Abstract
Neuropeptide Y is implicated in energy homeostasis, and contributes to obesity when hypothalamic levels remain chronically elevated. To investigate the specific role of hypothalamic Y2 receptors in this process, we used a conditional Y2 knockout model, using the Cre-lox system and adenoviral delivery of Cre-recombinase. Hypothalamus-specific Y2-deleted mice showed a significant decrease in body weight and a significant increase in food intake that was associated with increased mRNA levels for the orexigenic NPY and AgRP, as well as the anorexic proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) in the arcuate nucleus. These hypothalamic changes persisted until at least 34 days after Y2 deletion, yet the effect on body weight and food intake subsided within this time. Plasma concentrations of pancreatic polypeptide and corticosterone were 3- to 5-fold increased in hypothalamus-specific Y2 knockout mice. Germ-line Y2 receptor knockout also produced a significant increase in plasma levels of pancreatic polypeptide. However, these mice differed from conditional knockout mice in that they showed a sustained reduction in body weight and adiposity associated with increased NPY and AgRP but decreased POMC and CART mRNA levels in the arcuate nucleus. The transience of the observed effects on food intake and body weight in the hypothalamus-specific Y2 knockout mice, and the difference of this model from germ-line Y2 knockout mice, underline the importance of conditional models of gene deletion, because developmental, secondary, or extrahypothalamic mechanisms may mask such effects in germ-line knockouts.
Keywords: neuropeptide Y‖pancreatic polypeptide‖cre-lox‖arcuate nucleus
Neuropeptide Y (NPY) in the hypothalamus is known to be a strong stimulus for food intake (1, 2), and induces many neuroendocrine and metabolic changes that favor energy storage. Such changes include decreased thermogenesis in brown adipose tissue, hyperinsulinemia, hypercorticosteronemia, and insulin hyperresponsiveness in white adipose tissue (3, 4). All of these neuroendocrine and metabolic effects of central NPY administration persist even when NPY-induced hyperphagia is prevented by pair-feeding (3, 4), demonstrating that hyperphagia is not the only mechanism by which central NPY increases adiposity.
Although numerous other hormones and peptides act within the hypothalamus to regulate energy homeostasis, many exert an important component of their effects via actions on the hypothalamic NPY-ergic system (5–7), demonstrating the pivotal role of NPY in coordinating energy homeostasis. However, it is not clear which of the 5 cloned Y-receptors (Y1, Y2, Y4, Y5, and y6) are responsible for these effects (8).
There is increasing evidence that Y2 receptors are involved in energy homeostasis. Over 80% of NPY-containing neurons in the arcuate nucleus (Arc) coexpress Y2 receptor mRNA (9). This presynaptic Y2 receptor has therefore been proposed to regulate the release of NPY (10, 11) and other colocalized neurotransmitters involved in energy homeostasis such as agouti related protein (AgRP) (12). The existence of such a feedback mechanism has been strengthened by recent reports that show that NPY release from rat hypothalamic slices is reduced by the Y2-preferring agonist NPY(13–36), and that this effect is blocked by the selective Y2 receptor antagonist (BIIE0246; ref. 13). Further evidence for a role of Y2 receptors in energy homeostasis comes from the finding that release of the catabolic alpha melanocyte-stimulating hormone (α-MSH), and the expression of its precursor, proopiomelanocortin (POMC) mRNA within neurons of the Arc are strongly down-regulated by NPY (14, 15). These effects are mimicked by NPY(13–36) but not by the Y1/Y5 agonist [L31,P34]NPY (14, 15).
The lack of a full complement of selective pharmacological tools for NPY receptors has made it difficult to determine which Y receptors are responsible for the effect of NPY on energy homeostasis. Moreover, germ-line knockout mice lacking NPY or the Y1, Y2, or the Y5 receptor surprisingly show either no change or increased body weight and/or adiposity (16–20). However, experience has also highlighted many pitfalls to a germ-line knockout approach, such as compensatory mechanisms arising during development, or secondary effects consequent to germ-line deletion.
Therefore, to more directly investigate the role of hypothalamic Y2 receptors in energy homeostasis, we have generated a conditional Y2 receptor knockout mouse model. This model, the first conditional knockout of a Y receptor, allows specific deletion of hypothalamic Y2 receptors in the adult, thus avoiding the potential complications resulting from germ-line knockout.
Materials and Methods
Targeting Vector Construction and Generation of Y2 Receptor Knockout Mice.
A 129/SvJ mouse genomic bacterial artificial chromosome (BAC) library (Genome Systems, St Louis) was screened under low stringency for the Y2 receptor gene by using a human Y2 cDNA probe (21). Positively hybridizing clones were isolated and mapped. An 8.5-kb BamHI fragment was subcloned into pBluescript and used to generate the targeting construct. A cassette containing the neomycin resistance gene (Neo) flanked on either side by a 34-bp-long Cre-recombinase (Cre) recognition (loxP) site orientated in the same direction was inserted into a unique Asp718 site downstream of the Y2 gene. A third loxP sequence was introduced by cloning two complementary 46-mer oligonucleotides into a NheI site in intron I, 1 kb upstream of the Y2 receptor gene initiation codon (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). A 1.2-kb PstI/BamHI fragment 5′ to the Y2 targeting construct was used for screening of positively targeted ES cell clones. Mice were genotyped by Southern blot analysis (see Results) and PCR using oligo-C (5′-TTAACATCAGCTGGCCTAGC-3′), oligo-D (5′-GGAAGTCACCAACTAGAATGG-3′) and oligo-E (5′-AGCATCCAGAGAAGTGCAAC-3′), with 35 cycles of 94°C for 45 s, 60°C for 1 min, and 72°C for 20 s.
