (PureGene kit, Gentra Systems) from an aliquot of transduced MSCs at the time the cells were harvested for infusion. DNA (500 ng) was amplified with Taq polymerase, and the relevant buffers (Fisher) according to the manufacturer's instructions, with addition of 1 μCi of [α-32P]dCTP (Amersham Pharmacia). The primers for neoR were: (forward) 5′-CAAGATGGATTGCACGCAGG-3′; and (reverse) 5′-CCCGCTCAGAAGAACTCGTC-3′. For γ-globin, they were (forward) 5′-ACACTCGCTTCTGGAACGTCTGAGGT-3′; (reverse) 5′-CACCTTCTTGCCATGTGCCT-3′. Amplification with the MJ Research (Cambridge, MA) DNA Engine consisted of an initial denaturation at 94°C for 4 min, followed by 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min, ending with a final elongation step of 5 min at 72°C. The PCR products were resolved by electrophoresis through a 7.2% polyacrylamide gel and analyzed with a PhosphorImager Storm 860 (Molecular Dynamics) and imagequant software. Transduction efficiency was determined by comparing the signal with a standard curve.
Osteogenic Induction Assay.
An aliquot of processed MSCs was maintained in culture with osteoinductive media (standard media supplemented with ascorbic acid, β-glycerol phosphate, and dexamethasone) for 10 days and then stained with Alizarin Red for mineral nodules, as previously described (25).
Engraftment Analysis.
Osteoblasts, marrow stromal cells, and skin fibroblasts were cultured from biopsy specimens as previously described (21). Conditions for PCR amplification of the isolated DNA were performed as described above. Primers (forward, 5′-AAGCCGGTCTTGTCGATCAG-3′; reverse, 5′-GCTTGCCAAACCTACAGGTG-3′) were used that span a sequence from the 3′ neoR cassette to the 3′ long terminal repeat (LTR), so that the G1PLII vector yields a 478-bp product and the LNc8 vector yields a 441-bp product. The level of donor MSC engraftment was estimated by comparing the PCR signal, analyzed with a PhosphorImager Storm 860 described above, with standards and adjusting the determination for the transduction efficiency of the infused MSCs.
Immune Response Assays.
For the chromium (51Cr) release cytotoxicity assays, we cocultured peripheral blood mononuclear cells for 5 to 6 days with irradiated, retrovirally transduced or mock-transduced donor MSCs as stimulator cells in sensitization media (RPMI medium 1640/10% FCS/50 mM 2-mercaptoethanol, 1 mmol sodium pyruvate, and nonessential amino acids). The ratio of mononuclear cells to marrow stromal stimulator cells was 4:1. After coculture, the nonadherent cells were harvested from the plates, washed, counted, and resuspended in fresh complete RPMI medium. Target MSCs (transduced or mock transduced) were labeled with 51Cr, washed, and resuspended in fresh medium. After 4 h of incubation at 37°C, the supernatant was harvested and the radioactive release was measured. Maximum release of the incorporated 51Cr was determined by Triton lysis of the MSC target cells. Spontaneous release of 51Cr was determined from the supernatant of control target cells. Each ratio represents the results of triplicate assays. ELISA for anti-FBS antibodies was performed exactly as described by Heim et al. (24).
Growth Evaluation.
Linear growth was assessed as previously described (21, 22). Briefly, each patient was measured from crown to heel by the same investigator (P.L.G.) 6 mo before, on the day of, and 6 mo after the first MSC infusion. Growth velocity is defined as the difference between measurements at two consecutive intervals and is reported as a percentage of the median growth velocity for age- and sex-matched healthy children (26).
Dual Energy X-Ray Absorptiometry.
Measurements of total body bone mineral content were done on a whole-body scanner with a pediatric platform (Hologic QDR 2000 Densitometer; Hologic, Waltham, MA), as described (22, 27).
Results
Isolation, Transduction, and Analysis of Marrow Stromal Cells.
