Abstract
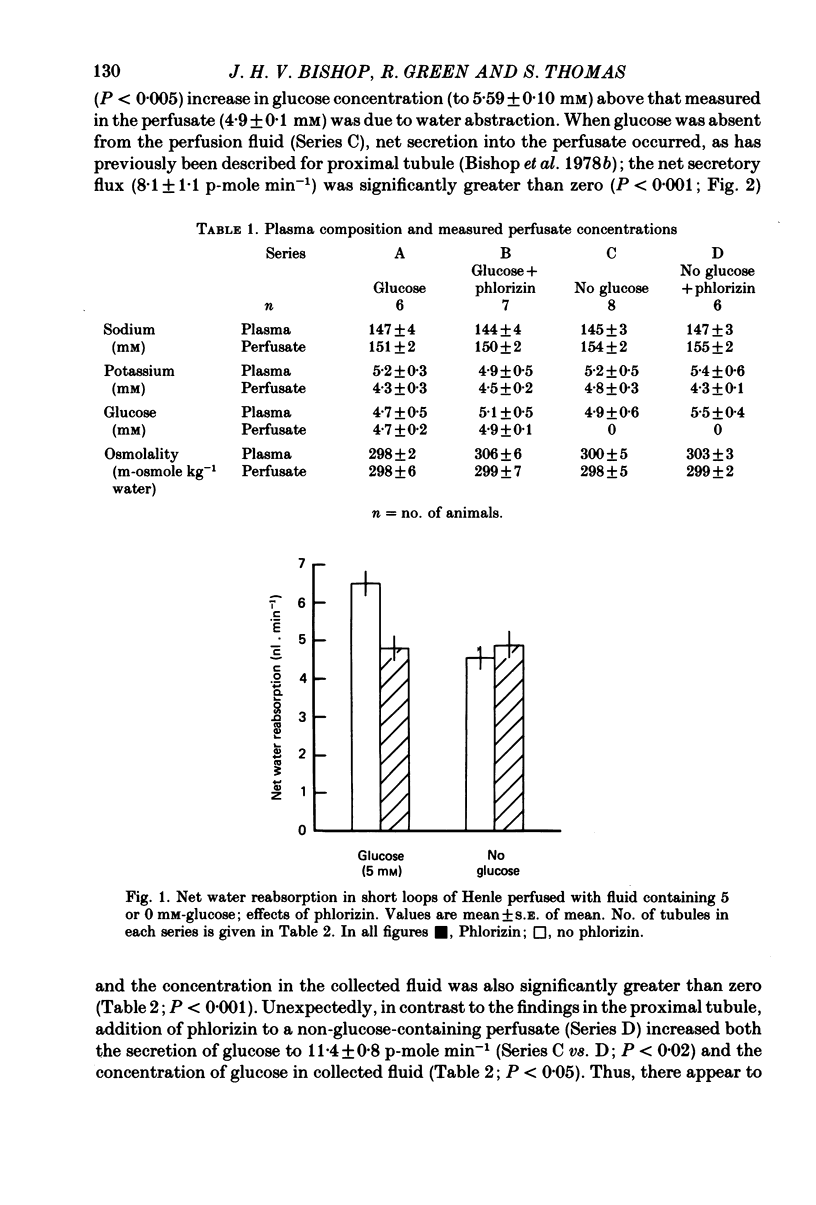
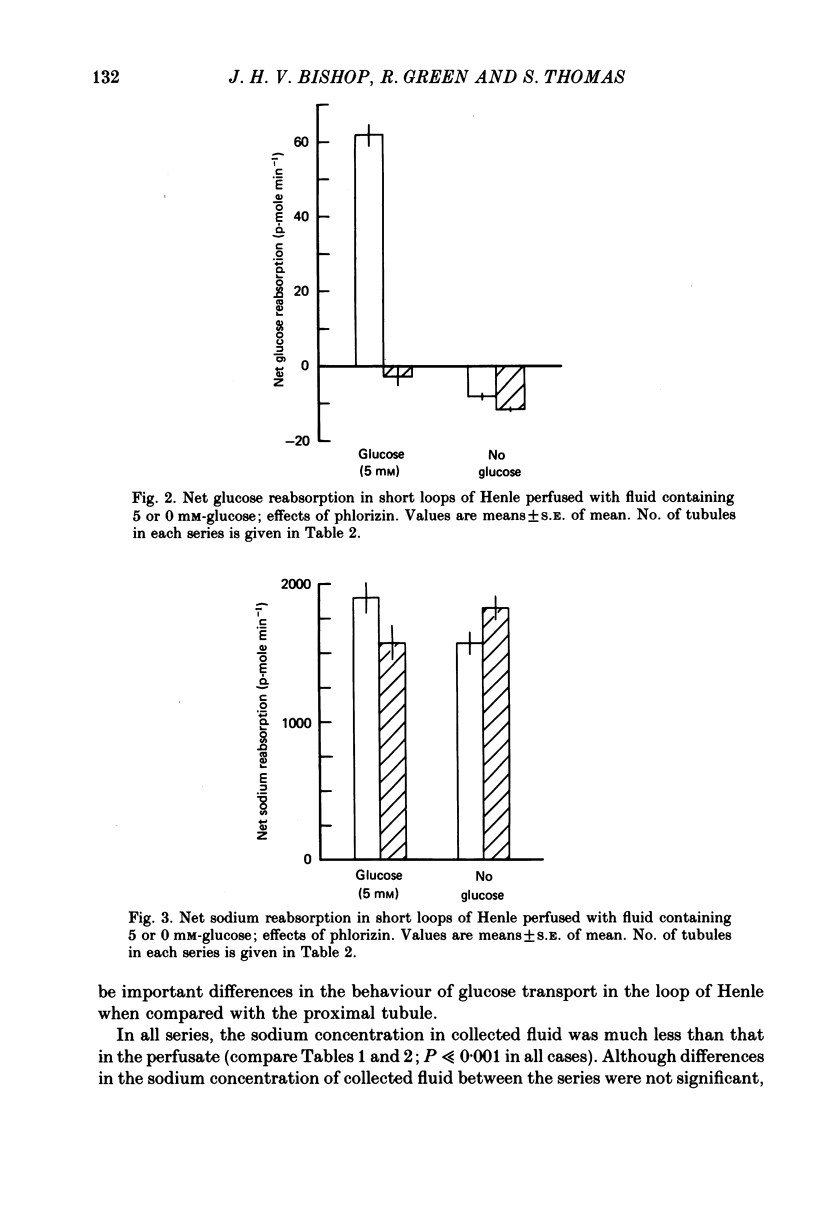
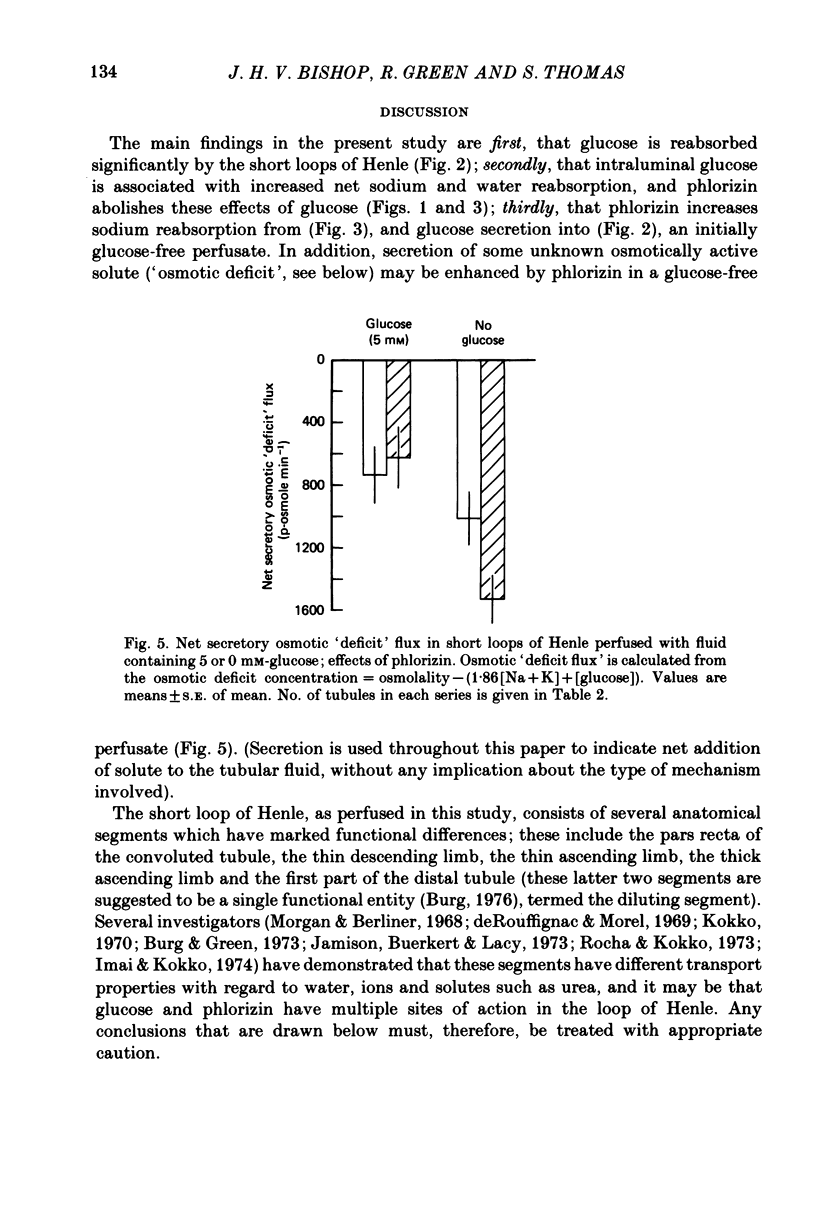
1. Short loops of Henle were artificially perfused with saline solutions containing 5 or 0 mM-glucose in the presence and absence of phlorizin. 2. Net fluid reabsorption was greater when glucose but no phlorizin was present than in all other series. 3. Glucose was reabsorbed from glucose-containing perfusate and this was abolished by phlorizin. Secretion of glucose occurred into the perfusate which initially contained no glucose and this secretion was enhanced by phlorizin. 4. Sodium reabsorption was inhibited by phlorizin, when glucose was present, but enhanced by phlorizin when glucose was absent. 5. It can be shown that there is secretion of some osmotically active solute in all series. Its secretion is enhanced by phlorizin in the absence of glucose.
Full text
PDF



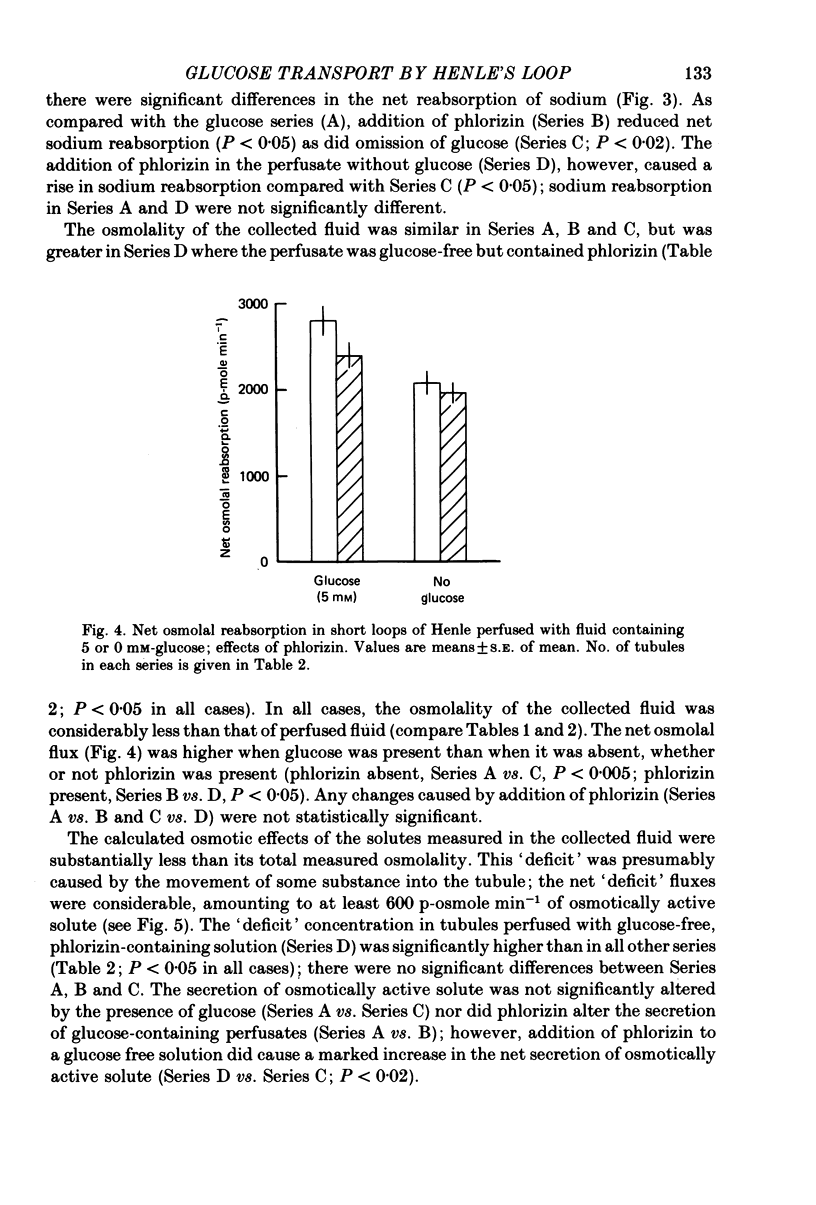




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Elegbe R., Green R., Thomas S. Effects of phlorizin on glucose, water and sodium handling by the rat kidney. J Physiol. 