Measurement of Food Intake and Body Weight.
Animals were group-housed and fed with standard chow. Body weight was monitored at the same time each week from 4 wk of age onwards. Food intake was measured over 7 days in individually housed mice at 8 and 12 wk of age.
Tissue Collection and Analysis.
At 16–18 wk of age, germ-line Y2 knockout mice were killed by cervical dislocation between 1000 and 1400 h, for collection of trunk blood. A subset of Y2+/+ and Y2−/− mice received corticosterone in the drinking water (25 mg/liter) for a period of 2 wk before sacrifice, during which time body weight, and food and water intake were monitored weekly. Conditional knockout mice were killed at 12–14 or 15–17 wk of age (12 or 34 days after Cre-expressing adenovirus injection). Mice were killed within 90 s of initial handling to avoid time-dependent increases in corticosteronemia. White adipose tissue (WAT) depots (right inguinal, right epididymal or periovarian, right retroperitoneal, and mesenteric), pancreas, stomach, liver, right kidney, heart, and right testis were collected and weighed. Plasma leptin and insulin levels were measured by RIA kits from Linco Research (St. Charles, MO), corticosteronemia was measured with a kit from ICN, and glycemia was determined with a glucose oxidase assay kit (Trace Scientific, Melbourne, Australia). Plasma pancreatic polypeptide (PP) was radioimmunoassayed by using guinea pig anti-rat PP serum, 2nd Antibody Precipitating System (Linco Research) and [125I]hPP (NEN).
Adenovirus Injection.
Ten- to 12-wk-old conditional Y2lox/lox and Y2+/+ mice were anesthetized with 100 mg/kg ketamine and 20 mg/kg xylazine (Parke-Davis-Pfizer; Bayer, Wuppertal, Germany) and injected with recombinant Cre- or green fluorescent protein (GFP)-expressing adenovirus (Riken, Fukyuka, Japan) using a stereotaxic table (Kopf Instruments, Tujunga, CA). Brain injection coordinates relative to bregma were posterior 2.3 mm, lateral ± 0.3 mm, ventral 5.6 mm, corresponding to the Arc (22). As additional controls, conditional Y2lox/lox mice were injected with Cre adenovirus into the CA3 region of the dorsal hippocampus (posterior 2.5 mm, lateral ± 3.0 mm, ventral 3.1 mm; ref. 22). Virus (1 × 109 plaque-forming units in 1 μl) was injected bilaterally over 10 min by using a 26-gauge guide cannula and a 33-gauge injector (Plastics One, Roanoke, VA) connected to a Hamilton Syringe and a syringe infusion pump (WPI Instruments, Waltham, MA). Mice were housed individually, and daily food and water intake and body weight were measured for the next 12–34 days.
In Situ Hybridization and Receptor Binding.
Coronal slices (20 μm) of fresh frozen brains were cut and thaw-mounted on charged slides. For in situ hybridization, DNA oligonucleotides complementary to mouse NPY (5′-GAGGGTCAGTCCACACAGCCCCATTCGCTTGTTACCTAGCAT-3′); mouse POMC (5′-TGGCTGCTCTCCAGGCACCAGCTCCACACATCTATGGAGG-3′); mouse cocaine- and amphetamine-regulated transcript (CART; 5′-TCCTTCTCGTGGGACGCATCATCCACGGCAGAGTAGATGTCCAGG-3′); and mouse AgRP (5′-AGCTTGCGGCAGTAGCAAAAGGCATTGAAGAAGCGGCAGTAGCAC-3′) mRNAs were labeled with [35S]thio-dATP (Amersham Pharmacia or NEN) using terminal deoxynucleotidyltransferase (Roche, Mannheim, Germany or Amersham Pharmacia). Matching sections from the same portion of the hypothalamus of knockout and control mice were assayed together, following the method of Young (23) with variations (24, 25). mRNA levels were evaluated by measuring silver grain densities over individual neurons from photo-emulsion-dipped sections.
Radio ligand binding studies were carried out as described recently (26), by using the Y2/Y5-selective ligand [125I]PYY(3–36). Autoradiographs were scanned, and relative optical density (ROD) values were determined over the stratum oriens CA3. Specific binding was calculated by subtracting nonspecific, obtained from sections incubated in 1,000-fold excess of cold ligand, from total binding. Background labeling was uniform and never exceeded 5% of total signal.
Statistical Analyses.
Differences among groups were assessed by ANOVA (factorial or repeated measures), followed by Fisher's post hoc tests, using statview version 4.5 (Abacus Concepts, Berkeley, CA). For all statistical analyses, P < 0.05 was accepted as being statistically significant.
Results
Generation of Germ-Line and Conditional Y2 Receptor Knockout Mice.
A targeting vector for the Y2 receptor gene was designed and generated that allowed the production of both germ-line (Y2−/−) and conditional (floxed, Y2lox/lox) knockout mice (Fig. 6A). In this construct, no original mouse genomic sequence was deleted or rearranged, thus keeping the targeted allele as similar as possible to the original gene.
