Abstract
1. Simultaneous recordings of membrane potential and edge movement were obtained in spontaneously beating chick embryonic myocardial cell aggregates, which are known to behave as an isopotential syncytium.
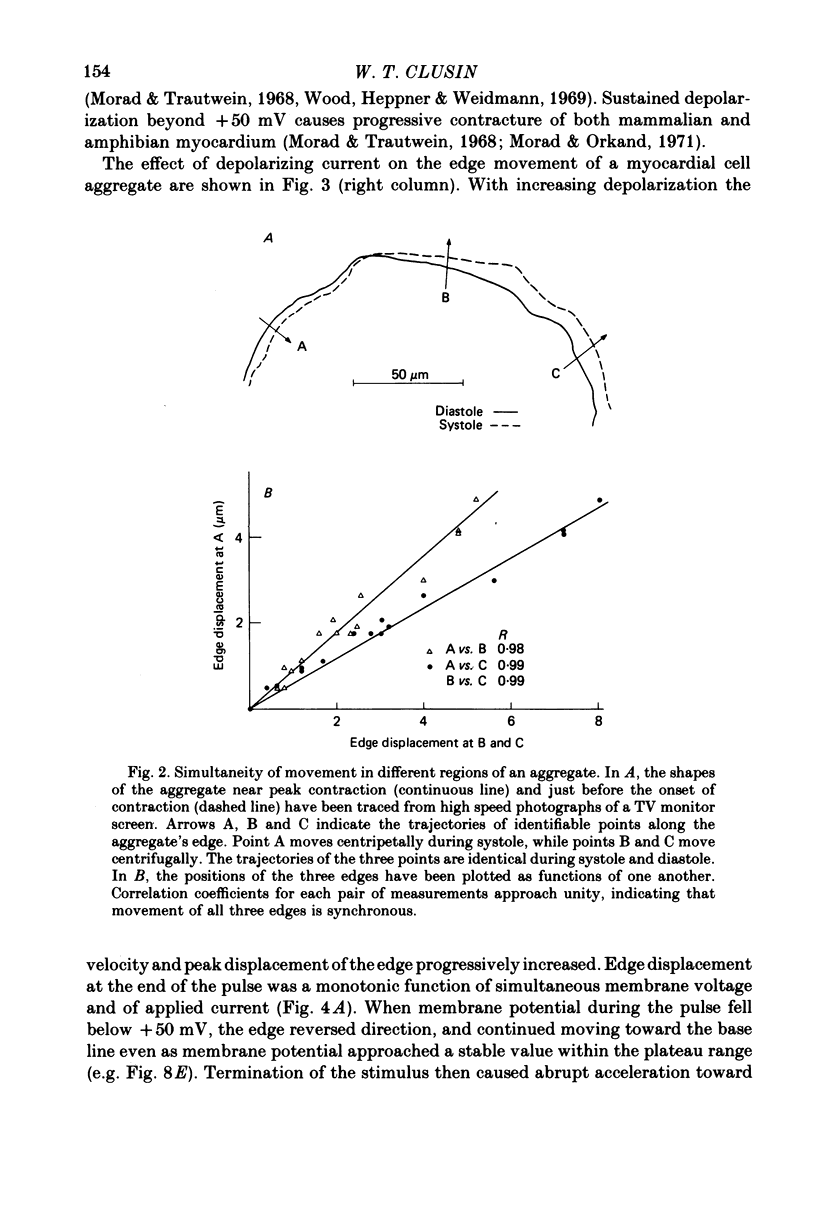
2. The time course of edge movement was similar in different aggregates, and in different regions of the same aggregate.
