Abstract
Although A/Hong Kong/156/97 (H5N1/97)-like viruses associated with the “bird flu” incident in Hong Kong SAR have not been detected since the slaughter of poultry in 1997, its putative precursors continue to persist in the region. One of these, Goose/Guangdong/1/96 (H5N1 Gs/Gd)-like viruses, reassorted with other avian viruses to generate multiple genotypes of H5N1 viruses that crossed to chickens and other terrestrial poultry from its reservoir in geese. Whereas none of these recent reassortants had acquired the gene constellation of H5N1/97, these events provide insight into how such a virus may have been generated. The recent H5N1 reassortants readily infect and kill chicken and quail after experimental infection, and some were associated with significant mortality of chickens within the poultry retail markets in Hong Kong. Some genotypes are lethal for mice after intra-nasal inoculation and spread to the brain. On this occasion, the early detection of H5N1 viruses in the retail, live poultry markets led to preemptive intervention before the occurrence of human disease, but these newly emerging, highly pathogenic H5N1 viruses provide cause for pandemic concern.
Influenza viruses pose significant challenges to both human and animal health. Highly pathogenic avian influenza virus infection can result in huge economic loss. Human pandemics are unrivalled in their rapid global impact, and the avian influenza gene pool is critical to their origin through genetic reassortment with the prevailing human virus/es (1–3). Both the 1957 and 1968 pandemics arose in southern China, a region regarded as an epicenter for the emergence of pandemic influenza (4). The H5N1 “bird flu” incident in the Hong Kong SAR in 1997 was the first confirmed instance of a purely avian virus causing respiratory disease and death in humans (5, 6). H5N1 disease in humans was associated with unusual severity (7), the molecular basis for which is still unclear (8, 9). The slaughter of 1.5 million chickens and other poultry across the SAR in December 1997 removed the source of virus infection for humans, and no human disease has been documented since. This incident was regarded by some as an incipient pandemic situation. If so, it was possibly the first instance where a potential human pandemic may have been averted (10).
A/Hong Kong/156/97 (H5N1/97)-like viruses are believed to be reassortants that acquired their hemagglutinin (HA) from H5N1 Gs/Gd (11) and the internal protein gene complement from Quail/HK/G1/97 [H9N2 (G1)] (12) or Teal/HK/W312/97 [H6N1 (W312)]-like (13) viruses. The neuraminidase (NA) of H5N1/97 is similar to that found in W312-like H6N1 viruses. Although viruses with the gene constellation of H5N1/97 have not been detected since the poultry slaughter in late 1997, its putative precursors continue to persist in the region—H5N1 Gs/Gd-like viruses in geese (14–16) and the H9N2 (G1)- and H6N1 (W312)-like viruses in quail (17, 18).
Given the local culinary preference for fresh poultry, live chicken and other poultry including quail, pigeon, pheasant, and Guinea fowl continue to be sold within the retail, live poultry markets of Hong Kong. To prevent the reemergence of an H5N1/97-like virus, central slaughtering of aquatic poultry (ducks and geese) commenced in Hong Kong in 1998, with the aim of keeping influenza viruses of aquatic avian hosts (including H5N1 Gs/Gd-like viruses) apart from the other precursors of H5N1/97, namely H9N2 and H6N1 (17, 18), found principally in quail, in the retail live poultry markets.
Whereas the identities of the putative precursors of H5N1/97 viruses have been deduced retrospectively, there was no direct information on the series of reassortment events that led to its genesis. In contrast, prospective virological surveillance and molecular epidemiology carried out on viruses isolated from terrestrial and aquatic poultry in Hong Kong between 1999 and 2001 now allows us to (i) document a series of virus reassortment events associated with a new interspecies transmission of H5N1 viruses from the aquatic avian influenza reservoir to terrestrial poultry, and (ii) gain insight into the genetic events that may have taken place in 1997. Until the year 2000, geese were the reservoir of H5N1 Gs/Gd-like viruses (14, 15). In 2000, these viruses also were isolated from ducks, and in December 2000, H5N1 Gs/Gd-like viruses isolated from a goose and a duck were found to have acquired internal protein genes (NS, PA, M, and PB2) through reassortment with other aquatic avian virus/es (16). However, since the slaughter of poultry in December 1997, H5N1 viruses have not been isolated from chickens or other terrestrial poultry in Hong Kong's retail poultry markets until February 2001. In this paper, we report the emergence of multiple genotypes of H5N1 viruses in terrestrial poultry between February and May 2001 leading to an avian influenza outbreak in chickens in retail markets in Hong Kong, and we document the molecular changes in the virus associated with this event.
