Abstract
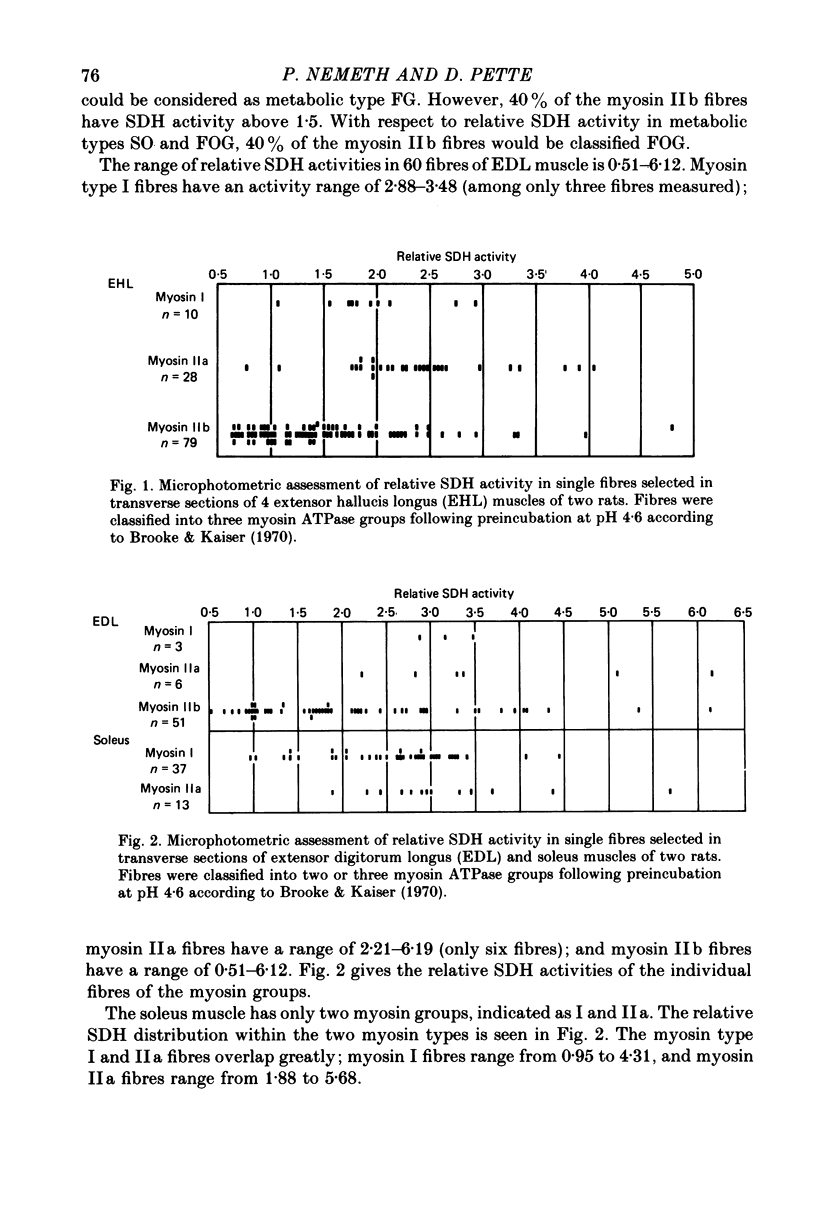
1. Succinate dehydrogenase (SDH) activity was assessed in situ in single fibres of cross-sectioned extensor hallucis longus, extensor digitorum longus, and soleus muscles of rat by means of microphotometric recordings of initial maximum reaction rates. 2. Each fibre assessed for SDH activity was subjectively classified into myosin subgroups by its histochemical reaction for myofibrillar actomyosin ATPase (myosin ATPase) following preincubation at pH 4.6 according to Brooke & Kaiser (1970). 3. The majority of fibres classified into myosin types I and IIa were highly reactive for SDH, such that those myosin groups could be interchangeable with the metabolic subgroups of Peter, Barnard, Edgerton, Gillespie & Stempel (1972); myosin I = slow-twitch oxidative, myosin IIa = fast-twitch oxidative glycolytic. 4. The myosin type IIb fibres, however, demonstrated marked variability in activity levels of SDH. Over 40% of those fibres had high SDH activity, and thus could not be equated with the metabolic subgroup fast-twitch glycolytic. 5. The histochemical reaction for myosin ATPase in muscle fibres therefore cannot be used as a reliable means to predict the fibres' metabolic characteristics.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977 Jan;99(1):123–125. doi: 10.1111/j.1748-1716.1977.tb10361.x. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Essén B., Jansson E., Henriksson J., Taylor A. W., Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975 Oct;95(2):153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Lowry C. V., Kaiser K. K., McKee D., Lowry O. H. Enzyme levels in individual rat muscle fibers. Am J Physiol. 1980 Sep;239(3):C58–C65. doi: 10.1152/ajpcell.1980.239.3.C58. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O. Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev. 1973;1:45–71. [PubMed] [Google Scholar]
- Lowry C. V., Kimmey J. S., Felder S., Chi M. M., Kaiser K. K., Passonneau P. N., Kirk K. A., Lowry O. H. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978 Nov 25;253(22):8269–8277. [PubMed] [Google Scholar]
- Nemeth P. M., Pette D. The interrelationship of two systems of fiber classification in rat EDL muscle. J Histochem Cytochem. 1980 Feb;28(2):193–193. doi: 10.1177/28.2.6444433. [DOI] [PubMed] [Google Scholar]