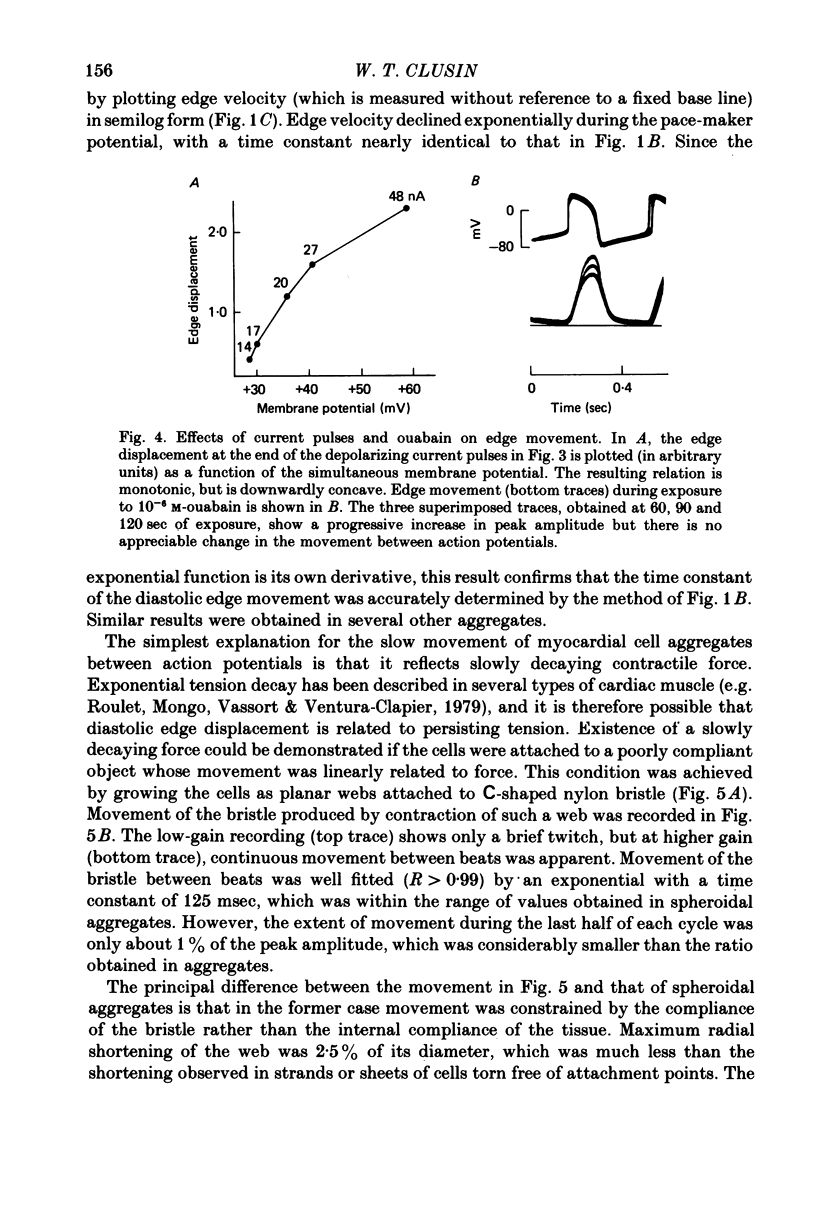
3. Peak amplitude was increased by 10-6 m-ouabain, and by rapid reduction of the external sodium concentration.
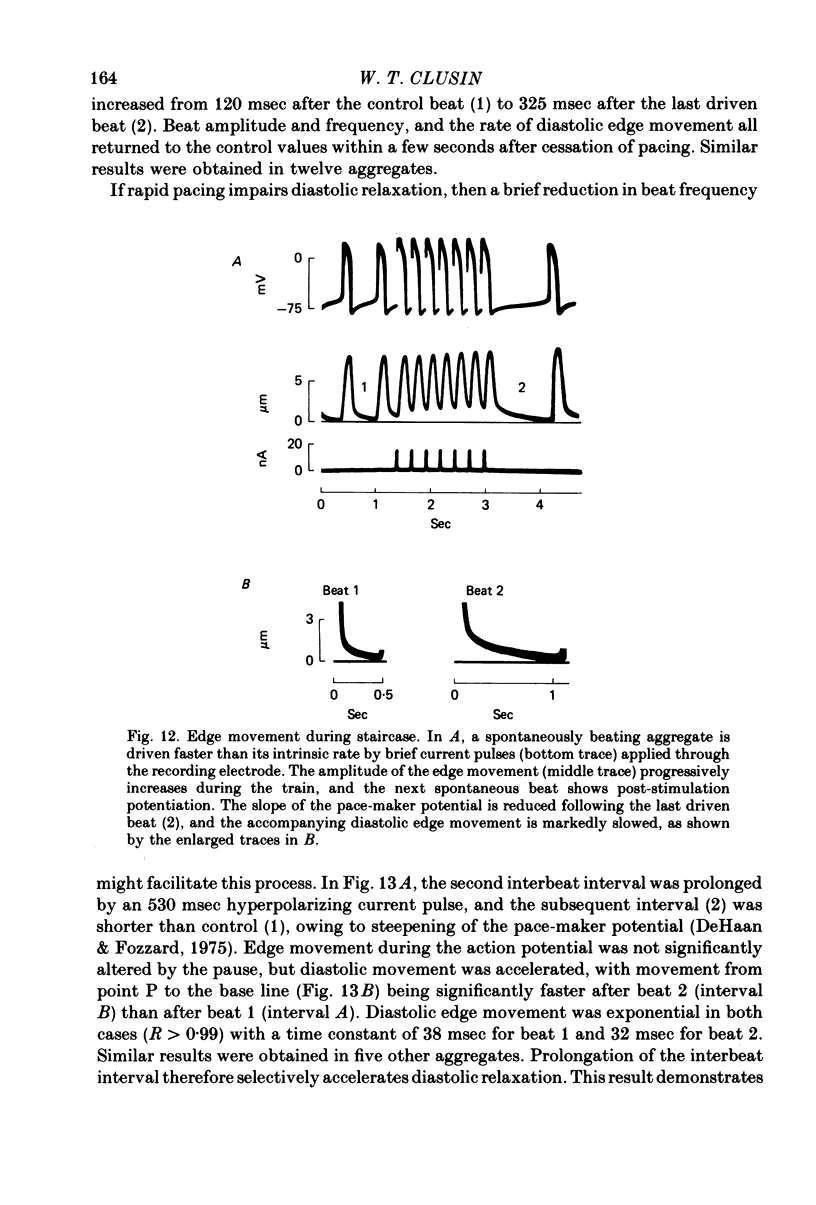
4. Peak amplitude was decreased during single premature action potentials, but sustained rapid pacing produced an ascending staircase.