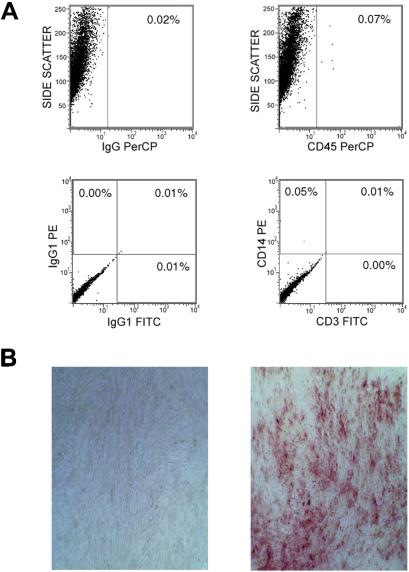
Fifty milliliters of bone marrow were harvested from the four sibling donors and two unrelated donors of six children who had undergone standard BMT for OI. The adherent MSCs were divided into two fractions, which were transduced with either the G1PLII or LNc8 retroviral vector after the first passage in culture. The transduction efficiency ranged from 2% to 25% (median, 19%) for both vectors and all six patients. One fraction was infused into the patients at a dose of 106 cell/kg of body weight after a minimal time in culture, whereas the other was expanded over three passages and infused at an intended dose of 5 × 106 cells/kg (actual median dose, 4.69 × 106). The vectors used for the two fractions were alternated among the patients to avoid vector bias. Contamination of the infused cells by CD45+, CD14+, or CD3+ cells generally ranged from 0% to 1.6% (median, 0.1%; Fig. 1A), without an overall difference between the minimally cultured and expanded fractions. One exception was patient 3, whose minimally cultured cells had 7% CD14+ cells. Finally, the MSCs uniformly showed osteogenic differentiation potential when cultured in osteoinductive media (standard media supplemented with ascorbic acid, β-glycerol phosphate, dexamethasone; Fig. 1B).
Figure 1.
Analysis of MSCs before infusion. (A) Flow cytometric analysis of MSCs (from patient 5) to exclude contamination by lymphohematopoietic cells. Anti-CD45 perCP (peridinin-chlorophyll) was used to screen for mature and progenitor white blood cells, anti-CD14-PE (phycoerythrin) for monocytes/macrophages, and anti-CD3 FITC (fluorescein isothiocyanate) for T cells. The percentage of positive cells among the total analyzed is shown. Isotype controls are shown on the left. (B) Induction of osteogenic differentiation with osteoinductive medium as described in the text. The Alizarin Red stain reveals foci of mineral deposition in the induced cells (Right), contrasted with the lack of mineralized foci in noninduced control cells (Left).
Engraftment of Mesenchymal Cells.
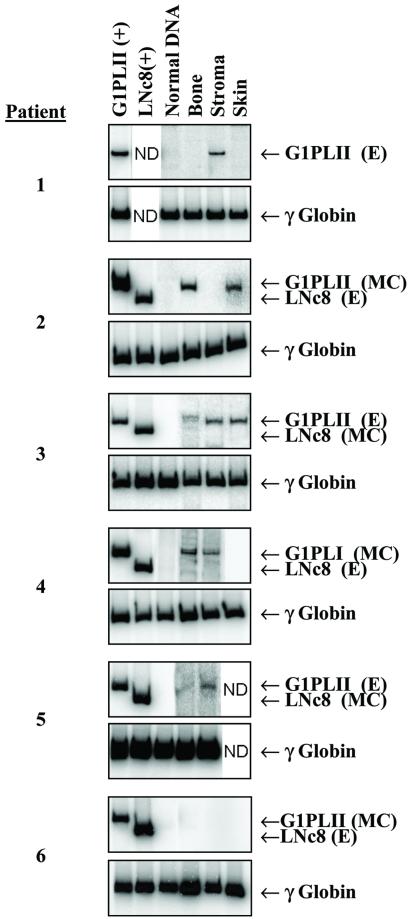
Between 4 and 6 weeks after the second infusion of MSCs, bone and skin biopsies and an aspirate of bone marrow were taken from each patient. Osteoblasts, skin fibroblasts, and marrow stromal cells were expanded in culture and assayed by flow cytometry for lymphohematopoietic cell contamination. DNA isolated from the cultured cells was analyzed by PCR for the presence of proviral sequences. Five of six children showed engraftment of the minimally cultured or expanded MSCs in at least one of the tissues examined (Fig. 2). Patients 2 and 4 had PCR signals indicative of the G1PLII marker (minimally cultured cells) in bone and skin and in bone and stroma, respectively. Patients 1, 3, and 5 had the G1PLII marker (expanded cells) in stroma, in bone, skin and stroma, and in bone and stroma, respectively. Patient 6 did not show engraftment of either population of MSCs in any tissue. None of the biopsy samples clearly contained sequences from the LNc8 vector, which expresses the neoR marker gene. Overall, the fraction of donor cells at any biopsy site never exceeded 1%.