Chimeras carrying the Y2 floxed gene were crossed with oozyte-specific Cre-expressing C57BL/6 mice (27) to obtain either heterozygotes carrying the floxed gene (conditional, Y2lox/+), or heterozygotes carrying the Cre gene and having the floxed gene already deleted (germ-line, Y2+/−). Absence of the Y2 gene in homozygote germ-line Y2−/− mice, which also provides verification for the functionality of the Cre-lox system, was confirmed by Southern analysis employing a Y2 receptor coding sequence-specific DNA fragment and PCR (Fig. 6 B and C). All further mice generated were maintained on this mixed C57BL/6–129/SvJ background, and littermates were used as controls. In the case of the germ-line Y2 receptor knockouts, animals that no longer carried the Cre-transgene were selected.
To confirm that introduction of the loxP sites and the Neo cassette did not alter expression of the Y2 receptor gene in our conditional line, we performed radioligand binding experiments on brain slices from wild-type Y2+/+, germ-line Y2−/−, and Y2lox/lox animals. As shown in Fig. 6D, whereas germ-line Y2−/− mice had negligible [125I]PYY(3–36) binding (relative optical density 0.02), the level of binding in uninduced conditional Y2lox/lox mice was comparable to that of wild types (relative optical density in CA3: 0.21 vs 0.27), making them suitable controls.
Hypothalamus-Specific Y2 Receptor Gene Deletion.
Refinement of the stereotaxic injection coordinates was monitored by the appearance of green fluorescence only in cells in the hypothalamus of GFP-injected animals. Cre-gene expression and subsequent Y2 receptor gene deletion were confirmed at 28 days postadenovirus injection by PCR analysis of DNA isolated from hypothalamic blocks. Only DNA isolated from the hypothalamus of Cre-injected Y2lox/lox animals, but not from other parts of the brain of the same animals, nor hypothalamic DNA from GFP-injected Y2lox/lox animals, gave rise to fragments specific for the Cre gene (Fig. 6C2), and fragments specific for deletion of the Y2 gene (Fig. 6C3). In the same DNA preparation, fragments specific for the homozygous floxed Y2 gene were strongly reduced to ≈10% relative to the controls (Fig. 6C1), further confirming successful deletion of the Y2 gene in the majority of the hypothalamic cells.
Effect of Conditional Y2 Receptor Deletion on Body Weight and Food Intake.
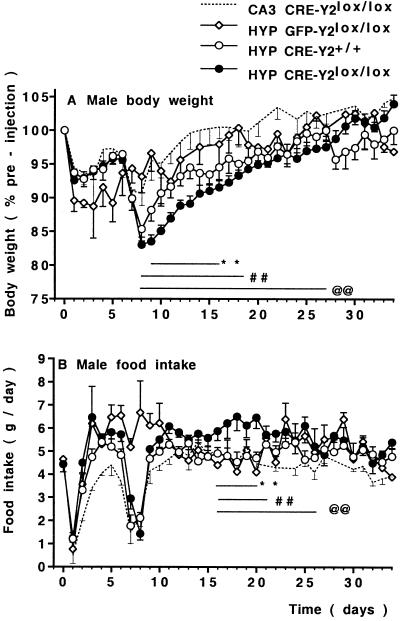
Hypothalamus-specific Y2 receptor deletion in HYP CRE-Y2lox/lox mice had significant effects on body weight and food intake. These effects were significantly different from the two groups of Cre-injected control mice (CA3 CRE-Y2lox/lox, and HYP CRE-Y2+/+), as well as GFP-injected controls (HYP GFP Y2lox/lox), as shown in Fig. 1. All groups of mice were of similar initial body weight (27.9 ± 0.6 g in males and 22.3 ± 0.4 g in females, n = 30–54 mice of each gender), and demonstrated an equivalent drop in body weight and food intake on the day after injection (Fig. 1), which is probably due to anesthesia and surgery. At 7–8 days postinjection, Cre- but not GFP-injected mice underwent a second drop in body weight and food intake (Fig. 1). However, compared with control mice, recovery to initial body weight was significantly delayed in the hypothalamus-specific Y2 receptor knockouts. Male CA3 CRE-Y2lox/lox and HYP CRE-Y2+/+ control mice returned to preinjection body weight within 14 to 22 days postinjection, respectively, whereas 100% recovery was delayed until 28 days in the male HYP CRE-Y2lox/lox knockouts (Fig. 1A). Female wild-type control mice returned to preinjection body weight within 16 days postinjection, but, in female knockouts, recovery to 100% body weight was delayed until 23 days postinjection (P < 0.01, n = 6–13 mice per group).
Figure 1.
Conditional Y2 receptor deletion reduces body weight. Body weight changes (A) and food intake (B) in male Y2lox/lox mice after injection of Cre-expressing adenovirus into the hypothalamus (HYP CRE-Y2lox/lox, n = 28) compared with control Y2lox/lox mice injected into the CA3 region of the hippocampus (CA3 CRE-Y2lox/lox, n = 6) or injected with control GFP-expressing adenovirus in the hypothalamus (HYP GFP-Y2lox/lox, n =6), as well as wild-type Y2+/+ controls injected in the hypothalamus (HYP CRE-Y2+/+, n = 15). Values are means ± SEM. **, P < 0.01 HYP CRE-Y2lox/lox vs. HYP CRE-Y2+/+; ##, P < 0.01 vs. GFP-injected controls; @@, P < 0.01 vs. CA3-injected controls.
