Abstract
New blood vessel formation in the cornea is an essential step in the pathogenesis of a blinding immunoinflammatory reaction caused by ocular infection with herpes simplex virus (HSV). By using a murine corneal micropocket assay, we found that HSV DNA (which contains a significant excess of potentially bioactive “CpG” motifs when compared with mammalian DNA) induces angiogenesis. Moreover, synthetic oligodeoxynucleotides containing CpG motifs attract inflammatory cells and stimulate the release of vascular endothelial growth factor (VEGF), which in turn triggers new blood vessel formation. In vitro, CpG DNA induces the J774A.1 murine macrophage cell line to produce VEGF. In vivo CpG-induced angiogenesis was blocked by the administration of anti-mVEGF Ab or the inclusion of “neutralizing” oligodeoxynucleotides that specifically oppose the stimulatory activity of CpG DNA. These findings establish that DNA containing bioactive CpG motifs induces angiogenesis, and suggest that CpG motifs in HSV DNA may contribute to the blinding lesions of stromal keratitis.
Keywords: pathogenesis‖HSV‖VEGF
CpG motifs are present at high frequency in the genomes of many bacteria and viruses. These motifs have been shown to stimulate multiple cellular components of the immune and central nervous systems (1–6). During efforts to determine the role of viral DNA in human disease states, we noted that the genome of herpes simplex virus (HSV) resembles bacterial DNA in having a high content of “CpG motifs” (1, 2).
HSV infection of the eye can result in the blinding lesions of stromal keratitis (SK) (7–9). A critical early event in the pathogenesis of herpetic SK is new blood vessel development in the normally avascular cornea (10, 11). Angiogenic sprouting from vessels at the corneal limbus can occur within 24 h of herpes infection, with angiogenesis reaching the central cornea during the ensuing 10–15 days (10). The mechanism by which HSV infection stimulates new blood vessel formation is unclear, because none of the proteins encoded by the virus are known to have angiogenic activity. We recently documented that the vascular endothelial growth factor (VEGF) group of angiogenic factors is up-regulated after HSV infection (10). Cells expressing VEGF proteins were detected in the infected corneal epithelium as well as in inflammatory cells in the underlying stroma (10). Because cells expressing viral antigen were not those producing VEGF, expression of these angiogenic factors may have been paracrine in nature (10).
We examined the possibility that potentially bioactive CpG motifs in HSV DNA and/or its breakdown products might contribute to angiogenesis. To assess this possibility, a murine corneal micropocket assay was used to examine whether purified HSV DNA, or synthetic oligodeoxynucleotides (ODN) containing CpG motifs, could stimulate angiogenesis. Results indicate that both CpG ODN and HSV DNA trigger new blood vessel formation, and that this effect is mediated by VEGF production. This angiogenesis can be prevented by administering anti-VEGF Abs or “neutralizing” ODN that specifically oppose the bioactivity of CpG DNA. These findings suggest that CpG motifs in HSV DNA may contribute to the angiogenesis characteristic of SK. They also raise the intriguing possibility that neutralizing ODN may be used to prevent HSV-induced SK, and that CpG ODN may be harnessed therapeutically to induce new blood vessel development in clinical situations requiring revascularization.
Materials and Methods
Reagents.
Phosphorothioate ODNs were synthesized at the Center for Biologics Evaluation and Research Core Facility, as described (12). The sequences of the stimulatory ODNs used in this study were: 1466, TCAACGTTGA, and 1555, GCTAGACGTTAGCGT (subsequent studies were conducted using an equimolar mixture of ODNs 1466 and 1555), the control ODN 1471 has the sequence TCAAGCTTGA, and the neutralizing ODN has the sequence GAGCAAGCTGGACCTTCCAT. There was no detectable endotoxin contamination in any of the ODNs, as monitored by Limulus amebocyte lysate assay (BioWhittaker). In some experiments, FITC was conjugated to the 5′ end of these ODN to monitor their distribution in vivo.
Herring sperm DNA (Boehringer Mannheim) was prepared by passage through Detoxi-Gel Endotoxin Removal Gel 20344 (Pierce) to reduce endotoxin levels to <6 endotoxin units per mg. Recombinant human VEGF165 (rhVEGF), recombinant mouse VEGF (rmVEGF), mouse VEGF-neutralizing antibody, and biotinylated rat anti-mouse VEGF were purchased from R & D Systems. Synthetic mesh and hydron polymer (type NCC) were purchased from Sefar America (Kansas City, MO) and Hydro Med Sciences (Cranbury, NJ), respectively. Sucralfate was kindly provided by Bulch Meditec (Vaerlose, Denmark). Lipopolysaccharide was purchased from Sigma, and streptavidin-PE from PharMingen.