Figure 2.
PCR analysis of engraftment. DNA isolated from osteoblasts, stromal cells, and skin fibroblasts of each patient, at 4 to 6 wk after the second cell infusion, was evaluated. DNA isolated from human cells transduced with either the G1PLII or LNc8 vector served as a positive control; normal human DNA was the negative control. Analysis of the γ-globin gene controlled for the quality and quantity of DNA. MC and E designate whether the signal represents minimally cultured cells or expanded cells, respectively. The vectors were alternated among the patients to avoid vector bias. ND, Not determined.
Immune Response to Transduced Mesenchymal Cells.
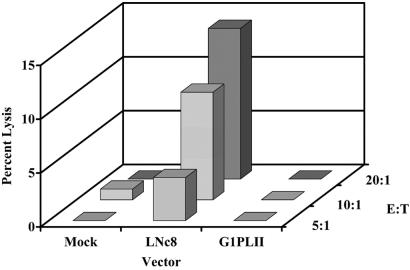
Failure to detect LNc8 proviral sequences after transplantation suggested immune recognition of MSCs expressing the neoR gene. We investigated this possibility by screening peripheral blood mononuclear cells from patients 2, 3, and 4 for evidence of cytotoxic T lymphocyte activity against the neoR protein, using a chromium release assay. Cryopreserved donor MSCs were expanded in culture and transduced with either the G1PLII or LNc8 vector (10% transduction efficiency) and tested for cytotoxic T lymphocyte-mediated lysis. At effector-to-target ratios of 10 and 20, the lymphocytes from patient 4 lysed 7% and 13% of the LNc8 transduced cells, respectively, in contrast to the lack of any appreciable lysis of G1PLII or mock transduced cells (Fig. 3). Neither of the two remaining patients showed evidence of an anti-neoR cytotoxic T lymphocyte response.
Figure 3.
T cell response against transduced MSCs. Cytotoxic T lymphocyte-mediated lysis of donor MSCs (patient 4) transduced with either the LNc8 or G1PLII retroviral vector or mock supernatant only. E:T designates the effector-to-target cell ratio. Each bar represents the mean of triplicate determinations.
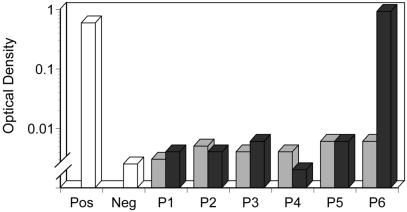
Five of six patients did not have detectable antibodies against FBS proteins after completion of the two infusions. Patient 6 showed a 150-fold increase in antibody titer after the second infusion, compared with his lack of detectable antibodies on the preinfusion assay (Fig. 4).
Figure 4.
Antibody response against FBS proteins. ELISA assay measuring antifetal bovine serum antibodies in the sera of patients before (░⃞) and after (■) both MSC infusions. Each bar represents the mean of triplicate determinations. Pos, positive control; Neg, negative control.
Clinical Responses.
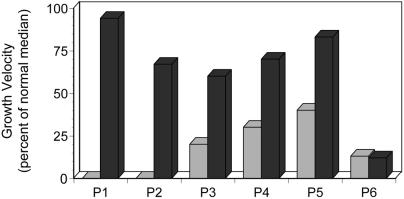
During the 6 mo before MSC therapy, the median growth velocity of patients 1 through 5 was 20% of that predicted for age- and sex-matched unaffected children (Fig. 5; ref. 26). Patients 1 and 2 did not grow at all during this interval, whereas patients 3, 4, and 5 showed growth velocities of 20% to 40%. Over the first 6 mo after MSC therapy, each patient had a striking increase in growth velocity, ranging from 60% to 94% of the predicted median. There was no change in the growth velocity of patient 6 after the MSC infusions (Fig. 5). The accelerated growth in patients 1, 2, 3, and 5 was not accompanied by increases in total body bone mineral content, measured at 3 mo after the first infusion. Patient 4 showed a substantial increase in mineralization, from 156 g to 209 g.
Figure 5.