Materials and Methods
Virus Surveillance in Poultry.
From April 1999 onward, fecal swabs were collected monthly from 8–9 representative live poultry retail markets for virus isolation. Fecal or cloacal swabs were similarly collected from imported geese and ducks at a central wholesale slaughter facility. The specimens were inoculated into embryonated eggs and virus isolates were characterized as described (17). Based on date, site and type of poultry sampled, representative virus isolates were chosen for genetic characterization. Viruses also were antigenically characterized by using the hemagglutination inhibition test (HI) and a set of reference antisera and monoclonal antibodies as described (16).
Genetic and Phylogenetic Analysis.
Viral RNA was extracted from virus-infected allantoic fluids with the RNeasy Mini Kit (Qiagen, Chatsworth, CA) and reverse transcribed, and PCR amplification was carried out (primer sequences available upon request). The PCR-amplified DNA was sequenced by using the Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems) on a Perkin-Elmer model 377 XL DNA sequencer. Sequence data were assembled and edited with the WISCONSIN PACKAGE Version 10.0 (Genetics Computer Group, Madison, WI; ref. 16). Alignment and homology analysis were performed with GENEDOC, Version 2.3 (available at http://www.psc.edu/biomed/genedoc/gddl.htm). The nucleotide sequences from each gene segment analyzed for this study were as follows: HA 41–1360; NA 41–1110; NP 45–1110; NS 41–866; M 32–1001; PB1 33–1490; PB2 970-2309; and PA 1366–2213.
Pathogenicity Tests.
Representative H5N1 influenza viruses belonging to each genotype isolated from terrestrial poultry were studied. Viruses isolated from geese in 1999 (Gs/Gd-like) were used for comparison. Pathogenicity tests on chickens were done by i.v. injection of virus-infected allantoic fluid, as described (19). Quail were infected by a combination of the oral, intranasal, and orbital routes (17). Tracheal and cloacal samples were collected on day 3 after infection and virus titrated in embryonated eggs. BALB/c mice were infected by the intra-nasal route (20).
Results
Epidemiology.
Since 1999, H5N1 viruses were intermittently isolated from geese (and since 2000, also from ducks) imported into the central slaughter house at the Western Wholesale Food Market (14–16). By contrast, between April 1999 and December 2000, no H5N1 virus was isolated from 8,626 routine surveillance swabs taken from terrestrial poultry in the retail, live poultry markets in Hong Kong. However, during April 2001, eleven H5N1 viruses were isolated from 531 fecal swabs collected from the pans below individual cages containing apparently healthy poultry. Of these, five isolates were from chickens, one from pigeon, one from quail, two from pheasant, and two from silky chickens. One additional virus was isolated from the water trough of a chicken cage. Retrospectively, an unidentified isolate obtained from feces in a chicken cage in February 2001 also was confirmed to be H5N1 virus. These H5N1 isolates were obtained from three of the eight retail markets under surveillance. This finding led to a more intensive virological surveillance of dead chickens in retail poultry markets across Hong Kong. Fifty-two additional H5N1 viruses were isolated from 161 cloacal swabs taken from dead chickens from 30 retail markets. There was no overt increase in mortality rates in chickens in most of these retail markets, but in mid-May, three markets reported greatly increased mortality of chickens. Taken together, these findings led to the decision to slaughter 1.3 million poultry in the retail markets and farms across Hong Kong in May 2001.
Eighteen representative H5N1 viruses isolated from chickens and one each from a quail, pheasant, pigeon, and silky chicken in the retail markets between February and May 2001 were selected for further characterization (Table 1).
Table 1.
