Abstract
Background:
Transfeminine people were assigned male at birth and experience a female or feminine gender identity. Many elect to undergo vaginoplasty, a surgical procedure that constructs a neovagina, typically using penile and scrotal tissue. Like cisgender females, transfeminine people experience gynecological symptoms, including pain, discharge, and malodor. In cisgender females, clinicians attribute these symptoms to bacterial dysbiosis and can be diagnosed by Nugent scoring of gram-stained vaginal smears. The Nugent score assesses the abundance of large gram-positive rod vs. small or curved gram-variable rod morphotypes, traditionally for the detection of Lactobacillusspp., Gardnerella vaginalis, and Mobiluncus spp. (curved rod), respectively. Although unvalidated for neovaginal samples, this method is frequently applied to diagnose dysbiosis in transfeminine people with vaginoplasty.
Objective:
This study assessed the Nugent score’s utility as a clinical tool for diagnosing neovaginal dysbiosis in transfeminine people who underwent penile inversion vaginoplasty.
Study Design:
As a part of the TransBiota study, n=39 transfeminine participants self-collected neovaginal smears. Smears were Gram stained and Nugent scored, and Nugent scores were correlated with existing data on neovaginal bacterial composition (16S rRNA gene sequencing), neovaginal cytokines (Luminex multiplex immunoassay), and self-reported symptoms.
Results:
More than 70% of smears fell in the 7–10 range that would indicate Bacterial Vaginosis in cisgender women. However, Nugent score failed to correlate with the abundance of Nugent-targeted bacteria. Bacteria with similar morphotypes, but not belonging to Lactobacillus, Gardnerella, or Mobiluncus, were highly abundant and prevalent in the neovagina. Nugent score also failed to predict local inflammation or clinical symptoms.
Conclusion:
The Nugent score is not an effective tool to identify neovaginal dysbiosis or indicators of health in transfeminine individuals. Clinicians need the development of accurate, evidence-based diagnostic tools for the neovagina.
Keywords: Bacterial Vaginosis, Gynecology, Microbiome, Transgender, Vaginoplasty
INTRODUCTION
Transfeminine individuals (TF) were assigned male at birth and experience a female/feminine gender identity. Numerous communities fall under this umbrella, including transgender women, non-binary, and other gender diverse individuals1,2. Many TF elect to undergo gender-affirming medical care, often through feminizing hormone therapy or surgery. Vaginoplasty is a widely performed gender-affirming surgery that creates a clitoris, vulva, and vaginal canal. The most prevalent technique is penile inversion vaginoplasty, involving orchiectomy, dissection of the space between the bladder and rectum, and lining the newly formed space with penile and scrotal tissue3. Although less common, sigmoid or peritoneal tissue may also be used to augment the vaginal canal4–6. Penile inversion vaginoplasty results in a vaginal canal that is visually and functionally similar to that of cisgender females (CF) but lined with soft-cornified skin. We use the term “vagina” to refer to the vaginal canal of those born with a vagina, and “neovagina” to refer to the surgically created vaginal canal of TF. Our aim in using two terms is to clinically distinguish between vaginal canals lined with epithelia of different origins.
In 2021, an estimated 20% of TF in the US had undergone genital surgery, with an additional 67% desiring it7. Like CF, TF with vaginoplasty often experience genital symptoms, including itching, burning, discharge, and malodor; however, the source of these symptoms has not been explored. To address this gap, we performed a study of the neovaginal microenvironments of 47 TF living in Canada8,9. In this study, 56% of participants reported neovaginal symptoms within the past 30 days10. In reproductive-aged CF (rCF), similar symptoms are caused by bacterial vaginal dysbiosis termed “bacterial vaginosis” (BV), which occurs when beneficial Lactobacillus spp. are replaced by a polymicrobial microbiome, including Gardnerella vaginalis, Prevotella spp., Ca. Lachnocurva vaginae, and other anaerobic bacteria11–13. Lactobacillus spp. play a critical role in rCF vaginal health by producing antimicrobial compounds and lactic acid, which lowers pH and inhibits colonization by pathogens14,15. Even without symptoms, dysbiotic microbiomes in rCF are associated with increased susceptibility to sexually transmitted infections, including chlamydia, gonorrhea, HSV-2, and HIV-1, underscoring the importance of the vaginal microbiome in sexual health14–23.
Clinicians usually diagnose BV in rCF using the Nugent score. The Nugent score is a Gram stain-based diagnostic measure that rates the relative abundance of gram-positive rods (indicative of beneficial Lactobacillus spp.) vs. small or curved gram-variable rods (morphotypes of BV-associated bacteria)12,24. Scores from 0–3 are considered optimal, 4–6 inconclusive or intermediate, and 7–10 indicative of BV. The Nugent score is often applied to diagnose TF experiencing genital symptoms; however, the neovaginal microbiota is distinct from the vaginal microbiota25–27, and results from TransBiota suggest bacteria associated with inflammation in the neovagina differ significantly from those in the rCF vagina8. Bacteria associated with neovaginal immune activation include Lawsonella, Howardella, Fusobacterium, and Parvimonas, while higher abundances of Ezakiella, Fastidiosipila, Murdochiella, and Peptoniphilus associated with reduced inflammation8. Core neovaginal bacteria are listed in Supplemental Table 1.