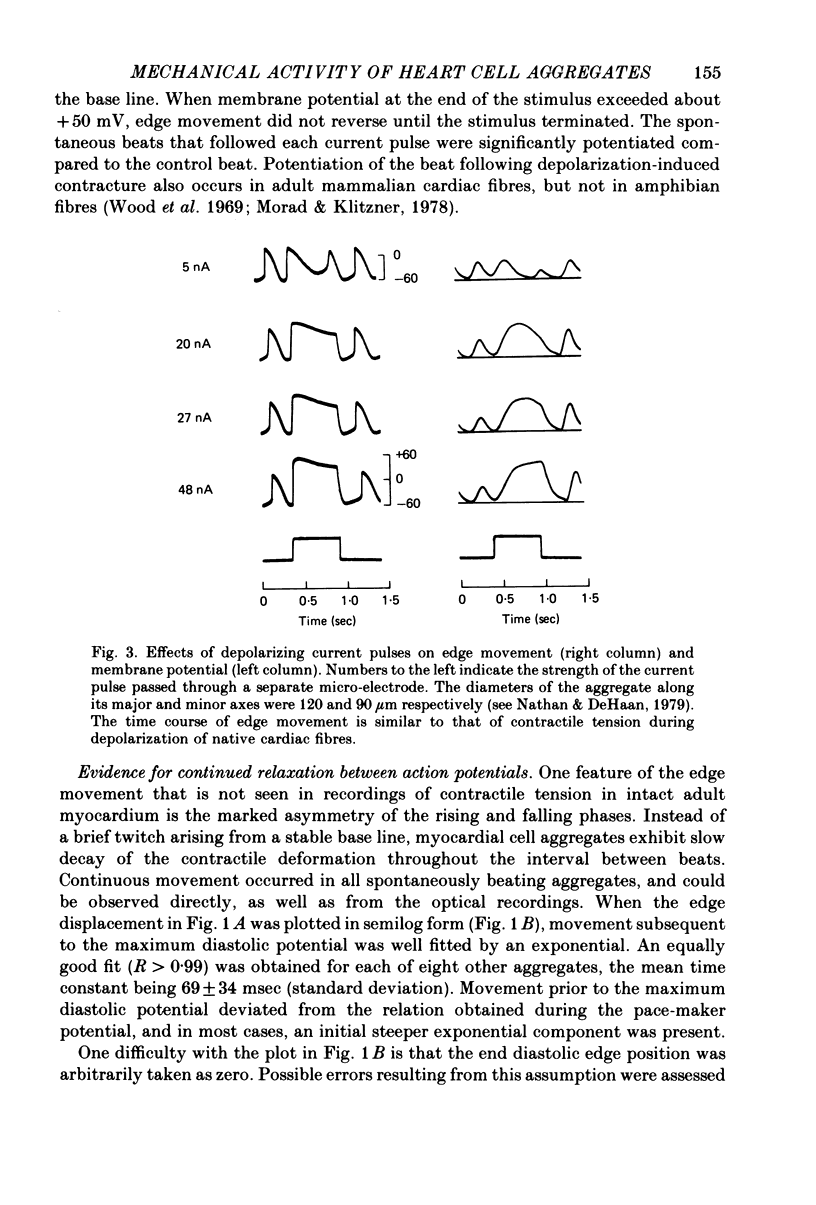
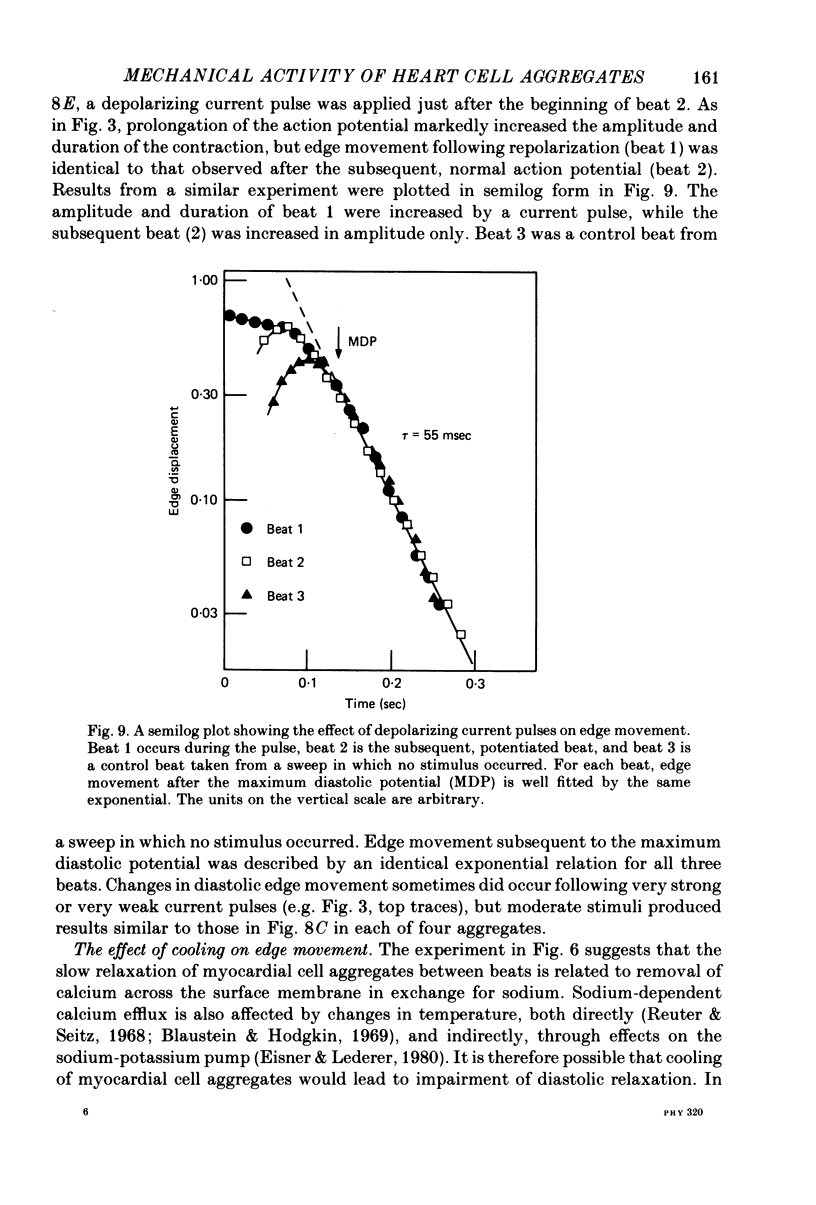
5. Depolarizing current pulses increased both the amplitude and duration of the contraction, and caused potentiation of the next spontaneous beat. Edge displacement during a series of pulses was a monotonic function of membrane potential.