H5N1 viruses isolated in 2001 characterized in this study
| Virus and type of poultry | Sampling date (M/D/Y) | Markets sampled* | Genotype |
|---|---|---|---|
| Ck/HK/FY77/01† | 2/14/01 | KL 1 | C |
| Ck/HK/YU562/01† | 4/4/01 | NT 1 | B |
| Ck/HK/YU563/01† | 4/4/01 | NT 1 | B |
| Ck/HK/FY150/01† | 4/20/01 | KL 1 | D |
| Ph/HK/FY155/01† | 4/20/01 | KL 1 | C |
| SCk/HK/SF189/01† | 4/25/01 | HKI 1 | A |
| Qa/HK/SF203/01† | 4/25/01 | HKI 1 | A |
| Pg/HK/SF215/01† | 4/25/01 | HKI 1 | A |
| Ck/HK/SF219/01† | 4/25/01 | HKI 1 | A |
| Ck/HK/715.5/01‡ | 4/26/01 | KL 2 | E |
| Ck/HK/751.1/01‡ | 5/5/01 | KL 1 | C |
| Ck/HK/822.1/01‡ | 5/15/01 | NT 1 | A |
| Ck/HK/829.2/01‡ | 5/16/01 | KL 1 | C |
| Ck/HK/830.2/01‡ | 5/16/01 | HKI 1 | A |
| Ck/HK/858.3/01‡ | 5/17/01 | KL 3 | A |
| Ck/HK/866.3/01‡ | 5/17/01 | KL 4 | A |
| Ck/HK/867.1/01‡ | 5/17/01 | KL 5 | A |
| Ck/HK/879.1/01‡ | 5/17/01 | HKI 2 | A |
| Ck/HK/873.3/01‡ | 5/18/01 | KL 2 | E |
| Ck/HK/876.1/01‡ | 5/18/01 | KL 6 | A |
| Ck/HK/891.1/01‡ | 5/18/01 | NT 2 | A |
| Ck/HK/893.1/01‡ | 5/18/01 | HKI 3 | A |
| Gs/HK/76.1/01† | 1/13/1 | WWFM | C |
| Gs/HK/ww100/01† | 3/601 | WWFM | B |
| Dk/HK/573.4/01† | 4/501 | WWFM | C |
| Dk/HK/646.3/01† | 4/14/01 | WWFM | B |
Different markets in each region [Hong Kong Island (HKI), Kowloon (KL) and New Territories (NT)] of the Hong Kong SAR are numbered. WWFM is the Western Wholesale Food Market to which geese and ducks are imported for central slaughter.
Virus isolated from fecal droppings from apparently healthy poultry in retail poultry markets.
Virus isolated from cloacal swabs of dead poultry in the retail poultry markets.
Antigenic Characterization of Virus Isolates.
To determine the antigenic inter-relationships of the H5N1 influenza viruses circulating in live poultry markets during 2001 with previous H5N1 viruses isolated in Hong Kong, representative strains were examined in hemagglutination inhibition tests by using polyclonal monospecific antiserum to the reference strain of H5 subtype virus A/Tern/South Africa/61 (H5N3) and monoclonal antibodies CP-24, CP-46, and CP-58 to the HA of Ck/Penn/1370/83 (H5N2; ref. 15). The polyclonal reference antiserum to Tern/SA/61 gave similar titers with the viruses isolated in 2001 and with Gs/HK/437–6/99, although there were discernible differences (>4-fold) between them and the H5N1 viruses isolated in 1997 (see Table 3, which is published as supporting information on the PNAS web site, www.pnas.org). The panel of monoclonal antibodies did not reveal major differences, although Ck/HK/822.1/01 failed to react with CP-46, as did Ck/HK/AFD258/97. Thus, the HA of these new viruses were related to H5N1 influenza viruses isolated from aquatic birds in Hong Kong during the last 2 years.
Phylogenetic Analysis.
Eighteen H5N1 viruses isolated from the retail markets between February and May 2001 were characterized genetically (Table 1). Each of eight gene segments of these viruses was partially sequenced, and their phylogenetic relationships were established (Figs. 1 and 2). For comparison, nine viruses isolated from ducks and geese between January and April 2001 also were genotyped. Two different genotypes were identified in these aquatic poultry, and more detailed genetic analysis was carried out on four viruses representing these two genotypes (Table 1).