The substantial differences in microbiome composition and associated inflammation found in the rCF vagina and the neovagina raise questions about the utility of the Nugent score as a diagnostic tool in TF. Nevertheless, clinicians lack alternative diagnostics and the Nugent score continues to be frequently employed28. This study evaluated the Nugent score’s relevance as a diagnostic tool for neovaginal dysbiosis by assessing its accuracy in identifying bacteria targeted by the score, and its ability to predict markers of bacterial dysbiosis (cytokines and symptoms) in the neovagina.
METHODS
Participants
TransBiota was a study investigating the genital microenvironments of trans and other gender diverse people receiving gender-affirming medical care8–10. Eligible TF participants were Canadian residents 18 years or older, who underwent vaginoplasty > 1 year prior to study entry. Research ethics board approval was obtained from Western University (REB #115503) and University of Maryland (IRB #HP-00096952), and all participants provided written informed consent. Participants were recruited online through social media and community groups, healthcare provider referral, and re-contact of consenting Trans PULSE Canada participants29. Participants were mailed a study kit containing instructions and self-collection materials. Demographic, behavior, and symptom data were collected through an online questionnaire. Participants (n = 47) returned three weekly neovaginal sample sets by mail using pre-paid envelopes. This analysis includes the first timepoint for each participant for which there is a corresponding 1) scorable gram-stained slide, 2) microbiome data, and 3) immune analyte data.
All research activities were conducted in accordance with institutional and ethical regulations, and participant data were deidentified prior to analysis.
Microbiota and Cytokine Analyses
Participants self-collected neovaginal swabs at each timepoint for Nugent scoring, microbiota analysis, and cytokine quantification. Sample collection and analysis of neovaginal microbiota and cytokines have been described in detail elsewhere8. In brief, participants were instructed to insert each swab (Puritan HydraFlock) 5cm into the neovaginal canal, rotate 3 times, and place in collection media. Swabs for microbiota analysis were collected into 1ml of Qiagen C2.1 solution. DNA extractions were performed using the MagAttract PowerMicrobiome DNA/RNA kit (Qiagen), and 16S rRNA gene V3-V4 region amplicon sequencing (amplified via two step PCR) was conducted at University of Maryland Institute for Genomic Sciences30. Reads were processed using a QIIME-dependent script with DADA2 to generate amplicon-sequence variants (ASVs). Taxonomy was assigned with the RDP naïve-Bayes classifier trained on the SILVA v138.2 16S rRNA database and refined species-level calls with SpeciateIT v2.0.0. ASVs sharing identical taxonomy were collapsed. Swabs for cytokine analysis were collected into 500μl of a PBS-based stabilizing buffer, and IL-1a, IL-1b, IL-6, IL-8, MIG, MIP-1b, and RANTES concentrations were quantified on a Luminex MAGPIX system.
Nugent Scoring
Participants rolled collection swabs onto charged glass microscope slides (USA Scientific) and returned them to Western University in secured plastic cases. Neovaginal smears were heat-fixed and Gram stained using standard techniques. In brief, slides were stained with crystal violet (1 minute), iodine mordant (1 minute), decolorizing solution (until solution ran clear), and safranin (30 seconds) at room temperature. Slides were rinsed with running tap water between steps. Slides were observed under a 100x oil-immersion light microscope. For each smear, bacterial morphologies were individually scored in 10 representative fields of view (FOVs) following Nugent criteria24. The criteria assign a decreasing score from 0–4 for the abundance of Lactobacillus-like gram-positive large rods, an increasing score from 0–4 for the abundance of Gardnerella-like short gram-variable straight rods, and an increasing score from 0–2 for the abundance of Mobiluncus-like gram-variable curved rods. Scores for each morphotype were summed to yield a total score from 0–10 for each FOV. Final mean score across the 10 FOVs was calculated for each participant. In rCF, scores of 0–3 are optimal, 4–6 inconclusive/intermediate, and 7–10 indicative of BV24.
Data Analysis
Multiple unpaired Mann-Whitney U nonparametric tests were used to assess differences in Nugent scores between asymptomatic and symptomatic participants. Fisher’s exact test was used to assess differences in bacterial prevalence, and Mann-Whitney U test was used to assess differences in relative abundance between asymptomatic and symptomatic participants. Associations between Nugent score and cytokines (IL-1α, IL-1β, IL-6, IL-8, MIG, MIP-1β, and RANTES) were assessed by Spearman’s correlation. Relationship between neovaginal bacteria taxa and cytokines, as well as bacterial taxa and Nugent score was also evaluated using Spearman’s correlations. Only taxa with a neovaginal prevalence of > 25% were included in analyses. GraphPad Prism 8 and R Studio (version 4.3.2) were used to create graphs and perform statistical analyses.
RESULTS
Of the n = 47 TransBiota participants, 8 were excluded (17%) due to no scorable slides from destruction during heat-fixation from smearing on the incorrect side of the slide; damage to slides during shipping; or insufficient sample for Nugent scoring (defined at < 30 bacteria/FOV). Demographics on the remaining n = 39 participants included in this study are displayed in Table 1. Median time on hormone therapy was 5.6 years and median time since vaginoplasty 2.8 years.
Table 1.