Male HYP CRE-Y2lox/lox mice showed a significantly greater food intake than mice in the three control groups (Fig. 1B). In female knockout mice, there was also a tendency to greater food intake in the postinjection period (5.4 ± 0.2 g/day vs. 5.0 ± 0.2 g/day in Cre-injected wild-type controls and 4.1 ± 0.2 g/day in GFP-injected Y2lox/lox controls, n = 6–10 mice per group).
Increased Plasma Corticosterone and PP Levels in Hypothalamus-Specific Y2 Knockout Mice.
Despite the transient reduction in body weight induced by hypothalamus-specific Y2 receptor deletion, at 34 days postinjection, these mice were not significantly different from control mice with respect to WAT mass, leptinemia, insulinemia, and glycemia (data not shown). At 12 days postinjection, at which time body weight of hypothalamus-specific knockouts was significantly less than the three groups of control mice, there was no significant difference between groups with regard to WAT mass, although the insulinemia of knockout mice tended to be less than control values at this time (58 ± 6 vs. 97 ± 19, n = 5–6 male mice per group).
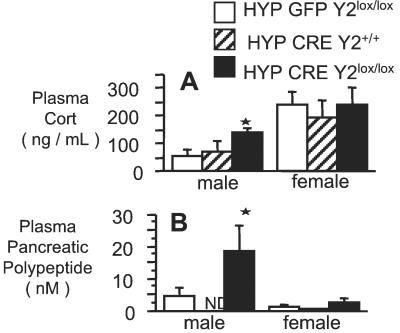
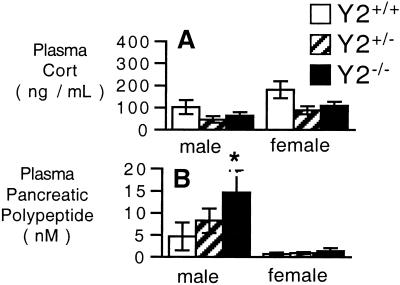
Corticosteronemia of male hypothalamus-specific knockouts was significantly increased over GFP- or Cre-injected control mice at 34 days postinjection (Fig. 2A), and this change was associated with a 4-fold increase in plasma PP levels (Fig. 2B). In contrast, levels of corticosterone in female mice, which were already higher than that of male mice under basal conditions, were not affected by Y2 receptor deletion in conditional knockout animals (Fig. 2B). The plasma levels of PP in female mice were not affected by hypothalamus-specific Y2 deletion (Fig. 2B).
Figure 2.
Plasma corticosterone and PP levels of male and female hypothalamus-specific Y2 receptor knockout mice. (A) Corticosteronemia and (B) plasma PP levels of Cre adenovirus-injected Y2lox/lox (knockout) mice vs. GFP adenovirus-injected Y2lox/lox or Cre adenovirus-injected Y2+/+ control mice. Data are means ± SEM of 5–13 mice per group. *, P < 0.05 vs. same-sex control mice: HYP GFP-Y2lox/lox and HYP CRE-Y2+/+ for A, and HYP GFP-Y2lox/lox for B.
Effect of Germ-Line Y2 Receptor Deletion on Body Weight and Food Intake.
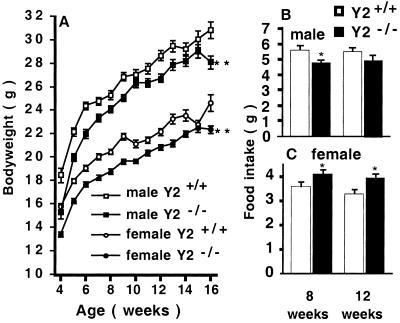
Male and female germ-line Y2−/− mice had identical birth-weights as control animals. However, knockout mice had a significantly lower body weight compared with heterozygous or wild-type littermates at 4 wk of age, and this difference persisted throughout the 16 wk of monitoring (Fig. 3A). The daily food intake of male germ-line Y2−/− mice was significantly less than that of wild-type controls at 8 wk but not at 12 wk of age (Fig. 3B). In contrast, significantly increased food intake was observed in female Y2−/− mice at both 8 and 12 wk of age (Fig. 3C).
Figure 3.
(A) body weight of germ-line male Y2−/− and female Y2−/− mice compared with combined wild-type (Y2+/+) and heterozygous (Y2±) controls. Values are means ± SEM+/− of 20–32 mice per group. **, P < 0.01 vs. same-sex controls. (B and C) Twenty-four-hour food intake of (B) male and (C) female germ-line Y2−/− mice vs. wild-type controls, measured at 8 and 12 wk of age. Values are means ± SEM of 12–14 mice per group. *, P < 0.05 vs. age-matched same-sex controls.
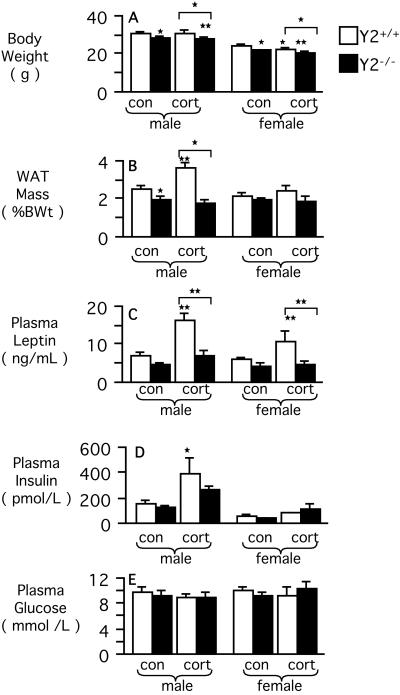
The reduced body weight of male germ-line Y2 knockout mice was associated with a significant reduction in relative WAT mass (Fig. 4 A and B), but there was no difference between male knockouts and controls with respect to leptinemia, insulinemia, nor glycemia (Fig. 4 C–E). Despite reduced body weight, WAT mass, leptinemia, insulinemia, and glycemia of female germ-line knockouts were no different from wild-type control values (Fig. 4 A–E). There was no difference between germ-line Y2 knockout and control mice with respect to mass of any of the organs weighed.