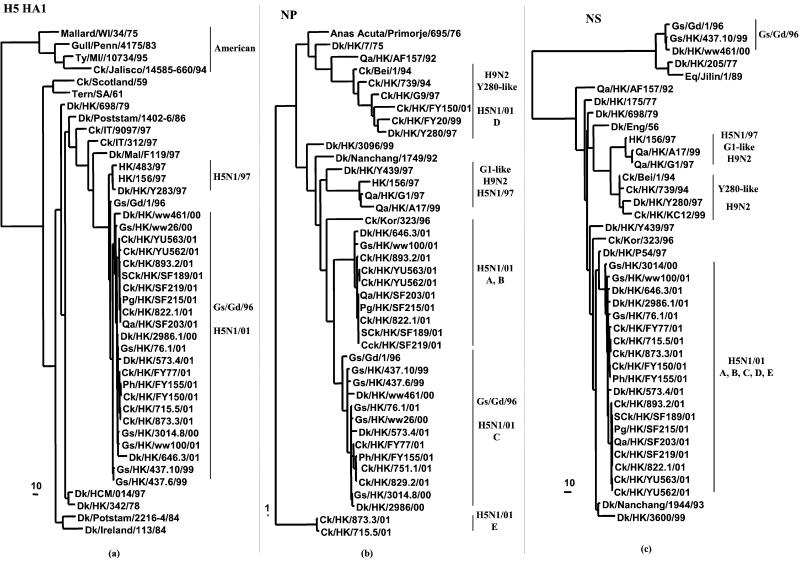
Figure 1.
Phylogenetic trees for the H5 HA1 (a), NP (b), and NS (c) genes of influenza A viruses. The nucleotide sequences were analyzed with PAUP by using a maximum-parsimony algorithm. Nucleotides 43 to 1,023 (981 bp) of the HA gene, 42 to 1,002 (961 bp) of the NP gene, and 41 to 824 (784 bp) of the NS gene were used for the phylogenetic analysis. The H5 HA1 phylogenetic tree is rooted to A/Japan/305+/57 (H2N2). The nucleotide trees of NP and NS gene are rooted to A/Equine/Prague/1/56 (H7N7). The lengths of the horizontal lines are proportional to the minimum number of nucleotide differences required to join nodes. Vertical lines are for spacing branches and labels. Names and abbreviations of viruses studied are listed in Table 1, and the abbreviations are denoted in the footnote. Other sequences can be found in GenBank.
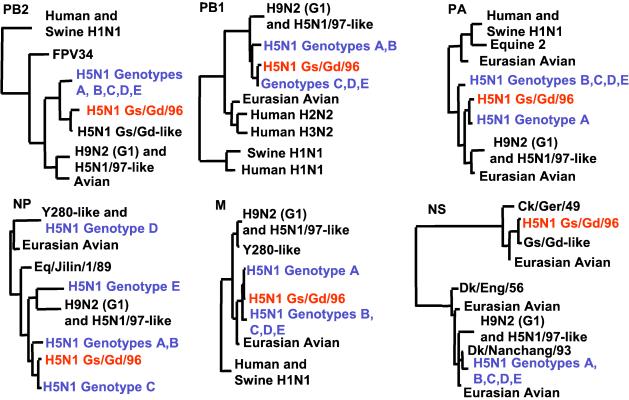
Figure 2.
Generalized phylogenies in diagrammatic form of PB2, PB1, PA, NP, M, and NS genes of H5N1 influenza viruses. The nucleotide sequences of PB2 (992 bp), PB1 (979 bp), PA (645 bp), NP (961 bp), and NS (784 bp) were used in the phylogenetic analysis. Only the major terminal branches of the Eurasian avian lineages of the trees are shown. The genes of H5N1 viruses isolated in 2001 are shown in blue, and those of the precursor Gs/Gd/1/96 are in orange.