Participant demographics.
| Participants (n = 39) | |
|---|---|
| Age, years (median, range) | 39 (26–67) |
| Ethnoracial Identity (%) | |
| White | 89.7% |
| Latin American | 2.6% |
| Jewish | 2.6% |
| Mixed Ethnicity | 5.1% |
| Months on hormone therapy (median, range) | 67 (35–265) |
| Months since vaginoplasty (median, range) | 33 months (12–229 months) |
| Circumcised prior to vaginoplasty (%) | 56.4% |
| Symptoms, past 7 days (%) | 23.1% |
| Bleeding | 5.1% |
| Discharge | 5.1% |
| Itching/burning | 2.6% |
| Malodor | 12.8% |
| Pain | 2.6% |
| pH (median, range) | 5.5 (4.5–8) |
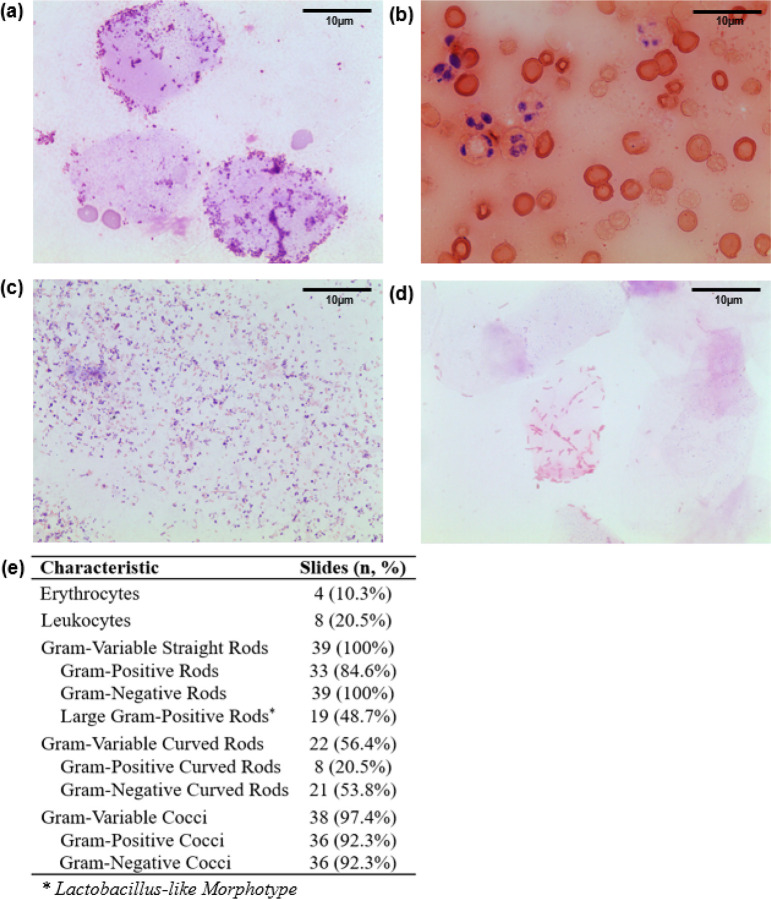
Neovaginal smears contained nucleate and anucleate epithelial cells, with most bacteria clustered near these epithelial cells (Fig. 1a, e). Four slides (10.3%) were visibly bloody with erythrocytes and leukocytes visible by microscopy (Fig. 1b, e). Gram-variable rods and cocci dominated the majority of smears (Fig. 1c, e). Although all smears contained gram-variable straight rods, only 48.7% of slides displayed large gram-positive rods (Lactobacillus-like morphotype), and just three (7.7%) were dominated by large gram-positive rods (Fig. 1d, e).
Figure 1. Representative images and descriptive characteristics of neovaginal smears.
Self-collected neovaginal smears (n=39) were heat-fixed, Gram-stained, and observed under a 100x oil-immersion light microscope. Ten fields of view (FOV) were scored per participant; representative FOVs showing (a) proximity of bacteria to epithelial cells, (b) abundance of red and white blood cells, (c)dominance with Gram-variable rods and cocci, and (d) dominance with Lactobacillus-like morphotype. Descriptive characteristics of neovaginal smears are shown in (e).
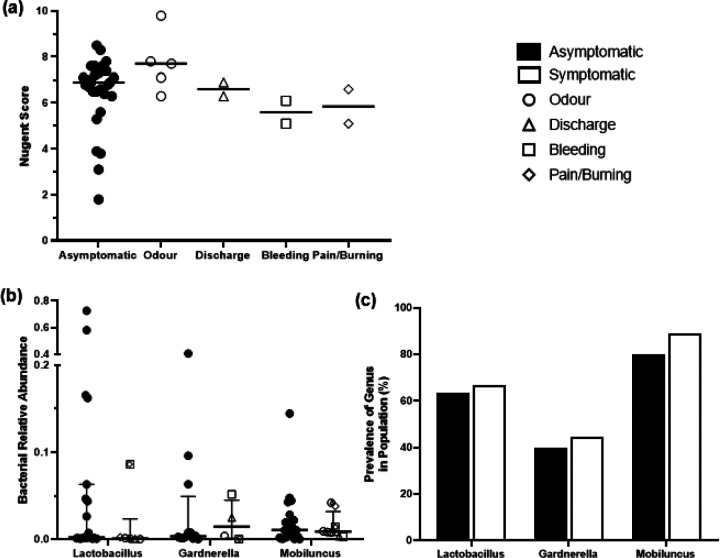
The majority of neovaginal smears (71.8%) fell within the BV Nugent range (7–10; Fig. 2a). The most common score was 7 (46.2% of participants) indicating abundant gram-variable rods and minimal Lactobacillus-like rods. Only two participants (5.1%) scored an optimal Nugent score range for rCF (0–3). Lactobacillus showed a moderate negative correlation with Nugent score (Fig. 2b) while Gardnerella and Mobiluncus showed no correlation (Fig. 2c, d).