Figure 4.
Effect of germ-line Y2 receptor deficiency on body composition and hormonal and metabolic parameters in male and female mice, under control conditions and after 2 wk of corticosterone (cort) administration. (A) Body weight. (B) Combined weight (as a percentage of body weight) of right inguinal, right epididymal or periovarian, right retroperitoneal, and mesenteric WAT. (C) Leptinemia. (D) Insulinemia. (E) Glycemia. Data are means ± SEM of 9–30 mice per group. *, P < 0.05; and **, P < 0.01 vs. same-sex Y2+/+ control mice, or between the groups indicated by horizontal bars above columns.
Germ-Line Y2 Receptor Knockout Attenuates the Obesity Syndrome Associated with Corticosterone Administration.
In male wild-type mice, 2-wk corticosterone administration induced an obesity syndrome characterized by significant increases in WAT mass, leptinemia, and insulinemia, but with no change in body weight or glycemia (Fig. 4 A–E). Food intake of corticosterone-treated male wild-type mice was not significantly different from untreated wild-type controls (data not shown). In contrast, these effects of corticosterone were absent in male germ-line Y2−/− mice (Fig. 4). Corticosterone administration significantly reduced body weight in female Y2+/+ and Y2−/− mice (Fig. 4). It also significantly increased leptinemia, in the absence of any significant effects on WAT mass, insulinemia, or glycemia in female Y2+/+, but not Y2−/−, mice (Fig. 4). There was no significant difference in corticosteronemia among control and corticosterone-administered mice (data not shown).
Increased Plasma PP Levels in Germ-Line Y2 Knockout Mice.
In contrast to the hypercorticosteronemia of male conditional knockout animals, plasma corticosterone levels of germ-line Y2−/− male and female mice showed a tendency to lower values compared with control mice (Fig. 5A). However, consistent with results from the conditional knockout mice, male germ-line Y2−/− showed a 3-fold increase in plasma PP levels compared with wild-type controls whereas, in female germ-line Y2−/− mice, plasma PP levels were not different from control values (Fig. 5B). As in conditional knockouts and controls, plasma concentrations of PP were around 10-fold higher in male than in female mice (Figs. 2B and 5B).
Figure 5.
Plasma corticosterone and PP levels of male and female germ-line Y2 receptor knockout mice. (A) Corticosteronemia and (B) plasma PP levels of germ-line Y2−/− mice vs. Y2+/− mice or Y2+/+ control mice. Data are means ± SEM of 9–19 mice per group. *, P < 0.05 vs. same-sex Y2+/+ control mice.
Altered Hypothalamic Neuropeptide Expression in Germ-Line and Conditional Y2 Receptor Knockout Mice.
Selective Y2 receptor deletion by Cre-adenovirus injection into the hypothalamus of Y2lox/lox animals caused significant increases in Arc NPY and AgRP mRNA expression, both at 12 days (by 36–42%) and at 34 days (by 36–50%) postinjection, compared with age-matched wild-type injected controls (Table 1). Interestingly, POMC mRNA levels were also increased in Arc neurons of hypothalamus-specific Y2 receptor deficient mice at 12 and 34 days postinjection, compared with wild-type injected animals (Table 1), as was the expression of CART mRNA (by 30%), although this difference attained statistical significance only at 34 days postinjection (Table 1). CART expression in the paraventricular nucleus (PVN) was unchanged (104 ± 3.8% and 98 ± 3.9% of control at 12 and 34 days, respectively). In line with increased food intake and recovery of body weight after the weight loss induced by Cre-adenovirus injection, wild-type animals investigated at 12 days postinjection exhibited significantly lower levels of POMC and CART mRNAs and slightly increased levels of NPY and AgRP mRNA in neurons of the Arc relative to wild-type littermates at 34 days postinjection (Table 1).
Table 1.
Expression levels of neuropeptide mRNAs in neurons of the Arc at 12 and 34 days after Cre-expressing adenovirus injection
| mRNA | Wild type, 12 days | Y2lox/lox, 12 days | Wild type, 34 days | Y2lox/lox, 34 days |
|---|---|---|---|---|
| NPY | 18.3 ± 1.34 (4) | 24.8 ± 2.25 (5)* | 16.0 ± 0.43 (5) | 24.0 ± 2.54 (4)** |
| AgRP | 16.6 ± 1.42 (4) | 23.5 ± 1.67 (5)** | 14.9 ± 1.00 (5) | 19.0 ± 0.83 (4)*† |
| POMC | 23.6 ± 1.10 (4) | 27.0 ± 1.41 (5)* | 33.3 ± 0.45 (5)‡ | 36.5 ± 0.74 (4)*‡ |
| CART | 12.9 ± 1.09 (4) | 16.8 ± 0.41 (5) | 30.5 ± 2.53 (5)‡ | 39.5 ± 3.27 (4)**‡ |
Data represent mean labeling intensity of neurons given as percentage coverage of neuronal surface by silvergrains ± SEM of the number of mice shown in parentheses.