Phylogenetic analysis of the HA gene revealed that the new H5 viruses from terrestrial and aquatic poultry were Gs/Gd/96-like and were descendants of H5N1 viruses isolated from aquatic birds in Hong Kong in 2000 (Fig. 1a). The NA gene tree shows a similar phylogenetic relationship (data not shown).
An analysis of the internal protein genes shows that the H5N1 viruses of 2001 had acquired their internal gene complex partly or completely from other aquatic avian influenza viruses (Figs. 1 and 2). The nucleoprotein (NP) gene tree showed that these genes had multiple sources of origin (Fig. 1b)—some of the viruses (designated genotype C) clustered directly into the Gs/Gd/96-like lineage, whereas other viruses (designated genotypes A and B) together form a sister-group relationship with the Gs/Gd/96-like lineage and derive from influenza viruses of other waterfowl. Two chicken isolates formed an independent lineage and became a third subgroup (genotype E), whereas another virus (Ck/HK/FY150/01 designated genotype D) obtained its NP gene from a virus belonging to the H9N2 virus lineage represented by Dk/HK/Y280/97 (Table 1, Fig. 1b).
The NS (nonstructural) gene tree showed that all of the terrestrial and aquatic H5N1 viruses of 2001 clustered within the A allele, whereas the Gs/Gd/96-like viruses all belong to the B allele. The NS genes of viruses isolated in 2001 were closely related to that of two reassortant H5N1 influenza viruses that were detected in December 2000 (Fig. 1c).
The phylogenetic relationships of the other four internal protein gene segments are shown in the cladograms (Fig. 2) and also indicate that the new viruses are reassortants of Gs/Gd/96-like viruses—some of the gene segments are derived from Gs/Gd/96-like virus and others from influenza viruses resident in waterfowl.
Five different genotypes, designated A, B, C, D, and E, were recognized among the H5N1 influenza viruses isolated in 2001 (Fig. 3 and Table 4, which are published as supporting information on the PNAS web site). The six internal protein genes of genotype B viruses were all different from Gs/Gd/96-like viruses and had been acquired through reassortment with one or more unknown avian influenza viruses of waterfowl. Genotype A had acquired the PB2, PB1, NP, and NS genes from this same novel source, whereas genotype C had PB2, PA, M, and NS genes similarly derived. Genotype C is essentially similar to the reassortant first isolated from a goose (Gs/HK/3014.8/00) and a duck (Dk/HK/2986.1/00) in December 2000 (ref. 16; Fig. 3). The new internal protein genes acquired by Genotype A, B, and C viruses are genetically related to each other and may have all come from a single donor virus. Genotype D is represented by one virus, Ck/HK/FY150/01, which is a genotype C virus that acquired an NP gene through further reassortment with Ck/HK/Y280/97-like H9N2 viruses (17). Genotype E viruses are genotype C viruses that have acquired a new NP gene from yet another unknown aquatic avian influenza virus.
Figure 3.
The genotypes of H5N1 reassortants and their derivation from 1997 to 2001. The eight gene segments in each schematic virus particle are represented in the order (top to bottom) PB2, PB1, polymerase (PA), hemagglutinin (HA), nucleoprotein (NP), neuraminidase (NA), matrix (M), and nonstructural (NS) genes. A different color is used to represent each distinct virus lineage. The virus abbreviations used are: H9N2 (G1), Quail/Hong Kong/G1/97; H6N1 (W312), Teal/Hong Kong/W312/97; H5N1 Gs/Gd, Goose/Guangdong/1/96; H9N2/Y280, Chicken/Hong Kong/Y280/97; and H5N1/97, A/Hong Kong/156/97-like viruses. Abbreviations used for poultry are explained in the footnote. The reassortment events that most likely led to the genesis of the pathogenic viruses of 1997 (H5N1/97) are included for reference. ? denotes that genotype A viruses have not so far been isolated from aquatic poultry in Hong Kong.
Chronology of H5N1 Genotypes Isolated.
The viruses isolated from ducks and geese in 2001 all belonged to genotype B or C; the precursor Gs/Gd/96-like virus was not detected. All five genotypes were present in the retail live poultry markets containing terrestrial poultry (Table 1).