Figure 2. Nugent scores and correlation with traditional Nugent-targeted taxa across neovaginal smears.
(a) Distribution of Nugent scores from n=39 TF participants. Scores represent the median score per participant over 10 fields of view (FOVs) acquired at 100x oil-immersion magnification. Lower scores from 0–3 are considered optimal, intermediate scores of 4–6 are inconclusive, and higher scores of 7–10 are considered indicative of BV. Spearman’s correlations are shown between the relative abundances of (b) Lactobacillus (p<0.03), (c) Gardnerella (p=0.67), (d) and Mobiluncus (p=0.85) within neovaginal smears and Nugent score.
Nugent Score and traditional Nugent-targeted taxa are not associated with neovaginal symptoms
Nine participants (23.1%) reported neovaginal symptoms (malodor, discharge, bleeding, pain/burning) within 7 days prior to sample collection. There was no difference in Nugent scores between symptomatic and asymptomatic participants (Fig. 3a). Additionally, no relationship was observed between prevalence (Fig. 3b) or proportional abundance (Fig. 3c) of the traditional Nugent score bacteria (Lactobacillus, Gardnerella, Mobiluncus) and the presence or absence of neovaginal symptoms.
Figure 3. No differences in Nugent score or traditional Nugent score bacteria between symptomatic and asymptomatic participants.
(a) Median Nugent score did not vary between asymptomatic participants and participants who self-reported neovaginal malodor, discharge, bleeding, or pain/burning in the last 7 days (Mann-Whitney U, asymptomatic participants vs. each symptom, all p>0.1). Comparison of the (b) prevalence and (c)relative abundance of Lactobacillus, Gardnerella, and Mobiluncusbetween asymptomatic and symptomatic participants (prevalences compared by Fisher’s exact test, relative abundances by Mann Whitney U, all p>0.8). Median and interquartile range are shown.
Nugent Score is not associated with neovaginal cytokines
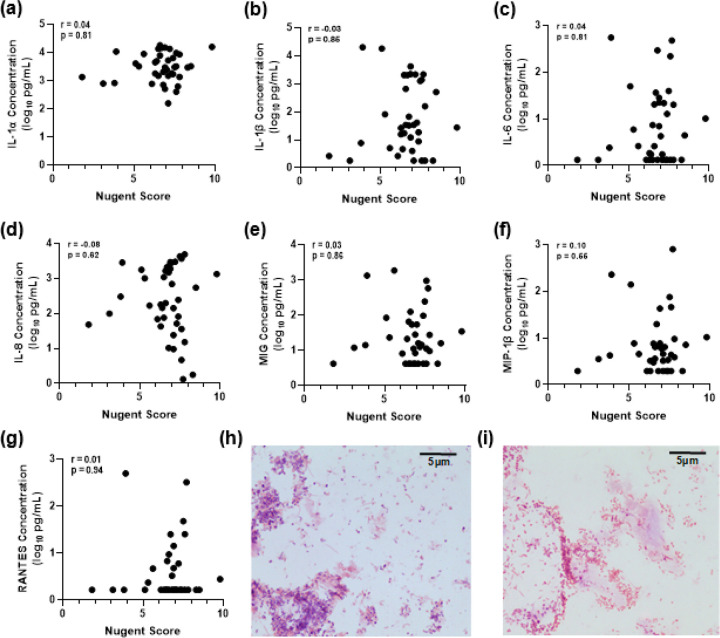
Nugent scores were analyzed in relation to pro-inflammatory cytokine concentrations, but no significant correlations were observed (Fig. 4a – g). Representative images are shown of gram-stained smears from one participant with high cytokine levels (87.8pg/ml IL-1α, 65.4pg/ml IL-1β, 34.7pg/ml IL-6, 85.3pg/ml IL-8, 61.1pg/ml MIG, 21.7pg/ml MIP-1β, 33.1pg/ml RANTES) and one participant with low cytokine levels (83.9pg/ml IL-1α, 35.6pg/ml IL-1β, 6.3pg/ml IL-6, 57.1pg/ml IL-8, 11.8pg/ml MIG, 21.7pg/ml MIP-1β, undetectable RANTES). Both participants had a Nugent score of 7 with neovaginal smears dominated by gram-negative rods (Fig. 4h – i).
Figure 4. Neovaginal cytokines do not correlate with Nugent score.
Self-collected neovaginal smears from transfeminine participants (n=39) were heat-fixed, Gram stained, and observed under a 100x oil-immersion light microscope. Cytokine concentrations in neovaginal swabs were measured by multiplex immunoassay (Luminex). Spearman’s correlations were used to assess associations between Nugent score and (a) IL-1α, (b)IL-1β, (c) IL-6, (d) IL-8, (e) MIG, (f) MIP-1β, and (g) RANTES. Representative images of gram-stained neovaginal smears from participants with low (h) and high (i) cytokine levels. Images have been cropped for visualization.