Isolation of HSV-1 DNA.
Virus was harvested from infected Vero cells when the cytopathic effects were maximal, as described (13, 14). Cells were suspended in sterile PBS and freeze-thawed three times to release viral particles. The virion-containing supernatant was then ultracentrifuged at 25,000 × g for 90 min at 4°C, and the pellet suspended in sterile PBS. Viral particles were precipitated in a solution of 7% polyethylene glycol 8000 in 2.3% NaCl overnight at 4°C and then centrifuged at 25,000 × g. DNA was isolated from virions by treatment with 200 μg/ml proteinase K and 1% sarcosyl in STE buffer overnight at 56°C. The DNA was purified by multiple phenol/chloroform/isoamyl alcohol extractions, precipitated, sedimented at 12,000 × g, dried, and resuspended in sterile STE buffer. RNA was removed by incubation with RNase (100 mg/ml; 5 Prime → 3 Prime) for 1 h at 37°C, and the DNA reextracted as described above. The resultant material provided a single band by electrophoresis and contained undetectable protein (checked spectrophotometrically) or cellular DNA (measured by PCR for β-actin DNA). All procedures were performed in a sterile environment, and all buffers and solutions were checked for the presence of lipopolysaccharide by using the Pyrogent plus test. No detectable protein, cellular RNA, or DNA, and less than 0.06 endotoxin units of endotoxin per mg of HSV DNA, were found.
Mice.
Female BALB/c (Harlan–Sprague–Dawley) were used for all experiments. Animals were housed and cared for as described (15).
Corneal Micropocket Assay.
The murine corneal micropocket assay used in this work observed the general protocol of Kenyon et al. (16). Pellets 0.4 × 0.4 × 0.2 mm3 composed of sucralfate and hydron polymer were prepared (16). Known amounts of VEGF, DNA, stimulatory or neutralizing ODN, and/or combinations thereof were added to these pellets before insertion into corneal pockets. The micropockets were placed 0.6–0.8 mm from the limbus (in the pericenter of the cornea at the lateral canthus of the eye) under stereomicroscopy (four eyes per group). In some experiments, anti-mVEGF-neutralizing antibody (5 μg in 5 μl of PBS) was injected subconjunctivally into the eyes of recipient mice just before and 2 days after pellet implantation.
Angiogenesis was quantitated at multiple times after pellet implantation under stereomicroscopy. The length of the neovessels generated from the limbal vessel ring toward the center of the cornea and the width of the neovessels presented in clock hours (each clock hour is equal to 30°C at the circumference) was measured (11). The angiogenic area was calculated according to the formula for an ellipse. A = [(clock hours) × 0.4 × (vessel length in mm) × π]/2.
Immunohistochemical Staining.
Eyes were removed and snap frozen in OCT compound (Miles). Sections (6-μm) were cut, air dried, and fixed in cold acetone for 10 min. The sections were blocked with 3% BSA and stained with biotinylated anti-mVEGF164. Sections were then treated with horseradish peroxidase-conjugated streptavidin (1:1,000) and 3,3′-diaminobenzidine (Vector), and counterstained with hematoxylin as described (10). Cellular infiltration was determined microscopically by counting the infiltrating cells in the corneal stroma. Each data point represents the mean total cellular infiltrate in four central corneal sections from two eyes.
VEGF Staining of J774A.1 Cells.
J774A.1 cells were plated and incubated in two-well chamber slides (Lab-Tek, Nalge Nunc International) or in 24-well plates [for later reverse transcription (RT)-PCR] in DMEM with 10% FBS overnight at 37°C in 5% CO2. The cells in chamber slides were cocultured with FITC-labeled CpG ODN (1555) or control ODN (1471) at a concentration of 2 μg/106 cells. The cells were washed twice with PBS and fixed in a 1:1 mixture of acetone/methyl alcohol at −20°C for 15 min. The cells were stained with biotinylated rat-anti-mVEGF 6–18 h after ODN stimulation and subsequently reacted with streptavidin-PE. Images were taken by using a fluorescence microscope (Hamamatsu, Ichinocho, Japan). The cells in 24-well plates were treated with 2 μg of ODN per 106 cells per ml. RNA from these cells was extracted for RT-PCR to detect VEGF mRNA (see RNA Extraction and RT-PCR).