Growth stimulation after MSC engraftment. Growth velocity of the patients during the 6 mo immediately before (░⃞) and after (■) the first MSC infusion. The values are percentages of the median growth of unaffected children of the same age and sex (23).
Toxicity.
Patient 6 developed an urticarial rash about 5 min after completion of the second MSC infusion. The rash rapidly resolved, without sequelae, after administration of hydrocortisone and diphenhydramine. There was no clinically significant toxicity during or after the MSC infusions among the other patients.
Discussion
Bone marrow transplantation offers curative therapy for many genetic disorders affecting the hematopoietic system (28), but its value in the treatment of nonhematopoietic genetic diseases (29, 30) has been limited. In two previous reports, we demonstrated the therapeutic potential of conventional BMT in children with severe OI (21, 22). Although the procedure resulted in substantial gains in body length and bone mineralization, it was not curative. The hypothesis suggested by this research was that primitive mesenchymal cells in transplanted whole marrow can engraft in skeletal sites in children with OI, where they differentiate into functional osteoblasts capable of contributing normal collagen matrix to the defective bone. In the study reported here, we sought to better understand mesenchymal cell transplantation biology and augment the beneficial effects of BMT by investigating the infusion of allogeneic MSCs isolated from the patients' original donors.
The principal finding was that allogeneic bone marrow-derived mesenchymal cells can engraft in bone, marrow stroma, and skin without the requirement for preparative chemotherapy and then produce clinically measurable benefits. Most striking were the 2 patients whose growth velocities after the MSC infusions were 67% and 94% of the predicted median, in contrast to their negligible linear growth during the 6 mo preceding MSC therapy. We attribute the growth acceleration in these patients and in the remaining three to the generation of normal osteoblasts from the mesenchymal cells engrafted in skeletal sites. Detection of gene-marked cells in each of these cases and the lack of potentially confounding variables, such as marrow-ablative chemotherapy, support this contention. Further support is offered by the course of patient 6. This child exhibited an urticarial reaction to the second infusion of MSCs, consistent with a systemic immune response. This patient was also the only one in whom we were unable to identify gene-marked cells in any tissue, the only one to have antifetal bovine serum antibodies, and the only one who did not show accelerated growth velocity. Although, the threshold level of donor MSC engraftment required to stimulate linear growth is unknown, the fraction of donor-derived osteoblasts in our biopsy specimens did not appear to exceed 1%. This finding suggests that low levels of MSC engraftment are adequate to produce objective clinical benefits, in agreement with our previous findings (21).
With one exception, we did not observe an increase in total body bone mineral content at 3 mo postinfusion, a time point associated with striking improvement in mineralization when the same patients underwent conventional BMT (21, 22). Although improved mineralization after BMT could reflect osteoclast inhibition because of the marrow ablative chemotherapy, continued posttransplantation increases in this measure after restoration of osteoclast activity (22) suggest a beneficial effect from the transplanted whole marrow that is not available from infusions of isolated donor MSCs. Alternatively, the follow-up times in this study were relatively short (3 to 6 mo), so that delayed increases in bone mineralization cannot be ruled out. Whatever the explanation, this finding strengthens our hypothesis that the mechanisms governing linear growth and bone mineralization are quite distinct. It also suggests that, if mesenchymal cell infusions are to be used as therapy without BMT, it may be necessary to include other treatments, perhaps bisphosphonate therapy or a yet-to-be defined modality, to ensure acceptable rates of bone mineralization during increases in body size.
Surprisingly, LNc8-transduced cells could not be detected in these patients, prompting screening studies to identify neoR-specific cytotoxic T lymphocyte populations. Among the samples tested, only those representing patient 4 elicited an anti-neoR response. However, peripheral blood mononuclear cells were collected at 6–9 mo postinfusion for patients 2 and 3, which may have limited our ability to detect low frequency cytotoxic T lymphocyte precursors with the relatively insensitive chromium release assay. The absence of LNc8 sequences in tissue biopsy specimens from the five patients and the cytotoxic T lymphocyte response demonstrated for patient 4 could have important implications for future gene therapy/marking trials with MSCs. In contrast to results for the neoR-expressing cells, there was no indication of an in vitro cytotoxic T lymphocyte response against mock-transduced cells or those transduced with the G1PLII retroviral vector, which does not express a foreign antigen. Moreover, none of the patients had clinical symptoms indicating an autoimmune response against the marrow microenvironment that could eventually manifest as a bone marrow failure syndrome. We conclude that donor MSCs can be safely used as therapeutic agents after BMT. If, however, such cells are to be exploited as vehicles for the delivery of therapeutic proteins, the expressed protein must be recognized by the patient's immune system as “self.” Expression of a foreign protein, such as neoR, may render the cells vulnerable to immune attack.