Bacteria with Nugent-targeted morphotypes in the neovagina
A comprehensive description of neovaginal bacterial communities of TransBiota participants has been previously published8. A summary of the most prevalent neovaginal bacteria (> 25% prevalence) are listed in Fig. 5, separated by the morphotypes targeted by Nugent scoring.
Figure 5. Neovaginal bacterial prevalence, relative abundance, and association with cytokines and Nugent score.
Taxa typically scored by Nugent criteria (Lactobacillus, Gardnerella, Fannyhessea, and Mobiluncus) at the start of each morphotype category. Bacterial taxa with a prevalence of >25% are included. Median relative abundance is measured among prevalent participants and interquartile range are shown. Bacterial prevalence and relative abundance were measured from neovaginal swabs (n=39) using 16S rRNA gene sequencing. Cytokine concentrations were measured from swab eluent by multiplex immunoassay (Luminex). Spearman’s correlations were used to assess associations between taxa and IL-1α, IL-1β, IL-6, IL-8, MIG, MIP-1β, RANTES and Nugent score. Spearman’s correlations p values are shown in Supplemental Table 3.
Traditional Nugent-targeted taxa (Lactobacillus, Gardnerella, and Mobiluncus) frequently appeared in the neovagina, but at low relative abundance (Fig. 5). Several other taxa detected in the neovagina had similar morphologies to Nugent-targeted genera. Lawsonella clevelandensis is a large gram-positive rod that can have similar appearance to Lactobacillus spp. on Gram staining31,32. Lawsonella clevelandensis (87.2% prevalence; 0.3% median abundance) outnumbered Lactobacillus (64.1%; 0.2%). Lawsonella abundance positively correlated with IL-1α, IL-1β, IL-8 and RANTES (p < 0.05), whereas Lactobacillus showed no correlation with cytokines, and correlated with lower Nugent scores (Fig. 5; p < 0.03). Three participants exhibited predominantly large rods; two had low Nugent scores of 2 (72.6% Lactobacillus relative abundance) and 3 (58.0% Lactobacillus relative abundance) and one scored 4 (16.5% Lactobacillus relative abundance), owing to mixed morphotypes.
Gardnerella vaginalis, was detected in 33.3% of samples at a median relative abundance of 0.4%. Fannyhessea vaginae (formerly Atopobium vaginae), although not a traditional Nugent bacteria, is also a BV-associated rod and may contribute to the diagnostic power of the Nugent score in rCF33. Fannyhessea was detected in 28.2% of samples at a median relative abundance of only 1.7%. Short straight rods in the neovagina were more likely to be Hoylesella (previously Prevotella; 97.4% prevalence, 9.7% relative abundance), Prevotella spp. (92.3%; 8.5%), or Porphyromonas spp. (97.4%; 8.3%). Prevotella was positively associated with increased cytokines (IL-1α; p < 0.01), while Gardnerella, Fannyhessea, Porphyromonas, and Hoylesella had no significant correlation. None had significant correlations with Nugent score.
Mobiluncus, the gram-variable curved rod traditionally targeted by Nugent scoring, was detected in 76.9% of samples at 0.8% median relative abundance. Other abundant neovaginal curved rods included Varibaculum (92.3% prevalence, 2.6% abundance) and Campylobacter (89.7%; 1.7%). Abundances of Campylobacter were inversely correlated with neovaginal cytokines (IL-1β, IL-8; p < 0.05) while Mobiluncus and Varibaculum showed no significant correlation with cytokines. Both Mobiluncus (p < 0.02) and Campylobacter (p < 0.02) correlated with higher Nugent scores.
Of note, in addition to F. vaginae, BV-associated Ca. Lachnocurva vaginae (formerly BVAB1; curved rod) and Sneathia amnii (curved Gram-negative rod) posses similar morphotypes to traditional Nugent bacteria and may contribute to the diagnostic power of the Nugent score in rCF34. However, both were absent from neovaginal samples.
As noted in Fig. 1E, many neovaginal smears contained high abundance of cocci, which are not considered by Nugent criteria. Based on V3-V4 16S rRNA gene sequences, 4/10 core neovaginal bacteria have morphotypes not considered during Nugent scoring (Supplemental Table 1). Peptoniphilus, Ezakiella and Anaerococcus are all gram-positive cocci. Of these, Ezakiella is associated with reduced neovaginal cytokines (IL-8, MIG, p < 0.05), while Anaerococcus is associated with increased cytokines (IL-6, MIG, MIP-1β; p < 0.05) (Fig. 5).
DISCUSSION
Principal findings
This study provides strong evidence that the Nugent score is not suitable for clinical diagnosis of neovaginal dysbiosis in TF with penile inversion vaginoplasty. Most neovaginal bacteria belong to taxa not targeted by the Nugent score, and the score does not associate with predictors of genital dysbiosis, including neovaginal symptoms and cytokines.