Fluorescence-Activated Cell Sorter Staining of VEGF-Expressing Cells.
J774A.1 cells were treated with 0–8 μg/ml of ODN for 6–12 h. The cells were then fixed in paraformaldehyde, blocked with FCS, and stained for VEGF by using biotinylated rat-anti-mVEGF164 antibody followed by streptavidin-PE. Positive cells were identified by flow cytometry.
RNA Extraction and RT-PCR.
For 3–6 h, 106 J774A.1 cells were cultured with 2 μg of ODN. The cells were harvested in Tri-reagent (Molecular Biology, Cincinnati) and total RNA extracted as recommended by the manufacturer. Total RNA (10 μg) was reverse transcribed, and aliquots of cDNA were used in a 25:1 PCR reaction as described (10). The amplification profile was 94°C for 1 min, 65°C for 1 min, and 72°C for 15 min for 30 cycles. The primer sequences for VEGF were: 5′-GCGGGCTGCCTCGCAGTC-3′ (sense) and 5′-TCACCGCCTTGGCTTGTCAC-3′ (antisense), respectively. RT- PCR products were 716 bp (mVEGF188), 644 bp (mVEGF164), and 512 bp (mVEGF120), respectively.
Statistical Analysis.
Significant differences between groups were evaluated by using the Student's t test. P ≤ 0.05 was regarded as significant difference between two groups.
Results
Purified HSV DNA Stimulates Angiogenesis.
HSV infection of mice provides a model for studying the blinding lesions of SK (7–9). To determine whether viral DNA plays a role in the pathogenesis of these lesions, virions were isolated, lysed, and HSV DNA prepared. This DNA was introduced into hydron pellets and surgically inserted into corneal micropockets established in the eyes of BALB/c mice. New blood vessel formation in the corneal limbus (emanating from the margin of the limbal vessel ring) was monitored daily. Initial experiments showed that HSV DNA elicited significant angiogenesis, as did pellets containing VEGF protein, but not those pellets containing control herring sperm DNA (Fig. 1).
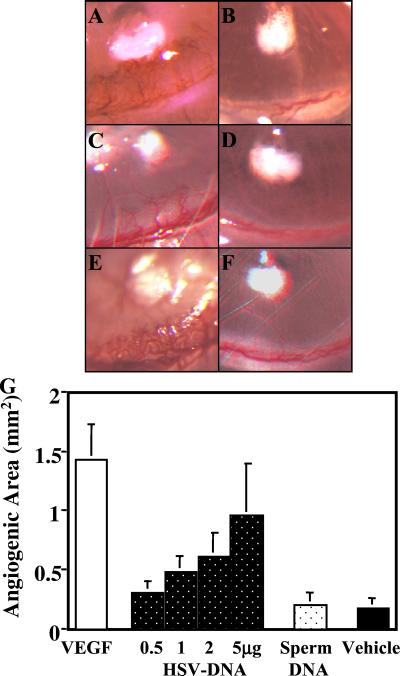
Figure 1.
HSV DNA and CpG ODN induce angiogenesis. Pellets containing 90 ng of VEGF (A); vehicle alone (B); 2 μg of HSV DNA or control herring sperm DNA (C and D); or 1 μg of CpG or control ODN (E and F) were inserted into corneal micropockets. The degree of angiogenesis 4 days after pellet implantation is shown (40×). (G) The average degree of neovascularization induced by various amounts of HSV DNA is shown vs. 5 μg of herring sperm DNA, 90 ng of VEGF, or vehicle alone (four mice per group).
Dose–response studies established that 90 ng of rhVEGF and 2 μg of HSV DNA triggered significant blood vessel formation (Figs. 1 and 2). Angiogenesis developed within 24 h of implantation, with the magnitude of new blood vessel formation progressing until the experiment was terminated on day 4. Optimal amounts of HSV DNA induced approximately one-half as much angiogenesis as did purified VEGF (Fig. 1), significantly exceeding the effect of empty hydron pellets or pellets containing control DNA (Fig. 1).