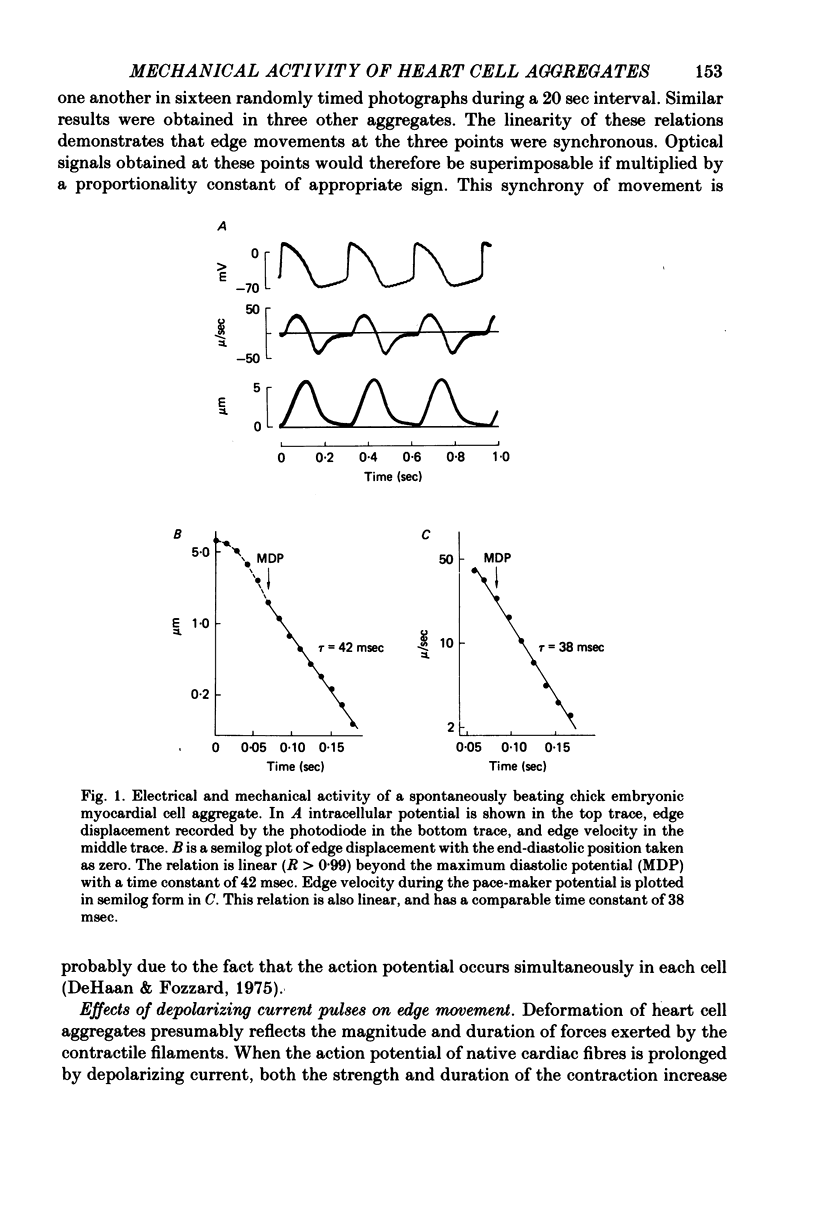
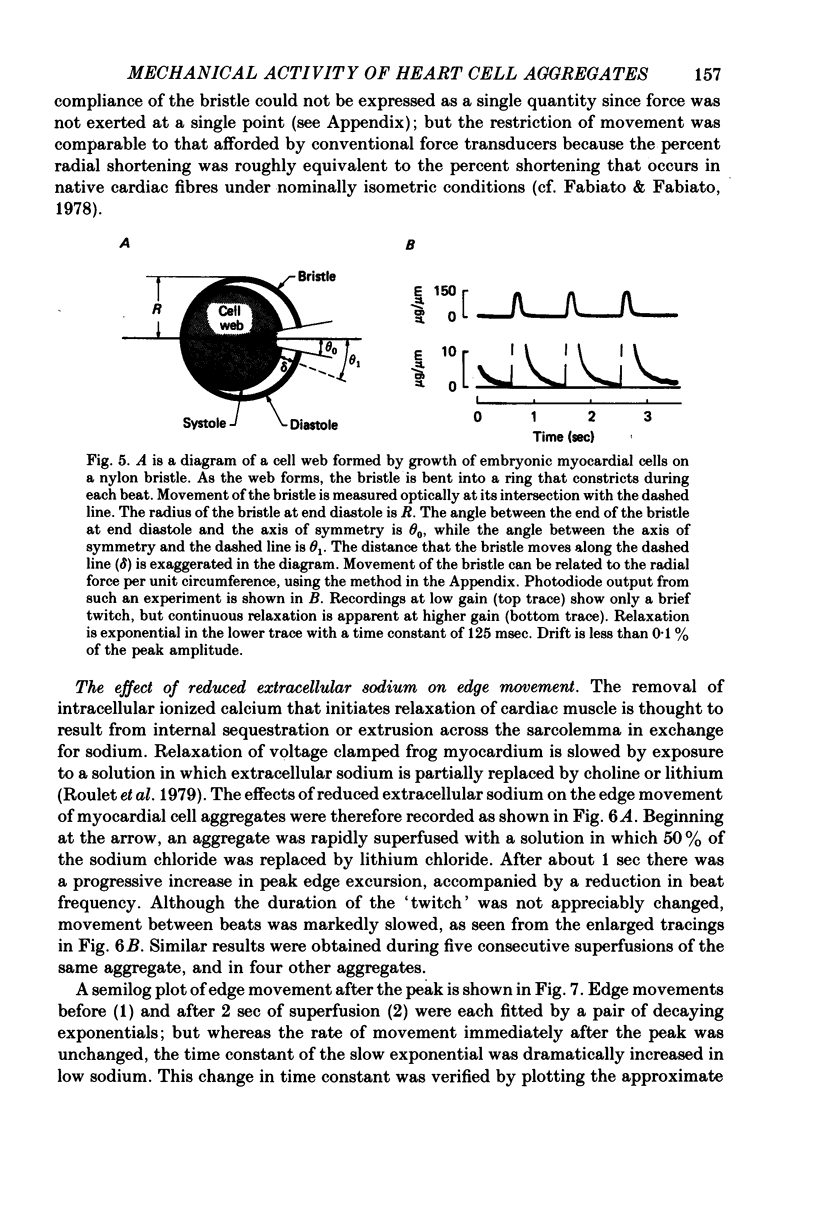
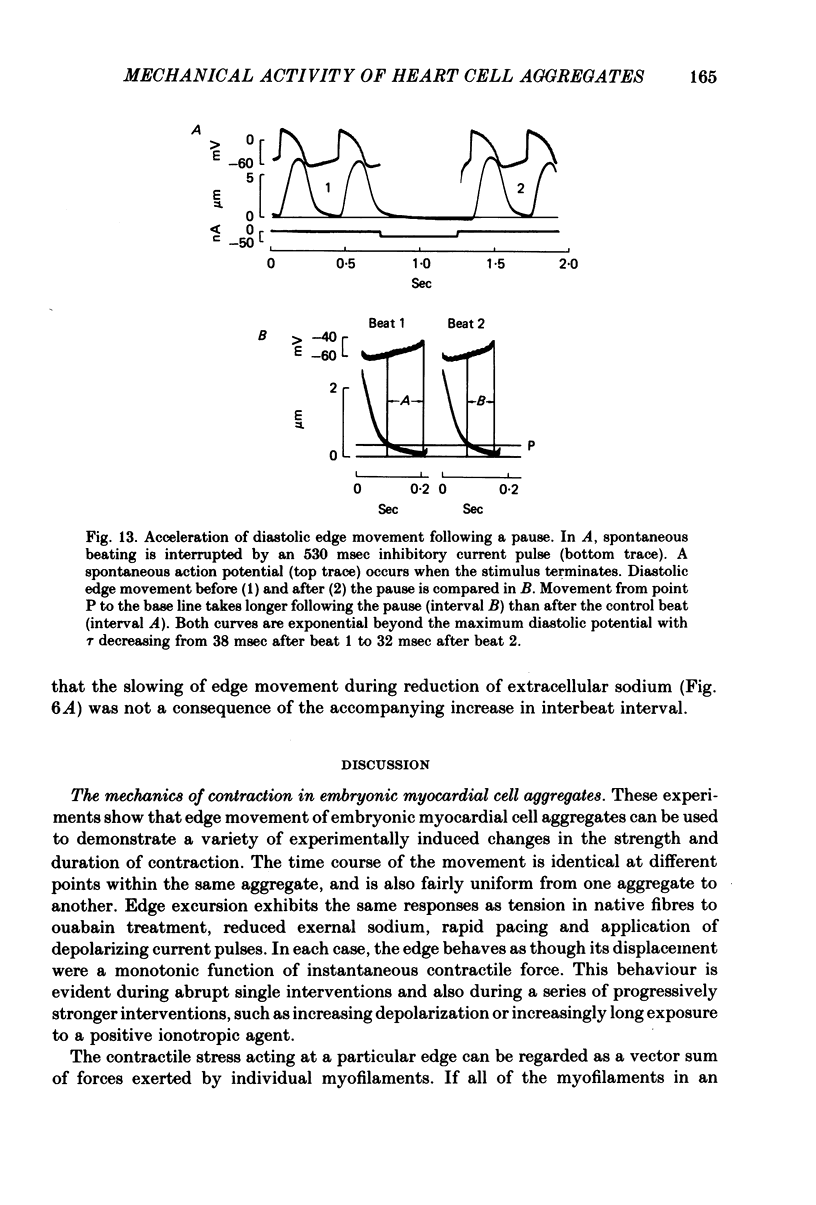
6. Edge movement between action potentials (diastolic movement) was well fitted by an exponential with a mean time constant of 69 msec. Diastolic edge movement was due to a weak, slowly decaying contractile force, which was demonstrated in cells grown on a linear-elastic nylon bristle.