Nugent scoring in rCF relies on the eubiotic nature of Lactobacillus spp. predominance, and vaginal polymicrobialism as dysbiotic. However, Lactobacillus spp. predominance in the neovagina is very rare8,27,28,35, potentially due to differences in carbon sources available in the rCF vagina and TF neovagina. Vaginal epithelial cells in rCF are rich in glycogen and constantly shed into the vaginal lumen15,36,37. In contrast, neovaginal epithelium derived from penile skin lacks glycogen despite exposure to estrogen levels similar to rCF, and is instead soft-cornified with a lipid-rich extracellular matrix15,36–39. In rCF, vaginal Lactobacillus spp. metabolize glycogen products to produce lactic acid with anti-inflammatory properties, inhibiting colonization by non-lactobacilli, including pathogens40,41. In our cohort Lactobacillus rarely dominated and showed similar abundance in symptomatic and asymptomatic TF. Future research is warranted to determine if the neovaginal epithelium can support Lactobacillus predominance, and if this confers any benefit.
Further, there is no evidence that neovaginal microbiomes rich in gram-variable rods are necessarily dysbiotic. Neovaginal abundances of traditional Nugent morphotypes Gardnerella and Mobiluncus were not different between symptomatic and asymptomatic TF and did not correlate with increased cytokines. While the relationship between neovaginal inflammation and sexual health outcomes has not been adequately explored, genital inflammation is strongly correlated with negative health outcomes in cisgender individuals16,26,42–45. In rCF, Mobiluncus and Gardnerella are positively associated with vaginal cytokines such as IL-1β and IL-842,46–48. Instead in the neovagina, Campylobacter, a more abundant curved rod, showed negative correlations with cytokines. More abundant short gram-variable rods such as Hoylesella (predominantly H. timonensis and H. buccalis, both previously Prevotella), Porphyromonas and Dialister lacked any association with inflammation, while Fenollaria correlated inversely with cytokines. Other bacteria that normally contribute to the diagnostic power of the Nugent score in rCF (Fannyhessea, Sneathia amnii, Ca. L. vaginae) showed no correlation to cytokines or were absent from the neovagina. These data suggest that gram-variable rod abundance on neovaginal smears does not provide interpretive information on neovaginal health.
Lawsonella abundance correlated strongly with inflammation, yet Nugent scoring classifies Lawsonella morphotypes under the “beneficial rod” category. Likewise, pro-inflammatory cocci such as Anaerococcus lay outside the Nugent framework. These findings are consistent with a study by Weyers et al. examining neovaginal cytology, reporting that, despite over 50% of participants being diagnosed with BV, no correlation was observed with neovaginal inflammation49. Additionally, Weyers et al. noted a significant presence of inflammatory cells in the neovagina. In our study, we frequently observed blood cells, suggesting participants may be experiencing epithelial erosion.
Clinical implications
Nugent scoring of neovaginal smears risks misdiagnosis and promotes futile antibiotic use. Nearly all neovaginal smears in this study fell outside the optimal Nugent range (0–3), indicating most would be classified as dysbiotic and indicative of BV. BV in rCF is commonly treated with metronidazole, which spares Lactobacilli but is bactericidal for BV-associated gram-negative anaerobes, including Gardnerella50,51. However, the gram-positive bacteria associated with inflammation in the neovagina, such as Lawsonella and Anaerococcus, are unlikely to be susceptible to metronidazole50,51. TF who receive a Nugent score in the BV range may feel distress or use products or home remedies aimed at treating BV and restoring Lactobacilli in rCF. TransBiota participants who used diverse solutions for douching (povidone-iodine, soapy water, vinegar) were more likely to have high abundances of inflammation-associated bacteria8. Many also reported using oral probiotics or probiotic suppositories designed for rCF when experiencing neovaginal symptoms, and referred to having BV in their questionnaires10. Additional research is urgently needed to better characterize the causative agents of neovaginal inflammation and symptoms, and to design effective diagnostic tools to identify them in a clinical setting. However, these findings apply chiefly to mature penile-skin lined neovagina, and microbial dynamics might differ in bowel-segmented or peritoneal graft neovaginas.
Limitations
This study’s mail-in methodology enabled broader participation, but resulted in unusable smears due to inadequate sampling or slide damage during shipping. This study also did not address sampling location within the neovaginal canal; while participants were instructed to collect swabs 5cm into the neovaginal canal, sampling depth may have varied between participants and microbiomes vary by distance from the introitus. Although participants were asked to refrain from inserting anything into their neovagina 24h prior to sampling, variable practices outside this window may have introduced variability52,53. Unmeasured behavioral or hormonal variables may also confound associations.
Conclusions
The Nugent score is an ineffective tool for predicting neovaginal dysbiosis in TF with penile inversion vaginoplasty. Bacteria traditionally targeted by the Nugent score are rare in the neovagina, while other taxa with similar morphotypes are abundant. Nugent scores did not correlate with inflammation or symptoms in the neovagina. Using the Nugent score on neovaginal smears may result in misdiagnosis, inappropriate antibiotic use, and misplaced efforts by TF and clinicians to “correct” neovaginal microbiomes, possibly disrupting an optimal microbiome. These findings highlight that vaginal dysbiosis differs fundamentally between rCF and TF and underscores the need to establish evidence-based neovaginal diagnostics.
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
ACKNOWLEDGEMENTS
The authors would like to thank Jason Hallarn and Greta Bauer for their contributions establishing TransBiota.