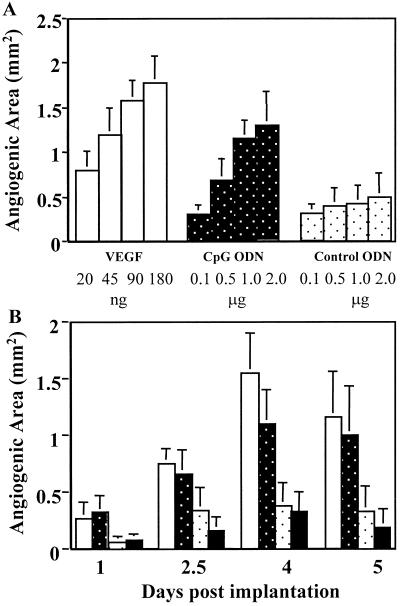
Figure 2.
Dose and kinetics of the angiogenic response to CpG DNA. (A) Dose-response of new blood vessel formation was monitored by using the corneal micropocket assay 4 days after implantation. (B) The kinetics of neovascularization was measured 1–5 days after implantation. Open bar, VEGF; white dot bar, control ODN; black dot bar, CpG ODN; black solid bar, vehicle. All results represent the mean of four animals per group. The different weight units of x axis (nanograms and micrograms) represent the amount of the materials incorporated into the pellets.
CpG DNA Induces Angiogenesis.
The DNA sequence of HSV was analyzed to identify potentially proangiogenic motifs. The frequency of bioactive CpG motifs present in the HSV genome was similar to that of bacterial DNA and significantly higher than that of murine DNA (Table 1). To determine whether these CpG motifs [which activate cells of the immune and central nervous systems (1–6)] contribute to HSV-dependent angiogenesis, hydron pellets infused with ≥1 μg of CpG ODN induced significant angiogenesis (≈75% of that elicited by an optimal concentration of VEGF). The kinetics of new blood vessel formation induced by CpG ODN was indistinguishable from that of HSV DNA (Fig. 2). In contrast, control ODN (in which the CpG motif was eliminated by inversion) had no greater effect than empty hydron pellets (Fig. 2).
Table 1.
CpG expression frequency in HSV-1 vs. murine DNA
| Motif | Expression frequency
|
Fold difference | ||
|---|---|---|---|---|
| E. coli | Mouse | HSV-1 | ||
| GACGTT | 1.3 | 0.11 | 0.82 | 7.5 |
| AGCGTT | 1.7 | 0.17 | 0.46 | 2.7 |
| AACGTC | 0.6 | 0.11 | 0.81 | 7.4 |
| AGCGTC | 1.3 | 0.15 | 0.95 | 6.3 |
| GGCGTC | 1.4 | 0.15 | 4.50 | 30.0 |
| GGCGTT | 2.5 | 0.15 | 1.68 | 11.2 |
| Average | 1.53 | 0.14 | 1.54 | 11.0 |
The frequency with which each CpG hexamer is expressed in the genome of Escherichia coli, mice and HSV-1 was determined by using published sequence data. Accession numbers are from the National Center for Biotechnology Institute. (The mouse chromosome data were a composite of chromosomes 1–3. The accession numbers for mouse chromosomes 1, 2, and 3 are: NT_025524, NT_019187, and NT_015485. The accession numbers for E. coli-K12 and HSV-1 complete genome are NC_000913 and X14112.) Note the significant overexpression of immunostimulatory GCGTC and GCGTT motifs in the HSV genome vs. the expression of the mouse (fold difference).
Neutralizing ODN Inhibits HSV DNA-Induced Angiogenesis.
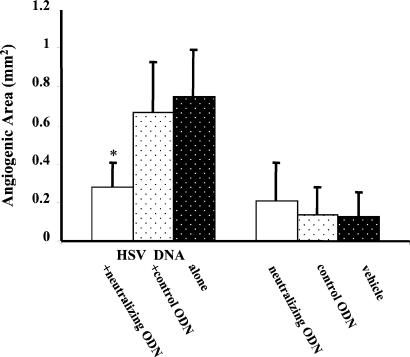
To investigate further whether CpG motifs in HSV DNA were responsible for the angiogenesis, HSV DNA was mixed with “neutralizing” or control ODN and the effect on angiogenesis recorded. The neutralizing ODN contained multiple G-tetrad-forming motifs that other studies have shown to neutralize the immunostimulatory effects of CpG DNA (refs. 17–19; D.M.K., unpublished data). In these experiments, hydron pellets were prepared that contained HSV DNA along with neutralizing or control ODN. Levels of angiogenesis were recorded at 4 days after implantation. As is evident, pellets that incorporated control ODN plus HSV DNA induced the same level of angiogenesis as did HSV DNA pellets alone. In contrast, pellets containing neutralizing ODN plus HSV DNA induced significantly less (≈60% inhibition) angiogenesis (P < 0.01, Fig. 3).