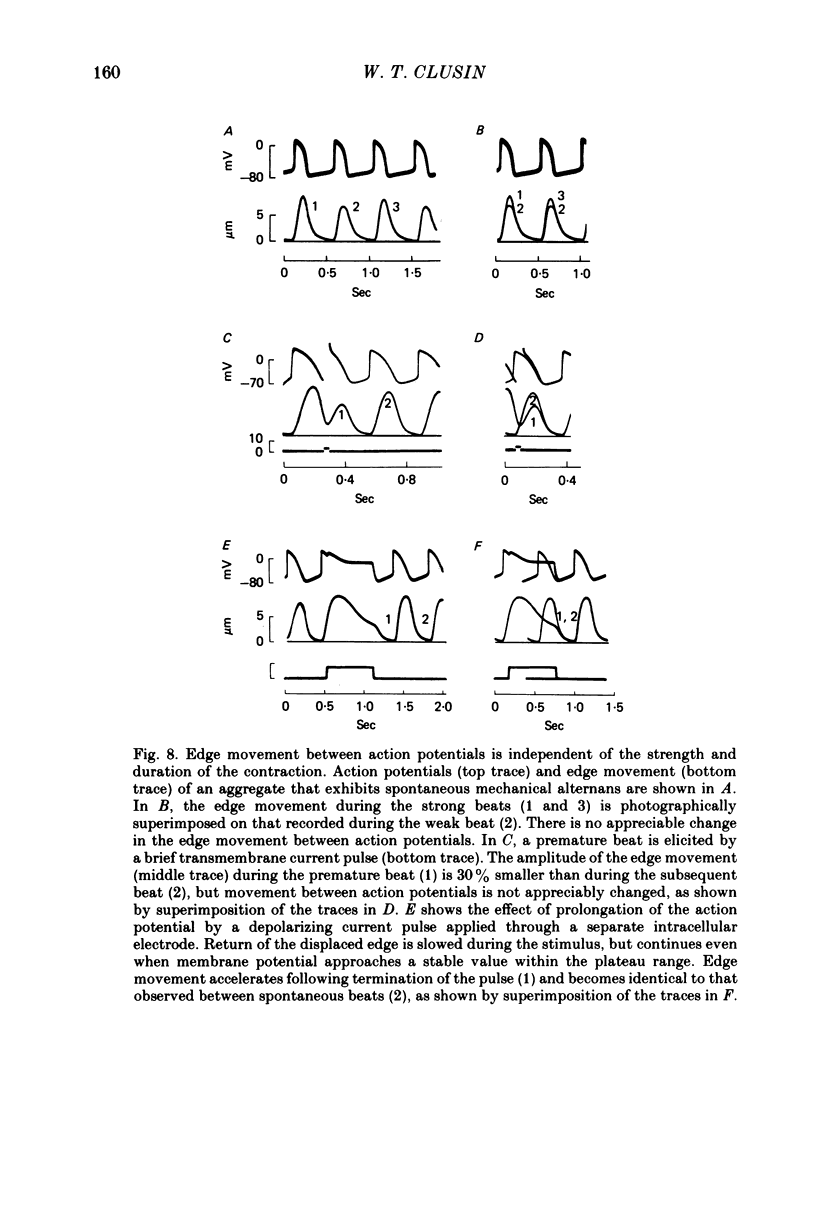
7. The time course of diastolic edge movement remained constant, or nearly constant, during variations in peak amplitude that resulted from prematurity of the action potential, exposure to 10-6 m-ouabain, spontaneous mechanical alternans, or prolongation of the action potential by current pulses.