, P < 0.05;
, P < 0.01 vs. respective wild-type control group. †, P < 0.05 vs. respective group at 12 days. ‡, P < 0.01 vs. respective group at 12 days.
In germ-line Y2 receptor knockout mice, mRNA levels relative to wild-type controls in the Arc were 120 ± 4.4% (P = 0.001) for NPY and 140 ± 9.6% (P = 0.003) for AgRP, consistent with the changes seen in conditional knockout mice. In contrast to the hypothalamus-specific knockouts, POMC and CART expression were significantly reduced in germ-line Y2−/− mice compared with wild-type controls (POMC, 83 ± 4.3%, P = 0.007; CART, 73 ± 4.6%, P = 0.01). No significant changes in neuropeptide mRNA levels were observed in other hypothalamic (PVN) and subthalamic areas (CART 98 ± 4.6%; melanin concentrating hormone 100 ± 7.0%; orexin 102 ± 1.8%). Data are mean percentage of control ± SEM of 6–7 mice per group.
Discussion
This study provides in vivo evidence for the involvement of hypothalamic Y2 receptors in energy homeostasis, because specific and permanent deletion of these receptors by central injection of Cre-expressing adenovirus in Y2lox/lox adult mice resulted in reduced body weight in association with increased food intake. Moreover, adaptation to these effects of hypothalamic Y2 receptor deletion occurred within 23–28 days.
We also demonstrate that endogenous Y2 receptors are involved in tonic inhibition of NPY and AgRP expression in neurons of the Arc, because conditional Y2 receptor deletion significantly increased the mRNA levels of these colocalized (12) peptides. This change may have contributed to the increased food intake observed in the hypothalamus-specific Y2 receptor knockout mice. The transiently decreased body weight of conditional Y2 receptor knockout mice was associated with increased mRNA expression of the anorexic POMC and CART, in keeping with a causal relationship. These data show a role (direct or indirect) of endogenous Y2 receptors in regulating POMC and CART expression in vivo.
Conditional Y2 receptor knockout resulted in a robust increase in the plasma concentrations of PP specifically in male but not female mice, suggesting hypothalamic regulation of the plasma levels of this hormone. PP is released from F-cells in the islets of Langerhans in response to stimuli such as ingestion of food, hypoglycemia, or neuroglucopenia, predominantly via a vagal, muscarinic mechanism (28, 29). PP may contribute to the counterregulatory response to hypoglycemia by stimulating adrenal glucocorticoid secretion, as seen in vitro and in vivo (30, 31). The increased plasma PP levels of male hypothalamus-specific knockout mice may have contributed to the associated increase in corticosteronemia in these mice.
This study has also revealed effects of Cre adenovirus injection in control mice on body weight and food intake. These effects were probably due to Cre expression per se (32) and were not observed after GFP adenovirus injection. Toxicity of Cre at high but not low expression levels has been reported in vitro (33) and is probably due to interaction of the enzyme with pseudo-loxP sites. This finding exemplifies the importance of using adeno-Cre-injected control mice to determine specific effects of adeno-Cre-induced gene deletion.
Our direct comparison of the phenotype of conditional vs. germ-line Y2 receptor knockout mice has revealed differences that are likely to be due to either compensatory mechanisms arising during development, secondary effects consequent to germ-line deletion, or effects of deleting Y2 in peripheral as well as hypothalamic tissue. Notably, in contrast to male conditional knockouts, male germ-line Y2 knockout mice displayed significantly reduced body weight, food intake, and WAT mass. These effects may be the result of long-term elevations in plasma PP levels seen specifically in male Y2−/− mice. This result is supported by findings that PP-overexpressing transgenic mice exhibit reductions in body weight, food intake, and fat mass (34). Furthermore, human obesity syndromes, as well as genetically obese ob/ob mice, demonstrate reduced plasma levels of PP, whereas aspects of these obese phenotypes, including hyperphagia, are attenuated by exogenous PP administration (35–37).
A further difference between conditional and germ-line Y2 knockout was the effect on corticosteronemia. Unlike conditional knockout of Y2, germ-line knockout did not increase the plasma concentrations of this hormone. This difference may be related to the fact that prolonged activation of the hypothalamo-pituitary-adrenal axis can eventually lead to down-regulation of this axis at the level of the hypothalamus (4). Of note is the observation that Y2 receptor deficiency also reduced the responsiveness of mice to exogenous corticosterone administration. In wild-type mice, corticosterone administration resulted in an obesity syndrome, which has been observed by other investigations of rats (6) and mice (38). This finding may be related to the fact that glucocorticoids are known to increase NPY mRNA and/or peptide levels and Y receptor expression in the hypothalamus in vivo (6, 39) and in vitro (39). The present study demonstrates that glucocorticoid excess mediates at least part of its effects through Y2 receptors. This finding, in conjunction with altered corticosteronemia in hypothalamus-specific Y2 receptor knockout mice, suggests involvement of Y2 receptors in regulation of hypothalamo-pituitary-adrenal axis output and responses.
Further differences between conditional and germ-line Y2 knockout mice were observed at the level of hypothalamic peptide expression. In contrast to conditional knockouts, germ-line knockout mice showed increased NPY and AgRP but decreased POMC and CART mRNA levels in the Arc, despite the reduced body weight of the latter model. This hypothalamic profile, which has been observed in obese rodent models (40, 41), may be a secondary adaptation to germ-line Y2 receptor knockout that reduces further weight loss, and underscores the importance of time-controlled conditional knockout models for the analysis of energy homeostasis.