Figure 3.
Neutralizing ODN inhibits HSV DNA-induced angiogenesis. Pellets containing 1 μg of HSV DNA alone or in combination with 1 μg of control or neutralizing ODN were implanted into corneal micropockets. The figure shows the average degree of angiogenesis 4 days after pellet implantation (four to five mice per group). *, P < 0.01.
Production of VEGF Characterizes CpG DNA-Induced Angiogenesis.
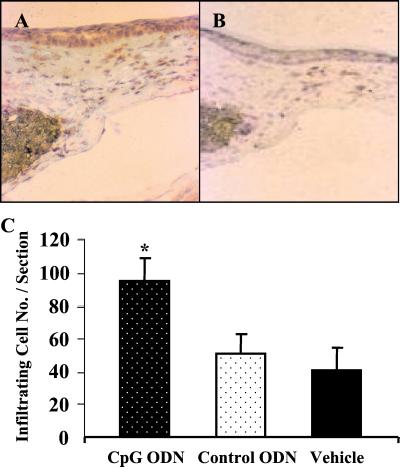
On the basis of evidence that HSV-associated angiogenesis involved the production of VEGF (10), the ability of CpG DNA to stimulate VEGF secretion was evaluated. Histologic analysis of the region surrounding the corneal micropockets of animals treated with both HSV DNA and CpG ODN revealed that numerous inflammatory cells had infiltrated the site of pellet implantation (Fig. 4C, P ≤ 0.01). These cells were primarily polymorphonuclear leukocytes and macrophages (Fig. 4). Staining these sections with anti-VEGF Ab revealed that the infiltrating cells, and cells in the epithelium, were producing VEGF protein. Significantly fewer VEGF-expressing cells were present in the eyes of mice treated with control ODN or empty pellets (Fig. 4 A and B).
Figure 4.
CpG DNA induces inflammation and VEGF expression in corneal micropockets. Pellets containing 1 μg of CpG (A) or control (B) ODN were implanted into mouse corneas. Frozen sections from these eyes were stained for VEGF-expressing cells (dark-brown stain) 4 days later. Positive cells are present in the ipsilateral site of the pellet-implanted cornea (200×). (C) Cellular infiltration was quantified by enumerating infiltrating cells in the corneal stroma. Each point represents the mean total cellular infiltrate from four central corneal sections from two eyes.
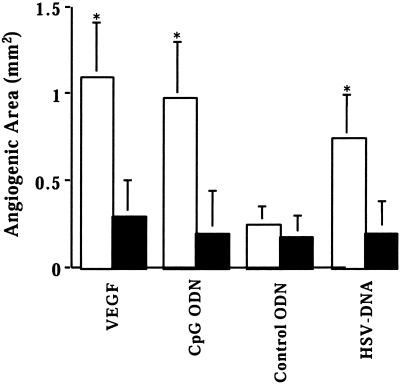
To determine whether the VEGF being produced by cells at the site of CpG DNA administration contributed to new blood vessel formation, neutralizing anti-VEGF antibody was administered subconjunctivally to these mice. As seen in Fig. 5, anti-VEGF Ab inhibited the angiogenesis induced by HSV DNA, CpG ODN, and VEGF by ≈70%. In contrast, anti-VEGF Ab had no effect on the background levels of angiogenesis observed by using empty pellets or pellets containing control ODN.
Figure 5.
Anti-mVEGF Ab suppresses CpG DNA-induced angiogenesis. Pellets containing 45 ng of rmVEGF164, 1 μg of ODN, or 2 μg of HSV or herring sperm DNA were placed in corneal pockets. Anti-mVEGF antibody (5 μg in 5 μl of PBS) was injected subconjunctivally into the eyes just before and 2 days after pellet implantation. The eyes were observed under stereomicroscopy and neovascularization measured 4 days after pellet implantation in four mice per group. Black solid bar, +anti-mVEGF Ab.
CpG DNA Stimulates VEGF Expression in Vitro.