Despite the therapeutic potential of ex vivo expanded MSCs, results of a recent in vitro study raise the possibility that prolonged culturing will compromise the engraftment and differentiation capacity of such cells (31). The stronger PCR signals in bone specimens from patients 2 and 4 (engraftment of gene-marked minimally cultured cells) compared with those in samples from patients 1, 3, and 5 (engraftment of gene-marked ex vivo expanded cells) support this concern. Thus, it will be important to consider alternative forms of cell therapy in which the mesenchymal progenitors have increased potential to generate large quantities of osteoblasts long term. One promising candidate is the small rapidly self-renewing (RS) cells described by Colter et al. (32). These small, round, rapidly cycling adherent marrow progenitors can theoretically produce over 1 × 1013 MSCs from 20 ml of bone marrow. A second candidate is the CD34low/neg hematopoietic stem cell, first identified by Goodell et al. (33) within a murine bone marrow “side population” stained with the vital dye Hoechst 33342, and subsequently found in humans (34). These so-called SP cells have strong reconstituting ability and remarkable plasticity in terms of regenerating nonhematopoietic tissues (M. A. Goodell, personal communication). Thus, if more immature mesenchymal progenitors, or perhaps true pluripotent stem cells, can be substituted for marrow stromal cells, it may be possible to overcome the problem of low-level engraftment and limited therapeutic effects currently associated with allogeneic MSC transplantation.
Gene-marked cells were found in skin biopsies from two of our patients. Although MSCs may have lodged in dermal capillaries and persisted in an undifferentiated state, we interpret these data to suggest that the infused MSC populations contained either rare pluripotent stem cells able to differentiate in ectodermal pathways or mesenchymal progenitors capable of crossing embryonic germ layer barriers and differentiating to skin fibroblasts. If the latter interpretation holds, our finding would be the first to demonstrate MSC differentiation across the embryonic germ layers in humans
Infusions of bone marrow-derived stromal cells are under active investigation as a means to facilitate hematopoietic stem cell engraftment (35) and as therapy for children with metabolic storage disorders (36). The data presented here indicate that allogeneic MSCs can be safely administered to children with severe OI, will engraft in genetically defective bone, and will differentiate to osteoblasts capable of extending the clinical benefits of BMT. These properties could be exploited in the development of new therapeutic approaches to other genetic or acquired disorders. Still required, however, are methods that will promote long-term tissue-specific proliferation and differentiation, thus ensuring maximum clinical benefits from this cell-based treatment strategy.
Acknowledgments
We gratefully acknowledge Drs. Malcolm Brenner and Darwin Prockop for important discussions throughout this study and are indebted to Dr. Cynthia Dunbar for use of the G1PLII vector, to Dr. Richard Morgan for permission to use the clinical grade retroviral vector supernatants, and to Lillith Reeves at the Vector Production Facility at Indiana University for preparation of the clinical supernatants. We also thank the nurses in the Bone Marrow Transplant Outpatient Clinic for excellent care of our patients, John Gilbert for his editorial review, and Ginger Hoskins for assistance in preparation of the manuscript. This work was supported by the Doris Duke Charitable Foundation (T99102B), by National Cancer Institute Cancer Center Support CORE Grant P30 CA 21765, and by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- MSCs
marrow stromal cells
- OI
osteogenesis imperfecta
- BMT
bone marrow transplantation
- neoR
neomycin phosphotransferase gene
References
- 1.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavillo F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 2.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone B, Hering T M, Caplan A I, Goldberg V M, Yoo J U. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 4.Malaval L, Modrowski D, Gupta A K, Aubin J E. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara H, Goldberg V M, Caplan A I. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 6.Pereira R F, Halford K W, O'Hara M D, Leefer D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz E J, Alexander G M, Prockop D J, Azizi S A. Hum Gene Ther. 1999;10:2539–2549. doi: 10.1089/10430349950016870. [DOI] [PubMed] [Google Scholar]