1978 Feb;275:467–480. doi: 10.1113/jphysiol.1978.sp012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Green R., Thomas S. Effects of glucose on water and sodium reabsorption in the proximal convoluted tubule of rat kidney. J Physiol. 1978 Feb;275:481–493. doi: 10.1113/jphysiol.1978.sp012202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. H., Green R., Thomas S. Free-flow reabsorption of glucose, sodium, osmoles and water in rat proximal convoluted tubule. J Physiol. 1979 Mar;288:331–351. [PMC free article] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Tubular chloride transport and the mode of action of some diuretics. Kidney Int. 1976 Feb;9(2):189–197. doi: 10.1038/ki.1976.20. [DOI] [PubMed] [Google Scholar]
- Frasch W., Frohnert P. P., Bode F., Baumann K., Kinne R. Competitive inhibition of phlorizin binding by D-glucose and the influence of sodium: a study on isolated brush border membrane of rat kidney. Pflugers Arch. 1970;320(3):265–284. doi: 10.1007/BF00587458. [DOI] [PubMed] [Google Scholar]
- Frohnert P. P., Höhmann B., Zwiebel R., Baumann K. Free flow micropuncture studies of glucose transport in the rat nephron. Pflugers Arch. 1970;315(1):66–85. doi: 10.1007/BF00587238. [DOI] [PubMed] [Google Scholar]