Funding:
This work was supported by the Canadian Institute of Health Research [PJT 180322] and the National Institutes of Health [R21 AI157912]. JLP is supported by the Canada Research Chairs Program [CRC-2020-00175]. AS is supported by a Canada Graduate Scholarship from the Canadian Institute of Health Research. This research was undertaken, in part, thanks to funding from the Canada Foundation for Innovation [CFI 42343]. The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Funding Statement
This work was supported by the Canadian Institute of Health Research [PJT 180322] and the National Institutes of Health [R21 AI157912]. JLP is supported by the Canada Research Chairs Program [CRC-2020-00175]. AS is supported by a Canada Graduate Scholarship from the Canadian Institute of Health Research. This research was undertaken, in part, thanks to funding from the Canada Foundation for Innovation [CFI 42343]. The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Footnotes
There are no conflicts of interest or financial disclosures to declare.
Contributor Information
Jessica Prodger, Western University.
Reeya Parmar, University of Western Ontario.
Bern Monari, University of Western Ontario.
Emery Potter, University of Western Ontario.
Jorge Rojas-Vargas, University of Western Ontario.
Hannah Wilcox, University of Western Ontario.
David Zuanazzi, University of Western Ontario.
Annabel Poon, University of Maryland, Baltimore.
Ainslie Shouldice, University of Maryland, Baltimore.
Vonetta Edwards, University of Maryland, Baltimore.
Yonah Krakowsky, University of Maryland, Baltimore.
Jacques Ravel, University of Maryland, Baltimore.
DATA AVAILABILITY
All source codes used to analyze the data and generate the figures presented are available in GitHub at github.com/prodgerlab/TransBiota/tree/main/Nugent_scoring_paper. The cytokine, microbiome, and Nugent scoring datasets used in this study are also accessible through this repository.
References
- 1.James S. E., Herman J., Keisling M., Mottet L. & Anafi M. 2015 U.S. Transgender Survey (USTS): Version 1. ICPSR - Interuniversity Consortium for Political and Social Research; 10.3886/ICPSR37229.V1 (2019). [DOI] [Google Scholar]
- 2.Standards of Care for the Health of Transgender and Gender Diverse People, Version 8 - PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC9553112/.
- 3.Papadopulos N. A. et al. Quality of Life and Patient Satisfaction Following Male-to-Female Sex Reassignment Surgery. The Journal of Sexual Medicine 14, 721–730 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Jun M. S., Gonzalez E., Zhao L. C. & Bluebond-Langner R. Penile Inversion Vaginoplasty with Robotically Assisted Peritoneal Flaps. Plast Reconstr Surg 148, 439–442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanon C. D. G. et al. Laparoscopy Assisted Peritoneal Pull-Through Vaginoplasty in Transgender Women. Urology 166, 301–302 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Salgado C. J., Nugent A., Kuhn J., Janette M. & Bahna H. Primary Sigmoid Vaginoplasty in Transwomen: Technique and Outcomes. Biomed Res Int 2018, 4907208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tristani-Firouzi B. et al. Preferences for and barriers to gender affirming surgeries in transgender and non-binary individuals. International Journal of Transgender Health 23, 458–471 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas-Vargas J. et al. The Neovaginal Microbiota, Symptoms, and Local Immune Correlates in Transfeminine Individuals with Penile Inversion Vaginoplasty. 2025.03.14.643288 Preprint at 10.1101/2025.03.14.643288 (2025). [DOI] [Google Scholar]
- 9.Monari B. et al. The vaginal microbiota, symptoms, and local immune correlates in transmasculine individuals using sustained testosterone therapy. 2025.03.14.643255 Preprint at 10.1101/2025.03.14.643255 (2025). [DOI] [Google Scholar]
- 10.Hallarn J. et al. Gynecological concerns and vaginal practices and exposures among transfeminine individuals who have undergone vaginoplasty. The Journal of Sexual Medicine 20, 1344–1352 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hanlon D. E., Gajer P., Brotman R. M. & Ravel J. Asymptomatic Bacterial Vaginosis Is Associated With Depletion of Mature Superficial Cells Shed From the Vaginal Epithelium. Front Cell Infect Microbiol 10, 106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma B., Forney L. J. & Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66, 371–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschenbach D. A. et al. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 158, 819–828 (1988). [DOI] [PubMed] [Google Scholar]
- 14.Martin H. L. et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180, 1863–1868 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Mirmonsef P. et al. Free Glycogen in Vaginal Fluids Is Associated with Lactobacillus Colonization and Low Vaginal pH. PLOS ONE 9, e102467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson L. et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 90, 580–587 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Masson L. et al. Inflammatory cytokine biomarkers to identify women with asymptomatic sexually transmitted infections and bacterial vaginosis who are at high risk of HIV infection. Sex Transm Infect 92, 186–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norenhag J. et al. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127, 171–180 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Brotman R. M. et al. Bacterial Vaginosis Assessed by Gram Stain and Diminished Colonization Resistance to Incident Gonococcal, Chlamydial, and Trichomonal Genital Infection. J Infect Dis 202, 1907–1915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cauci S. et al. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9, 53–58 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Wiesenfeld H. C., Hillier S. L., Krohn M. A., Landers D. V. & Sweet R. L. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 36, 663–668 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Peters S. E. et al. Behaviors Associated with Neisseria gonorrhoeae and Chiamydia trachomatis: Cervical Infection Among Young Womena Attending Adolescent Clinics. Clin Pediatr (Phila) 39, 173–177 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Cherpes T. L., Meyn L. A., Krohn M. A., Lurie J. G. & Hillier S. L. Association between Acquisition of Herpes Simplex Virus Type 2 in Women and Bacterial Vaginosis. Clinical Infectious Diseases 37, 319–325 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Nugent R. P., Krohn M. A. & Hillier S. L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29, 297–301 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravel J. et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences 108, 4680–4687 (2011). [Google Scholar]