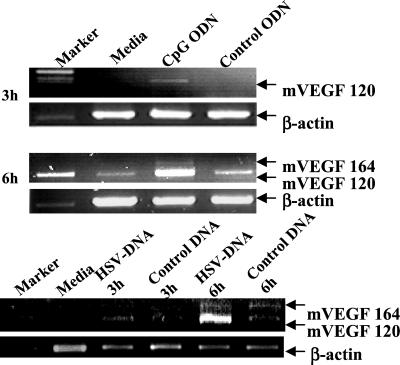
To verify that CpG DNA directly induces cells to produce VEGF, the J774A.1 murine macrophage cell line (which produces VEGF when infected with HSV; unpublished data) was treated in vitro with control or CpG-containing DNA. As seen in Fig. 6, mRNA encoding the 120 isotype of VEGF was up-regulated within 3 h of exposure to HSV DNA and CpG ODN, whereas little or no VEGF-mRNA was induced by herring sperm DNA or control ODN. By 6 h, expression of both the 120 and 164 isoforms of VEGF were induced by HSV DNA and CpG ODN. In contrast, cells cultured in medium alone, with herring sperm DNA, or with control ODN, expressed minimal levels of VEGF mRNA.
Figure 6.
CpG DNA ODN up-regulates VEGF mRNA expression in J774A.l cells. J774A.l cells were incubated for 3–6 h with 3 μg/ml of HSV DNA, herring sperm DNA, CpG, or control ODN. Total RNA was extracted from 106 cells, reverse transcribed, and PCR amplified to detect the 120, 164, 188 isoforms of VEGF. β-actin served as the positive control and standard for this RT-PCR.
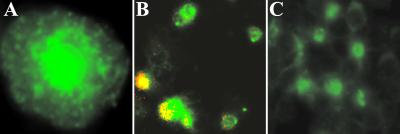
A second series of experiments measured the production of VEGF protein by J774A.1 cells stimulated with CpG ODN. Fewer than 0.3% of untreated J774A.1 cells were producing VEGF (Table 2). The number of VEGF-expressing cells increased within 6 h of CpG ODN stimulation, with 20–26% of cells treated with 3 μg/ml CpG ODN producing protein after 24 h (Table 2). This significantly exceeded the number of cells triggered to produce VEGF by control ODN. To determine whether VEGF production correlated with CpG ODN uptake, cultures were stimulated with fluorescein-labeled CpG ODN and simultaneously monitored for VEGF expression. All VEGF-producing cells stained positive for CpG ODN, suggesting that CpG DNA directly triggered these cells to produce this angiogenic protein (Fig. 7).
Table 2.
Expression of VEGF after exposure of J774A.1 cells to CpG DNA
| Treatment | Dose, μg/ml | VEGF-positive cells, %
|
|
|---|---|---|---|
| 6 h | 24 h | ||
| CpG ODN | 0.1 | 0.3 | 1.2 |
| 1.0 | 9.1 | 21.7 | |
| 3.0 | 10.1 | 26.2 | |
| 8.0 | 12.6 | 15.3 | |
| Control ODN | 3.0 | 4.5 | 5.3 |
| Media | 0.1 | 0.3 | |
J774A.1 cells were treated in vitro with 0.1–8.0 μg/ml of CpG ODN for 6 or 24 h. Cells expressing VEGF were identified by staining with rat-anti-mVEGF Ab. Results are representative of three independent experiments.
Figure 7.
CpG ODN induces VEGF production. J774A.l cells were incubated in two-well chamber slides with 2 μg/ml of FITC-CpG ODN (A and B) or control (C) FITC-ODN for 18 h. A represents one J774A.1 cell with internalized FITC–CpG ODN (1,000×). The cells (B and C) were fixed and stained for mVEGF. Approximately 70% of the cells internalized FITC-CpG ODN (green), and every cell expressing VEGF (color range: yellow to red) also stained with FITC-CpG ODN (400×).
Discussion
New blood vessel formation can be of benefit (by improving tissue implantation and treating small-vessel disease) or deleterious (by reducing visual acuity or hastening tumor growth). This work establishes that DNA containing bioactive CpG motifs can support neovascularization, thus adding to the spectrum of biological effects mediated by CpG motifs (1–6). Our work also shows that “neutralizing” motifs, which selectively block the activity of CpG DNA, can significantly reduce this neovascularization.