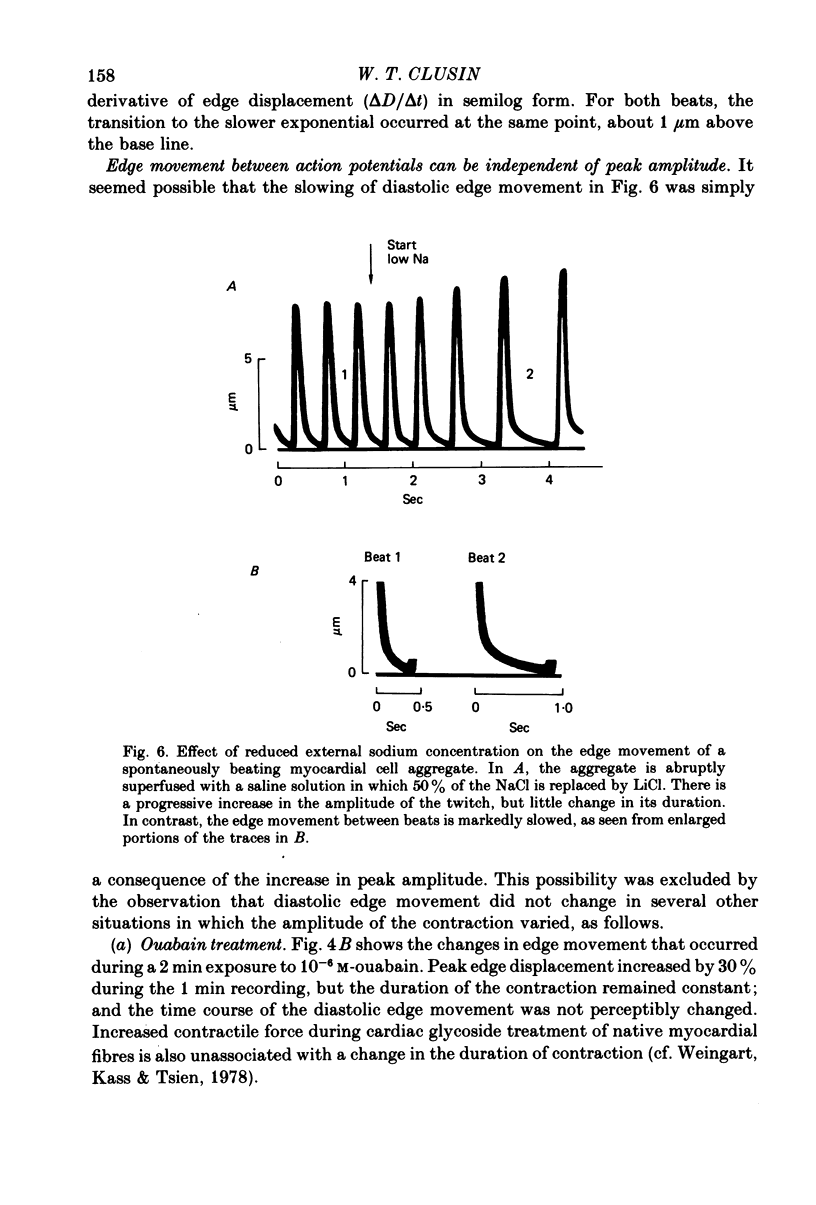
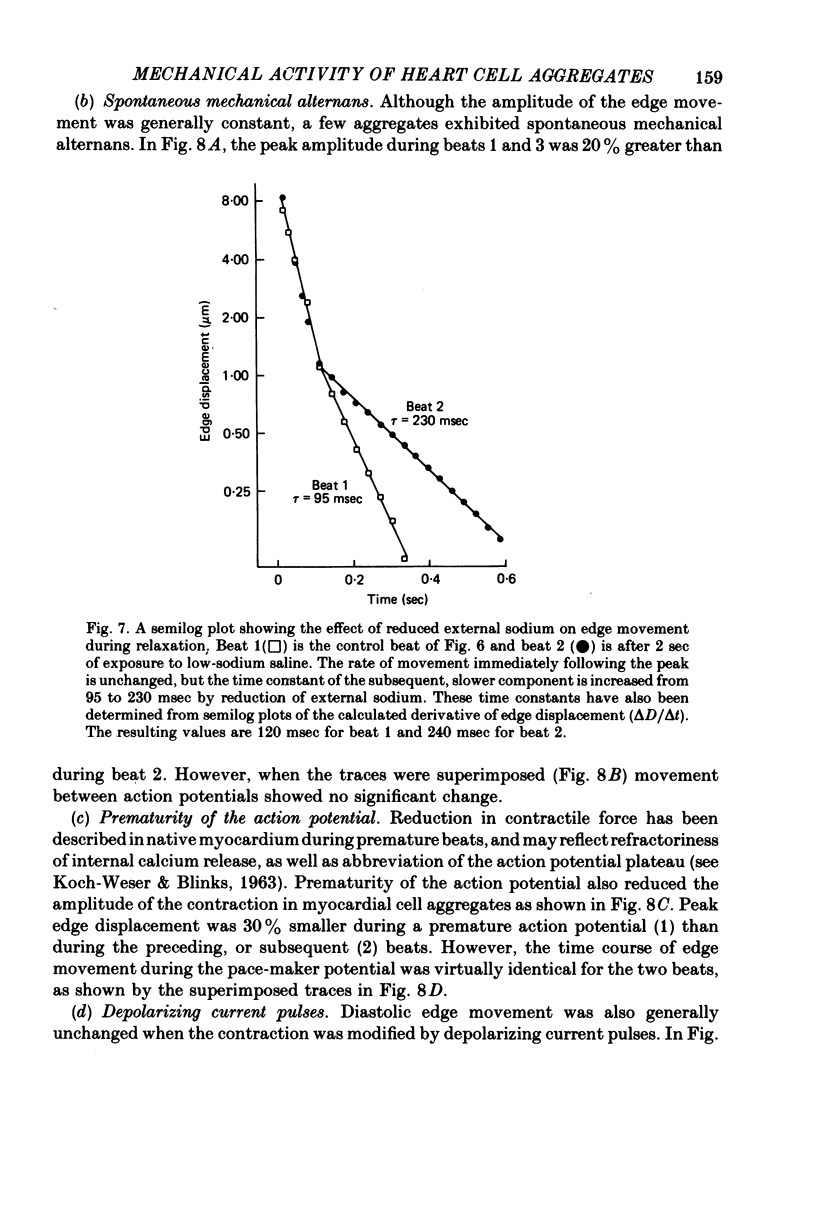
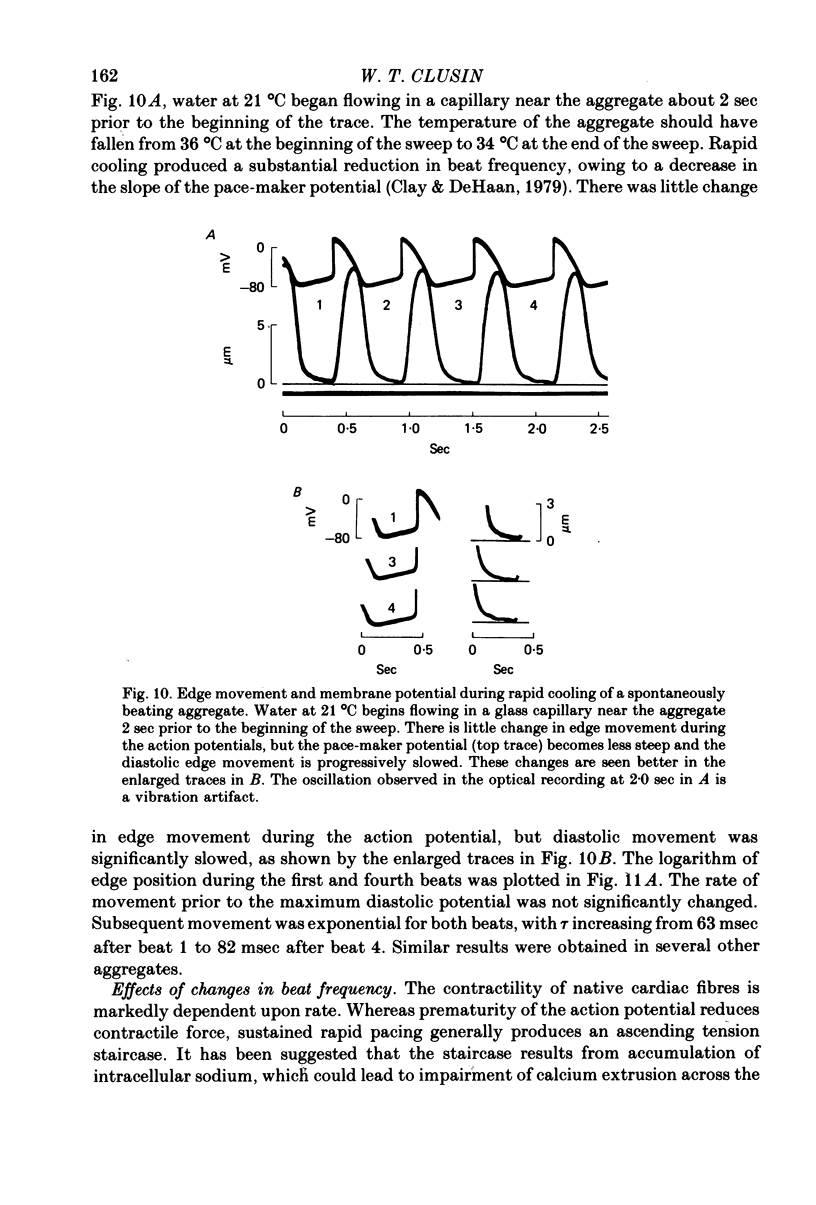
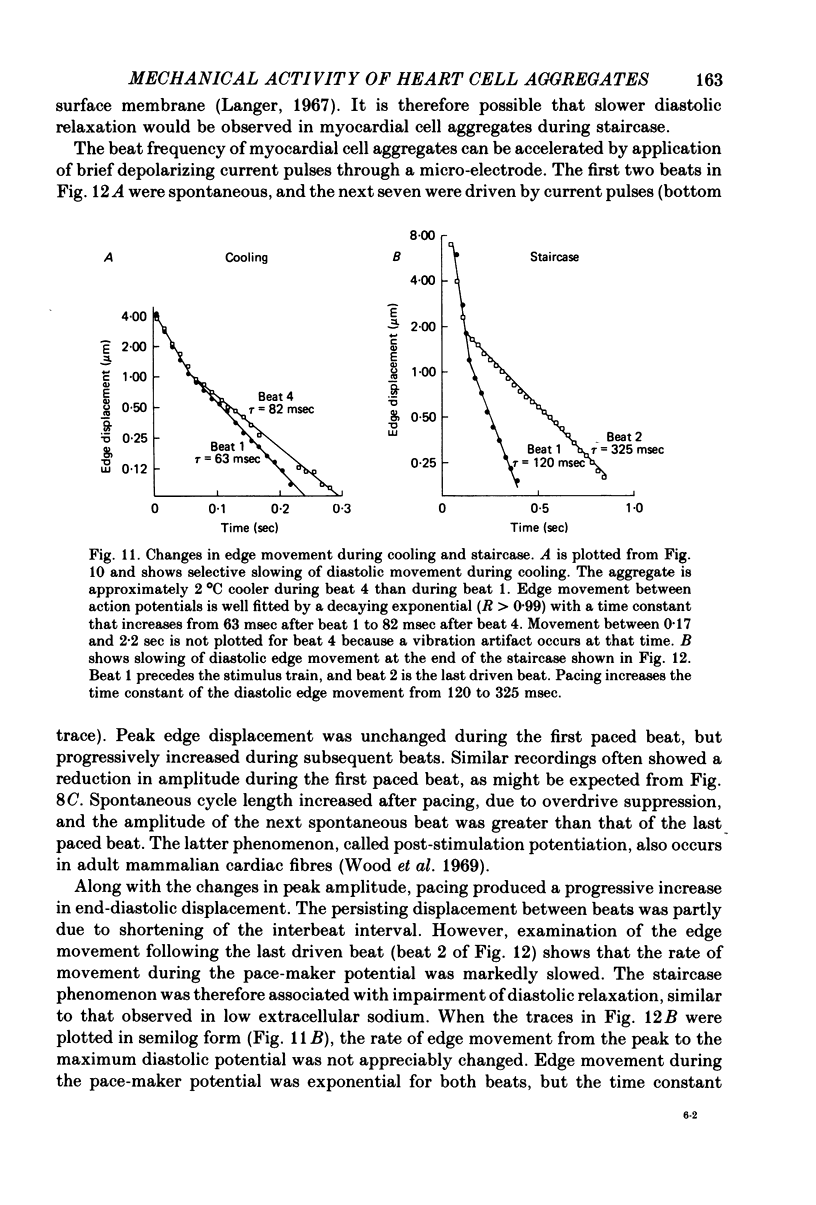
8. In contrast, reduction of the external sodium concentration produced marked, selective slowing of the diastolic edge movement. Similar slowing occurred during cooling and during staircase. Diastolic edge movement was selectively accelerated when the preceding interbeat interval was prolonged by a hyperpolarizing current pulse.