- GERLACH E., DEUTICKE B., DUHM J. PHOSPHAT-PERMEABILITAET UND PHOSPHAT-STOFFWECHSEL MENSCHLICHER ERYTHROCYTEN UND MOEGLICHKEITEN IHRER EXPERIMENTELLEN BEEINFLUSSUNG. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jul 30;280:243–274. [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L., Buerkert J., Lacy F. A micropuncture study of Henle's thin loop in Brattleboro rats. Am J Physiol. 1973 Jan;224(1):180–185. doi: 10.1152/ajplegacy.1973.224.1.180. [DOI] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of segments of thin loop of Henle in the rat. Am J Physiol. 1968 Jul;215(1):236–242. doi: 10.1152/ajplegacy.1968.215.1.236. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Solomon A. K. Interaction between phloretin and the red blood cell membrane. J Gen Physiol. 1976 Apr;67(4):381–397. doi: 10.1085/jgp.67.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 1970 Oct;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOTSPEICH W. D. Phlorizin and the cellular transport of glucose. Harvey Lect. 1960;56:63–91. [PubMed] [Google Scholar]
- Marsh D. J., Azen S. P. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol. 1975 Jan;228(1):71–79. doi: 10.1152/ajplegacy.1975.228.1.71. [DOI] [PubMed] [Google Scholar]