This study has revealed sex differences in several parameters of energy homeostasis, as well as in responses to conditional or germ-line Y2 receptor knockout. For example, plasma PP concentrations were higher in male than in female mice, and Y2 deletion led to significant increases in the plasma levels of this hormone only in male animals. The reason for these gender differences is not clear from the current study. However, it is likely that the differential effect of Y2 deletion on plasma PP levels in male and female mice may account for some of the other gender differences found in Y2 knockouts, such as the differential effect of conditional Y2 deletion on corticosteronemia in male and female mice, and the sex differences in food intake and adiposity in germ-line Y2 knockouts. A further gender difference observed in this study was that basal corticosteronemia was higher in female than in male wild-type mice, and females were less responsive to the effect of exogenous corticosterone in inducing an obesity syndrome. This finding extends previous observations of sexual dimorphism in function of the hypothalamo-pituitary-adrenal axis in rodents (42).
There are differences between the reduced body weight of our germ-line Y2−/− animals and the recently published results from another germ-line Y2 receptor knockout model (17), in which female knockouts exhibited increased food intake, body weight, and adiposity. The phenotypic differences of male mice were not discussed in detail in that report. However, the genetic backgrounds of the two knockout models differ. Our mice are on a mixed C57BL/6 × 129/SvJ background, whereas the other model is on a mixed 129/SvJ × BALB/c background, which makes direct comparisons difficult. Perhaps more importantly, the design of our construct leads to complete removal of the Y2 receptor gene, including the initiation codon as well as the Neo selection gene. In the previous study, the targeting construct allowed for translation of parts of the amino-terminal end of the Y2 receptor protein (which could interfere with other functions of the cell) and furthermore, it still carried the Neo gene with its strong promoter, which could alter the function of nearby genes.
In summary, we have established a mouse model that allows the investigation of a variety of Y2 receptor functions by selectively deleting the gene in any given tissue, both centrally as well as peripherally. This result is demonstrated by our hypothalamus-specific Y2 receptor knockout, which provides more direct insight into the role of hypothalamic Y2 receptors in energy homeostasis than germ-line knockouts. Investigation of these mice showed that hypothalamic Y2 receptors regulate expression of NPY, AgRP, POMC, and CART, and that these changes are associated with functional changes in food intake and body weight. Although deletion of the Y2 receptor in the hypothalamus of the conditional knockouts is permanent, and the effect of gene deletion on hypothalamic expression of peptides persists for at least 34 days, the effects on body weight and food intake are transient, consistent with adaptation to maintain homeostasis in these vital processes.
Supplementary Material
Acknowledgments
We thank Prof. I. Saito (University of Tokyo) for the Cre-adenovirus construct and Dr. Lee Carpenter for the GFP-expressing adenovirus. We thank Dr. Julie Ferguson for invaluable veterinary advice, and the staff of the Garvan Institute Biological Testing Facility. We are grateful to Profs. Don Chisholm, Peter Schofield, and John Shine, and Dr. Greg Cooney for critical review of this manuscript. This research was supported by a Garvan Project Grant donated by Ray Williams, by a National Health and Medical Research Council of Australia Centre Block Grant, and by a Peter Doherty PostDoctoral Fellowship (987122) to A.S. and an Auslands-Stipendium of the University of Innsbruck to C.S.
Abbreviations
- NPY
neuropeptide Y
- Arc
arcuate nucleus
- AgRP
agouti related protein
- POMC
proopiomelanocortin
- CART
cocaine- and amphetamine-regulated transcript
- GFP
green fluorescent protein
- Cre
Cre-recombinase
- Neo
neomycin
- PP
pancreatic polypeptide
- WAT
white adipose tissue
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Clark J T, Kalra P S, Crowley W R, Kalra S P. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 2.Stanley B G, Leibowitz S F. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 3.Billington C J, Briggs J E, Harker S, Grace M, Levine A S. Am J Physiol. 1994;266:R1765–R1770. doi: 10.1152/ajpregu.1994.266.6.R1765. [DOI] [PubMed] [Google Scholar]
- 4.Sainsbury A, Rohner-Jeanrenaud F, Cusin I, Zakrzewska K E, Halban P A, Gaillard R C, Jeanrenaud B. Diabetologia. 1997;40:1269–1277. doi: 10.1007/s001250050820. [DOI] [PubMed] [Google Scholar]
- 5.Erickson J C, Hollopeter G, Palmiter R D. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 6.Zakrzewska K E, Cusin I, Stricker-Krongrad A, Boss O, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Diabetes. 1999;48:365–370. doi: 10.2337/diabetes.48.2.365. [DOI] [PubMed] [Google Scholar]
- 7.Kask A, Schioth H B, Harro J, Wikberg J E, Rago L. Can J Physiol Pharmacol. 2000;78:143–149. [PubMed] [Google Scholar]