- 8.Woodbury D, Schwarz E J, Prockop D J, Black I B. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Dennis J E, Caplan A I. J Cell Physiol. 1996;167:523–538. doi: 10.1002/(SICI)1097-4652(199606)167:3<523::AID-JCP16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Grigoriadis A E, Heersche J N, Aubin J E. Dev Biol. 1990;142:313–318. doi: 10.1016/0012-1606(90)90352-j. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriadis A E, Heersche J N, Aubin J E. J Cell Biol. 1998;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynesworth S E, Goshima J, Goldberg V M, Caplan A I. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar M K, Thiede M A, Mosca J D, Moorman M, Gerson S L. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Wakitani S, Saito T, Caplan A I. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 15.Byers P H. In: The Metabolic and Molecular Bases of Inherited Disease. Schriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 4029–4077. [Google Scholar]
- 16.Marini J C, Gerber N L. J Am Med Assoc. 1997;277:746–750. [Google Scholar]
- 17.Sillence D O. In: Emery and Rimoin's Principles and Practice of Medical Genetics. Rimoin D L, Connor J M, Pyeritz R E, editors. New York: Churchill Livingstone; 1997. pp. 2817–2835. [Google Scholar]
- 18.Bembi B, Parma A, Bottega M, Ceschel S, Zanatta M, Martini C, Ciana G. J Pediatr. 1997;131:622–625. doi: 10.1016/s0022-3476(97)70074-x. [DOI] [PubMed] [Google Scholar]
- 19.Glorieux F, H, Bishop N J, Plotkin H, Chabot G, Lanoue G, Travers R. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 20.Plotkin H, Rauch F, Bishop N J, Montpetit K, Ruck-Gibis J, Travers R, Glorieux F H. J Clin Endocrinol Metab. 2000;85:1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz E M, Prockop D J, Fitzpatrick L A, Koo W W, Gordon P L, Neel M, Sussman M, Orchard P, Marx J C, Pyeritz R E, Brenner M K. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 22.Horwitz E M, Prockop D J, Gordon P L, Koo W W, Fitzpatrick L A, Neel M D, McCarville M E, Orchard P J, Pyeritz R E, Brenner M K. Blood. 2001;97:1227–1231. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 23.Miller A D. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- 24.Heim D A, Hanazono Y, Giri N, Wu T, Childs R, Sellers S E, Muul L, Agricola B A, Metzger M E, Donahue R E, et al. Mol Ther. 2000;1:533–544. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 25.Marx J C, Allay J A, Persons D A, Nooner S A, Hargrove P W, Kelly P F, Vanin E F, Horwitz E M. Hum Gene Ther. 1999;10:1163–1173. doi: 10.1089/10430349950018157. [DOI] [PubMed] [Google Scholar]
- 26.Hamill P V, Drizd T A, Johnson C L, Reed R B, Roche A F, Moore W M. Am J Clin Nutr. 1979;32:607–629. doi: 10.1093/ajcn/32.3.607. [DOI] [PubMed] [Google Scholar]
- 27.Koo W W, Bush A J, Walters J, Carlson S E. J Am Coll Nutr. 1998;17:65–70. doi: 10.1080/07315724.1998.10720457. [DOI] [PubMed] [Google Scholar]
- 28.O'Marcaigh A S, Cowan M J. Curr Opin Oncol. 1997;9:126–130. [PubMed] [Google Scholar]
- 29.Krivit W, Aubourg P, Shapiro E, Peters C. Curr Opin Hematol. 1999;6:377–382. doi: 10.1097/00062752-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Krivit W, Peters C, Shapiro E G. Curr Opin Neurol. 1999;12:167–176. doi: 10.1097/00019052-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 32.Colter D C, Class R, DiGirolamo C M, Prockop D J. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodell M A, Brose K, Paradis G, Conner A S, Mulligan R C. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodell M A, Rosenzweig M, Kim H, Marks D F, DeMaria M, Paradis G, Grupp S A, Sieff C A, Mulligan R C, Johnson R P. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 35.Koc O N, Gerson S L, Cooper B W, Dyhouse S M, Haynesworth S E, Caplan A I, Lazarus H M. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 36.Koc O N, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, Kolodny E H, Yoseph Y B, Gerson S L, Lazarus H, et al. Exp Hematol. 1999;27:1675–1681. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]