CpG ODNs express a wide range of biological activities. They are potent vaccine adjuvants and anti-allergens, and they trigger a protective innate immune response (20–23). Several recent reports indicate that CpG ODN also stimulate cells of the central nervous system (6). Despite this variety of biological effects, the ability of CpG DNA to induce angiogenesis has not been recognized. This report documents that bioactive CpG motifs induce dose-dependent neovascularization in the corneas of mice engrafted with hydron pellets containing CpG ODN or HSV DNA. These results were obtained by using a mixture of two ODN that expressed different CpG motifs, because such combinations are more broadly stimulatory than individual ODN and are more representative of the multiple motifs found in HSV DNA (24).
The mechanism by which CpG motifs induce angiogenesis has not been fully elucidated. Rather than directly triggering new blood vessel formation, our results suggest that CpG motifs stimulate host cells to secrete VEGF, which in turn induce neovascularization. Several findings are consistent with this model. First, CpG ODN was taken up by the same inflammatory cells that expressed VEGF. Second, exposure to CpG motifs directly stimulated J774A.1 murine macrophages to produce VEGF. Finally, local administration of anti-VEGF Ab to CpG DNA-treated eyes significantly inhibited corneal angiogenesis. Studies are underway to determine whether toll-like receptor 9, which is involved in CpG recognition by immune cells (25, 26) is expressed by cells in the cornea. We also plan to analyze whether CpG DNA induces the production of other proangiogenic molecules.
These studies were undertaken to elucidate the mechanism by which ocular infection with HSV causes angiogenesis, an essential event in the pathogenesis of herpetic stromal keratitis (10, 11). Neovascularization anywhere along the visual axis poses a threat to ocular function. HSV infection of the eye is associated with new blood vessel formation in the normally avascular cornea (10, 11). The molecular mechanism(s) underlying this effect is/are poorly defined, although evidence suggests that VEGF [a highly potent angiogenic host protein (27–29)] is involved, at least indirectly (10). Current studies provide evidence that HSV DNA, likely through its content of bioactive CpG motifs, contributes to virus-induced ocular angiogenesis.
The degree of angiogenesis elicited by HSV DNA and CpG ODN was ≈75% of that induced by the potent angiogenic factor VEGF. This activity was motif-specific, because (i) ODN in which the critical CpG dinucleotide was inverted to a GpC lacked function and (ii) CpG-induced angiogenesis was blocked by ODN containing neutralizing motifs that selectively oppose the action of CpG motifs (17–19; D.M.K., unpublished data). These findings suggest a mechanism by which HSV infection might induce angiogenesis, an essential event in the pathogenesis of herpetic stromal keratitis (10, 11). Consistent with this conclusion, codon usage in the HSV genome is biased toward usage of C and G (30) and as a consequence the HSV genome contains a significantly higher frequency of bioactive CpG motifs than does vertebrate DNA (Table 1). In addition, a search of the HSV genome sequence data revealed none of the suppressive 8mer motifs found frequently in the mouse genome. This pattern of CpG expression is reminiscent of bacterial DNA, which has well established proinflammatory effects (1, 2).
It is premature to conclude that HSV DNA is solely responsible for SK. The mechanism identified in this report may be part of a more complex series of events that culminate in neovascularization after herpes infection of the eye. In this context, it is uncertain whether the local concentration of CpG DNA achieved during HSV infection is sufficient to up-regulate VEGF levels under physiologic conditions. The amount of HSV DNA incorporated into hydron pellets (2 μg) was equivalent in content to ≈109 viral particles. Whereas only a small fraction of the DNA in hydron pellets is released and taken up by target cells, HSV-infected cells are exposed to 100% of internally produced viral DNA. In this context, previous studies established that CpG DNA introduced directly into a cell is >100-fold more stimulatory than exogenously administered material (31).
Neither can we be certain that results from studies of murine angiogenesis are applicable to human HSV infection. However, other CpG-mediated effects are as intense in primates as they are in rodents (32–34), and ongoing clinical trials indicate that CpG ODN can be safely used as immunomodulatory agents. Should CpG ODN be effective at promoting angiogenesis in humans, they may have therapeutic value for uses ranging from improved vascularization of tissue grafts, to treatment of peripheral vascular disease or even baldness. To the extent that CpG motifs in HSV DNA contribute to the development of SK, our results also suggest that administration of neutralizing ODN may be of therapeutic value.