- Melnik E., Latorre R., Hall J. E., Tosteson D. C. Phloretin-induced changes in ion transport across lipid bilayer membranes. J Gen Physiol. 1977 Feb;69(2):243–257. doi: 10.1085/jgp.69.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Steggall M., Eyring E. M. The effect of phloretin on red cell nonelectrolyte permeability. J Membr Biol. 1974;19(1):79–92. doi: 10.1007/BF01869971. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K. F. On the mechanism of inhibition of the sulfate transfer across the human erythrocyte membrane. Biochim Biophys Acta. 1972 Sep 1;282(1):265–276. doi: 10.1016/0005-2736(72)90333-1. [DOI] [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B. Glucose transport by proximal renal tubules. Am J Physiol. 1971 Aug;221(2):580–585. doi: 10.1152/ajplegacy.1971.221.2.580. [DOI] [PubMed] [Google Scholar]
- Von Baeyer H. Glucose transport in the short loop of Henle of the rat kidney. Its characterisation by transport constants. Pflugers Arch. 1975 Sep 29;359(4):317–323. doi: 10.1007/BF00581442. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of henle in psammomys. J Clin Invest. 1969 Mar;48(3):474–486. doi: 10.1172/JCI106005. [DOI] [PMC free article] [PubMed] [Google Scholar]