- Nemeth P. M., Pette D., Vrbová G. Comparison of enzyme activities among single muscle fibres within defined motor units. J Physiol. 1981 Feb;311:489–495. doi: 10.1113/jphysiol.1981.sp013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth P., Hofer H. W., Pette D. Metabolic heterogeneity of muscle fibers classified by myosin ATPase. Histochemistry. 1979 Sep;63(2):191–201. doi: 10.1007/BF00644541. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D. Microphotometric measurement of initial maximum reaction rates in quantitative enzyme histochemistry in situ. Histochem J. 1981 Mar;13(2):319–327. doi: 10.1007/BF01006885. [DOI] [PubMed] [Google Scholar]
- Pette D., Müller W., Leisner E., Vrbová G. Time dependent effects on contractile properties, fibre population, myosin light chains and enzymes of energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pflugers Arch. 1976 Jul 30;364(2):103–112. doi: 10.1007/BF00585177. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Pette D., Wasmund H., Wimmer M. Principle and method of kinetic microphotometric enzyme activity determination in situ. Histochemistry. 1979 Nov;64(1):1–10. doi: 10.1007/BF00493350. [DOI] [PubMed] [Google Scholar]
- Pette D., Wimmer M. Kinetic microphotometric activity determination in enzyme containing gels and model studies with tissue sections. Histochemistry. 1979 Nov;64(1):11–22. doi: 10.1007/BF00493351. [DOI] [PubMed] [Google Scholar]
- Pette D., Wimmer M., Nemeth P. Do enzyme activities vary along muscle fibres? Histochemistry. 1980;67(3):225–231. doi: 10.1007/BF00692756. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activities of malate dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase and fructose-1,6-diphosphatase with regard to metabolic subpopulations of fast- and slow-twitch fibres in rabbit muscles. Histochemistry. 1979 Feb 26;60(1):9–19. doi: 10.1007/BF00495725. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activity patterns of phosphofructokinase, glyceraldehydephosphate dehydrogenase, lactate dehydrogenase and malate dehydrogenase in microdissected fast and slow fibres from rabbit psoas and soleus muscle. Histochemistry. 1977 Jun 8;52(3):201–216. doi: 10.1007/BF00495857. [DOI] [PubMed] [Google Scholar]
- Streter F. A., Gergely J., Salmons S., Romanul F. Synthesis by fast muscle of myosin light chains characteristic of slow muscle in response to long-term stimulation. Nat New Biol. 1973 Jan 3;241(105):17–19. doi: 10.1038/newbio241017a0. [DOI] [PubMed] [Google Scholar]