9. The above observations are consistent with the hypothesis that edge displacement is a monotonic function of contractile force.
10. The slow relaxation between action potentials probably reflects removal of intracellular calcium across the surface membrane in exchange for sodium. Changes in the rate of calcium removal may play a role in the regulation of contractility in this tissue.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- BUCHER O. M. A photoelectric recording set for pulsation curves of heart muscle cultures in vitro. Exp Cell Res. 1957 Aug;13(1):109–115. doi: 10.1016/0014-4827(57)90050-2. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Pitzen R., Protas K., Harrison D. C. Inotropic effects of different calcium ion concentration on the embryonic chick ventricle. Comparison of single cultured cells and intact muscle strips. Circ Res. 1975 Jun;36(6):727–734. doi: 10.1161/01.res.36.6.727. [DOI] [PubMed] [Google Scholar]
- Biedert S., Barry W. H., Smith T. W. Inotropic effects and changes in sodium and calcium contents associated with inhibition of monovalent cation active transport by ouabain in cultured myocardial cells. J Gen Physiol. 1979 Oct;74(4):479–494. doi: 10.1085/jgp.74.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boder G. B., Harley R. J., Johnson I. S. Recording system for monitoring automaticity of heart cells in culture. Nature. 1971 Jun 25;231(5304):531–532. doi: 10.1038/231531a0. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., De Clerck N. M. Relaxation of mammalian single cardiac cells after pretreatment with the detergent Brij-58. J Physiol. 1978 Oct;283:481–491. doi: 10.1113/jphysiol.1978.sp012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., de Clerck N. M., Goethals M. A., Housmans P. R. Relaxation of ventricular cardiac muscle. J Physiol. 1978 Oct;283:469–480. doi: 10.1113/jphysiol.1978.sp012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Clay J. R., DeFelice L. J., DeHaan R. L. Current noise parameters derived from voltage noise and impedance in embryonic heart cell aggregates. Biophys J. 1979 Nov;28(2):169–184. doi: 10.1016/S0006-3495(79)85169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., DeHaan R. L. Fluctuations in interbeat interval in rhythmic heart-cell clusters. Role of membrane voltage noise. Biophys J. 1979 Dec;28(3):377–389. doi: 10.1016/S0006-3495(79)85187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shrier A. Developmental changes in subthreshold pace-maker currents in chick embryonic heart cells. J Physiol. 1981 Mar;312:491–504. doi: 10.1113/jphysiol.1981.sp013640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T. Correlation between relaxation and automaticity in embryonic heart cell aggregates. Proc Natl Acad Sci U S A. 1980 Jan;77(1):679–683. doi: 10.1073/pnas.77.1.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaan R. L., Fozzard H. A. Membrane response to current pulses in spheroidal aggregates of embryonic heart cells. J Gen Physiol. 1975 Feb;65(2):207–222. doi: 10.1085/jgp.65.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978 Nov;72(5):667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls steady state potassium conductance. Pflugers Arch. 1977 Oct 19;371(1-2):71–76. doi: 10.1007/BF00580774. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- KATZUNG B., FARAH A. Influence of temperature and rate on the contractility of isolated turtle myocardium. Am J Physiol. 1956 Mar;184(3):557–562. doi: 10.1152/ajplegacy.1956.184.3.557. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- Krueger J. W., Farber S. Sarcomere length 'orders' relaxation in cardiac muscle. Eur Heart J. 1980;Suppl A:37–47. doi: 10.1093/eurheartj/1.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Forletti D., Wittenberg B. A. Uniform sarcomere shortening behavior in isolated cardiac muscle cells. J Gen Physiol. 1980 Nov;76(5):587–607. doi: 10.1085/jgp.76.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A. Sodium exchange in dog ventricular muscle. Relation to frequency of contraction and its possible role in the control of myocardial contractility. J Gen Physiol. 1967 May;50(5):1221–1239. doi: 10.1085/jgp.50.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Sachs H. G., DeHaan R. L. Development of sensitivity to tetrodotoxin in beating chick embryo hearts, single cells, and aggregates. Science. 1972 Jun 16;176(4040):1248–1250. doi: 10.1126/science.176.4040.1248. [DOI] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(1):66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. In vitro differentiation of a fast Na+ conductance in embryonic heart cell aggregates. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2776–2780. doi: 10.1073/pnas.75.6.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulet M. J., Mongo K. G., Vassort G., Ventura-Clapier R. The dependence of twitch relaxation on sodium ions and on internal Ca2+ stores in voltage clamped frog atrial fibres. Pflugers Arch. 1979 Apr 30;379(3):259–268. doi: 10.1007/BF00581430. [DOI] [PubMed] [Google Scholar]
- Sinclair A. J., Miller H. A., Harrison D. C. An electrooptical monitoring technique for heart cells in tissue culture. J Appl Physiol. 1970 Nov;29(5):747–749. doi: 10.1152/jappl.1970.29.5.747. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., McLean M. J. Electrical properties of cultured heart cells. Recent Adv Stud Cardiac Struct Metab. 1976 May 26;12:645–666. [PubMed] [Google Scholar]
- Sperelakis N., Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972 Oct;60(4):430–453. doi: 10.1085/jgp.60.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M., Trank J. W., Leiffer P., Shepherd N. Sarcomere length-resting tension relation in single frog atrial cardiac cells. Circ Res. 1979 Oct;45(4):554–559. doi: 10.1161/01.res.45.4.554. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]