- 8.Blomqvist A G, Herzog H. Trends Neurosci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- 9.Broberger C, Landry M, Wong H, Walsh J N, Hökfelt T. Neuroendocrinology. 1997;66:393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- 10.Gehlert D R, Beavers L S, Johnson D, Gackenheimer S L, Schober D A, Gadski R A. Mol Pharmacol. 1996;49:224–228. [PubMed] [Google Scholar]
- 11.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. Proc Natl Acad Sci USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn T M, Breininger J F, Baskin D G, Schwartz M W. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 13.King P J, Williams G, Doods H, Widdowson P S. Eur J Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- 14.Blasquez C, Jegou S, Tranchand Bunel D, Fournier A, Vaudry H. Brain Res. 1992;596:163–168. doi: 10.1016/0006-8993(92)91544-o. [DOI] [PubMed] [Google Scholar]
- 15.Garcia de Yebenes E, Li S, Fournier A, St-Pierre S, Pelletier G. Brain Res. 1995;674:112–116. doi: 10.1016/0006-8993(94)01429-l. [DOI] [PubMed] [Google Scholar]
- 16.Erickson J C, Clegg K E, Palmiter R D. Nature (London) 1996;381:415–418. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 17.Naveilhan P, Hassani H, Canals J M, Ekstrand A J, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- 18.Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Proc Natl Acad Sci USA. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh D J, Hollopeter G, Kafer K E, Palmiter R D. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 20.Pedrazzini T, Seydoux J, Kunstner P, Aubert J F, Grouzmann E, Beermann F, Brunner H R. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- 21.Rose P M, Fernandes P, Lynch J S, Frazier S T, Fisher S M, Kodukula K, Kienzle B, Seethala R. J Biol Chem. 1995;270:22661–22664. doi: 10.1074/jbc.270.39.22661. [DOI] [PubMed] [Google Scholar]
- 22.Franklin K B, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 23.Young W S., 3rd Methods Enzymol. 1989;168:702–710. doi: 10.1016/0076-6879(89)68051-2. [DOI] [PubMed] [Google Scholar]
- 24.Schalling M, Seroogy K, Hökfelt T, Chai S Y, Hallman H, Persson H, Larhammar D, Ericsson A, Terenius L, Graffi J, et al. Neuroscience. 1988;24:337–349. doi: 10.1016/0306-4522(88)90335-1. [DOI] [PubMed] [Google Scholar]
- 25.Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. Neuroscience. 1997;80:1019–1032. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzer C, Kofler N, Sperk G. Mol Pharmacol. 1998;53:6–13. doi: 10.1124/mol.53.1.6. [DOI] [PubMed] [Google Scholar]
- 27.Schwenk F, Baron U, Rajewsky K. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havel P J, Akpan J O, Curry D L, Stern J S, Gingerich R L, Ahren B. Am J Physiol. 1993;265:R246–R254. doi: 10.1152/ajpregu.1993.265.1.R246. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz T W. J Auton Nerv Syst. 1983;9:99–111. doi: 10.1016/0165-1838(83)90134-0. [DOI] [PubMed] [Google Scholar]
- 30.Mazzocchi G, Malendowicz L K, Gottardo G, Meneghelli V, Nussdorfer G G. Life Sci. 1995;56:595–600. doi: 10.1016/0024-3205(94)00492-b. [DOI] [PubMed] [Google Scholar]
- 31.Rebuffat P, Malendowicz L K, Meneghelli V, Macchi V, Nussdorfer G G. Life Sci. 1998;62:1217–1222. doi: 10.1016/s0024-3205(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 32.Boulis N M, Bhatia V, Brindle T I, Holman H T, Krauss D J, Blaivas M, Hoff J T. J Neurosurg. 1999;90:99–108. doi: 10.3171/spi.1999.90.1.0099. [DOI] [PubMed] [Google Scholar]
- 33.Loonstra A, Vooijs M, Beverloo H B, Allak B A, van Drunen E, Kanaar R, Berns A, Jonkers J. Proc Natl Acad Sci USA. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno N, Inui A, Iwamoto M, Kaga T, Asakawa A, Okita M, Fujimiya M, Nakajima Y, Ohmoto Y, Ohnaka M, et al. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 35.Berntson G G, Zipf W B, O'Dorisio T M, Hoffman J A, Chance R E. Peptides. 1993;14:497–503. doi: 10.1016/0196-9781(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 36.Glaser B, Zoghlin G, Pienta K, Vinik A I. Horm Metab Res. 1988;20:288–292. doi: 10.1055/s-2007-1010817. [DOI] [PubMed] [Google Scholar]
- 37.Gettys T W, Garcia R, Savage K, Whitcomb D C, Kanayama S, Taylor I L. Pancreas. 1991;6:46–53. doi: 10.1097/00006676-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Walker H C, Romsos D R. Am J Physiol. 1992;262:E110–E117. doi: 10.1152/ajpendo.1992.262.1.E110. [DOI] [PubMed] [Google Scholar]
- 39.Corder R, Pralong F, Turnill D, Saudan P, Muller A F, Gaillard R C. Life Sci. 1988;43:1879–1886. doi: 10.1016/s0024-3205(88)80005-5. [DOI] [PubMed] [Google Scholar]
- 40.Makimura H, Mizuno T M, Roberts J, Silverstein J, Beasley J, Mobbs C V. Diabetes. 2000;49:1917–1923. doi: 10.2337/diabetes.49.11.1917. [DOI] [PubMed] [Google Scholar]
- 41.Kristensen P, Judge M E, Thim L, Ribel U, Christjansen K N, Wulff B S, Clausen J T, Jensen P B, Madsen O D, Vrang N, et al. Nature (London) 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 42.Chisari A, Carino M, Perone M, Gaillard R C, Spinedi E. J Endocrinol Invest. 1995;18:25–33. doi: 10.1007/BF03349692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.