Acknowledgments
We thank Dr. Steven Wilhelm and Leo Poorvin for their help in using the image system and Tommy Jordan for his excellent computer skills. This work was supported by National Institutes of Health Grant EY05093 (B.T.R.).
Abbreviations
- HSV
herpes simplex virus
- VEGF
vascular endothelial growth factor
- ODN
oligodeoxynucleotides
- SK
stromal keratitis
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballas Z D, Rasmussen W L, Krieg A M. J Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- 4.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 5.Sparwasser T, Koch E, Vabulas R M, Heeg K, Lipford G B, Ellwart J W, Wager H. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Takeshita S, Takeshita F, Haddad D E, Janabi N, Klinman D M. NeuroReport. 2001;12:3029–3032. doi: 10.1097/00001756-200110080-00010. [DOI] [PubMed] [Google Scholar]
- 7.Streilein J W, Dana M R, Ksander B R. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 8.Thomas J, Rouse B T. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande S P, Zheng M, Lee S, Rouse B T. Vet Microbiol. 2002;2267:1–10. doi: 10.1016/s0378-1135(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 10.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse B T. J Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M, Schwarz M A, Lee S, Kumaraguru U, Rouse B T. Am J Pathol. 2001;159:1021–1029. doi: 10.1016/S0002-9440(10)61777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verthelyi D, Ishii K J, Gursel M, Takeshita F, Klinman D M. J Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 13.Killington A, Stokes A, Hierholzor C. In: Virology Methods Manual. Mahy B W J, Kangro H O, editors. New York: Academic; 1996. pp. 71–88. [Google Scholar]
- 14.McCance D J. In: Virology Methods Manual. Mahy B W J, Kangro H O, editors. New York: Academic; 1996. pp. 191–197. [Google Scholar]
- 15.Gangappa S P, Babu J S, Thomas J, Daheshia M, Rouse B T. J Immunol. 1998;161:4289–4300. [PubMed] [Google Scholar]
- 16.Kenyon B M, Voest E E, Chen C C, Flynn E, Folkman J, D'Amato R J. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 17.Krieg A M, Wu T, Weeratna R, Efler S M, Love-Homan L, Yang L, Yi A K, Short D, Davis H L. Proc Natl Acad Sci USA. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenert P, Stunz L, Yi A K, Krieg A M, Ashman R F. Antisense Nucleic Acid Drug Dev. 2001;11:247–256. doi: 10.1089/108729001317022241. [DOI] [PubMed] [Google Scholar]
- 19.Pisetsky D S, Reich C F. Clin Immunol. 2000;96:198–204. doi: 10.1006/clim.2000.4897. [DOI] [PubMed] [Google Scholar]
- 20.Klinman D M. Antisense Nucleic Acid Drug Dev. 1998;8:181–184. doi: 10.1089/oli.1.1998.8.181. [DOI] [PubMed] [Google Scholar]
- 21.Davis H L, Weeranta R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 22.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen M D, Malek S, Van Uden J, Martin E, Gelf E W, Raz E. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 23.Krieg A M, Homan L L, Yi A K, Harty J T. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- 24.Verthelyi D, Kenney R T, Seder R A, Gam A A, Friedag B, Klinman D M. J Immunol. 2002;168:1659–1663. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. Nature (London) 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita F, Leifer C A, Gursel I, Ishii K, Takeshita S, Gursel M, Klinman D M. J Immunol. 2001;167:3555–3558. doi: 10.4049/jimmunol.167.7.3555. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 29.Yancopoulos G D, Davis S, Gale N W, Rudge J, Wieg S J, Holash J. Nature (London) 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 30.Roizman B. Cell. 1979;16:481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K J, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn L D, Klinman D M. J Immunol. 2001;167:2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 32.Davis H L. Curr Top Microbiol Immunol. 2000;247:171–184. doi: 10.1007/978-3-642-59672-8_12. [DOI] [PubMed] [Google Scholar]
- 33.Krieg A M, Davis H L. Curr Opin Mol Ther. 2001;3:15–24. [PubMed] [Google Scholar]
- 34.Davis H L, Suparto I I, Weeratna R R, Jumintarto, Iskandriati D D, Chamzah S S, Ma'ruf A A, Nente C C, Pwitri D D, Krieg A M, et al. Vaccine. 2000;18:1920–1924. doi: 10.1016/s0264-410x(99)00443-0. [DOI] [PubMed] [Google Scholar]