- 26.Prodger J. L. et al. Penile bacteria associated with HIV seroconversion, inflammation, and immune cells. JCI Insight 6, e147363 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birse K. D. et al. The neovaginal microbiome of transgender women post-gender reassignment surgery. Microbiome 8, 61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora R. M., Mehta P., Ziltzer R. & Samplaski M. K. Systematic Review: The Neovaginal Microbiome. Urology 167, 3–12 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Scheim A. I., Coleman T., Lachowsky N. & Bauer G. R. Health care access among transgender and nonbinary people in Canada, 2019: a cross-sectional survey. cmajo 9, E1213–E1222 (2021). [Google Scholar]
- 30.Holm J. B. et al. Ultrahigh-Throughput Multiplexing and Sequencing of >500-Base-Pair Amplicon Regions on the Illumina HiSeq 2500 Platform. mSystems 4, e00029–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell M. E. et al. Lawsonella clevelandensis gen. nov., sp. nov., a new member of the suborder Corynebacterineae isolated from human abscesses. Int J Syst Evol Microbiol 66, 2929–2935 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Morton A. B., Boyle E., Pettengill M. A. & Gancher E. The Brief Case: Strictly Anaerobic and Staining Acid Fast. J Clin Microbiol 61, e0015022 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muzny C. A. et al. Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J Infect Dis 218, 966–978 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan S. et al. More than meets the eye: associations of vaginal bacteria with gram stain morphotypes using molecular phylogenetic analysis. PLoS One 8, e78633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyers S. et al. Microflora of the penile skin-lined neovagina of transsexual women. BMC Microbiol 9, 102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruickshank R. The conversion of the glycogen of the vagina into lactic acid. J. Pathol. 39, 213–219 (1934). [Google Scholar]
- 37.Dinh M. H., Okocha E. A., Koons A., Veazey R. S. & Hope T. J. Expression of Structural Proteins in Human Female and Male Genital Epithelia and Implications for Sexually Transmitted Infections1. Biology of Reproduction 86, (2012). [Google Scholar]
- 38.Krakowsky Y. et al. The Effect of Gender-Affirming Medical Care on the Vaginal and Neovaginal Microbiomes of Transgender and Gender-Diverse People. Front. Cell. Infect. Microbiol. 11, 769950 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekker J. J. M. L., Hage J. J., Karim R. B. & Bloemena E. Do histologic changes in the skin-lined neovagina of male-to-female transsexuals really occur? Ann Plast Surg 59, 546–549 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Hearps A. C. et al. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 10, 1480–1490 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Delgado-Diaz D. J. et al. Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression. Microbiome 10, 141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell C. & Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71, 555–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Lu Y., Chen T. & Li R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 11, (2021). [Google Scholar]
- 44.Arnold K. B. et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIVsusceptible target cells. Mucosal Immunology 9, 194–205 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Prodger J. L. et al. Chemokine Levels in the Penile Coronal Sulcus Correlate with HIV-1 Acquisition and Are Reduced by Male Circumcision in Rakai, Uganda. PLoS Pathog 12, e1006025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph A. et al. Intrauterine colonization with Gardnerella vaginalis and Mobiluncus mulieris induces maternal inflammation but not preterm birth in a mouse model. Am J Reprod Immunol 90, e13749 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos A. C. C., Murta E. F. C., Michelin M. A. & Reis C. Evaluation of Cytokines in Endocervical Secretion and Vaginal pH from Women with Bacterial Vaginosis or Human Papillomavirus. ISRN Obstet Gynecol 2012, 342075 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anahtar M. N. et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965–976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weyers S. et al. Cytology of the ‘penile’ neovagina in transsexual women. Cytopathology 21, 111–115 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Verwijs M. C., Agaba S. K., Darby A. C. & van de Wijgert J. H. H. M. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am J Obstet Gynecol 222, 157.e1–157.e13 (2020). [Google Scholar]
- 51.Goldstein E. J. C., Tyrrell K. L. & Citron D. M. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin Infect Dis 60 Suppl 2, S98–107 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Muthusamy S., Varghese J., Raveendran V., Ezilarasan K. & Easow J. Evaluation of interobserver reliability of Nugent score for diagnosis of bacterial vaginosis. Indian J Sex Transm Dis 39, 120 (2018). [Google Scholar]
- 53.Mohanty S., Sood S., Kapil A. & Mittal S. Interobserver variation in the interpretation of Nugent scoring method for diagnosis of bacterial vaginosis. Indian J Med Res 131, 88–91 (2010). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All source codes used to analyze the data and generate the figures presented are available in GitHub at github.com/prodgerlab/TransBiota/tree/main/Nugent_scoring_paper. The cytokine, microbiome, and Nugent scoring datasets used in this study are also accessible through this repository.