The first H5N1 virus found in the retail live poultry markets was a genotype C virus isolated from chicken in Kowloon Market 1 in February 2001. Subsequently, this genotype was isolated from the same retail market in April and May but was not found in any other terrestrial poultry market. In late April, this virus seemed to have acquired an NP gene from a HK/Y280/97-like H9N2 virus to generate a virus designated genotype D. Two genotype B viruses were isolated from chicken in early April (New Territories Market 1), but this was the only sampling occasion where this virus has been detected in terrestrial poultry. A genotype E virus was isolated from Kowloon 2 market in April, and the same genotype was isolated from this market in mid-May (Table 1). Genotype A viruses were first detected in late April in the Hong Kong Island Market 1 but have since been isolated from many retail poultry markets across all three regions of Hong Kong SAR (Table 1).
The genotypes of viruses isolated from pheasant, quail, silky chicken, and pigeon essentially reflected those found in chickens in the same retail poultry market. None of these viruses had (yet) reassorted with H9N2 or H6N1 viruses containing the internal protein genes of H5N1/97 that are known to be particularly prevalent in quail within these markets (17, 18).
Molecular Characterization.
The alignment of the deduced amino acid sequences of the H5 HA genes from the H5N1 virus isolated in 2001 shows that, with one exception, they all have the same multiple basic amino acids at the connecting peptide between HA1 and HA2. This result is identical to that found in many H5N1 viruses isolated from 1997 to 2000: Gs/HK/437–6/99 and Gs/HK/437–10/99, for example. One duck isolate, Dk/HK/573.4/01, has a deletion from nucleotide 1056 to 1058 resulting in the loss of one R at the −4 position of HA1-connecting peptide. Based on the amino acid sequence at the HA1-HA2 connecting peptide, all these new H5N1 influenza viruses would be regarded as highly pathogenic influenza viruses for chickens (21).
Even though phylogenetic analysis suggests that the NA genes of the H5N1 viruses isolated in 2001 are all Gs/Gd/96-like, analysis of the deduced amino acid sequences revealed that all viruses from genotype A had a 20-aa deletion in the stalk region (position 49 to 68). This deletion is distinct from, but overlaps, the 19-aa deletion found in H5N1/97 viruses (position 54–72). None of the other genotypes had a similar deletion. It was noteworthy that genotype A was the most widespread virus in chickens and other terrestrial poultry in the retail live-poultry markets, and its appearance in May 2001 correlated with an overt increase in mortality in chickens in some markets. Alignment analysis of the internal protein gene segments also revealed that all five genotypes carry NS genes with an unique 5-aa deletion (position 80–84) in the middle of the NS1 protein. Whether these molecular markers are associated with the interspecies transmission from aquatic birds to terrestrial birds is still not determined.
Pathogenicity Tests.
A representative virus from each of the five genotypes of H5N1 influenza viruses isolated in 2001 was inoculated into chickens, quail, and mice. Two Gs/Gd/96-like viruses isolated from geese in 1999 were used for comparison. After experimental infection, the H5N1 Gs/Gd-like parental viruses as well as the recent reassortants infected and killed chicken and quail (Table 2), but genotypes A, B, and E are particularly pathogenic in chickens, killing all infected birds within a day. All H5N1 viruses tested are also highly pathogenic for quail. Genotype A and E seem to be more lethal for quail than other genotypes, killing all infected birds within 1 to 3 days.
Table 2.
Pathogenicity of H5N1 influenza viruses for animals following experimental infection
| Virus | Genotype | Avian infection: No. dead/no. inoculated (days to death)
|
Mouse infection
|
|||
|---|---|---|---|---|---|---|
| Chicken* | Quail* | No. dead/no. inoculated† | Virus in lung (mean log10 EID50)‡ | Virus in brain (mean log10 EID50)‡ | ||
| Gs/HK/437.6/99 | Gs/Gd/96-like | 8/8 (2–6) | 7/8 (4–7) | 0/10 | 4.2 | No |
| Gs/HK/437.10/99 | Gs/Gd/96-like | 7/8 (3–9) | 6/8 (4–7) | 4/8 | 2.3 | No |
| Ck/HK/YU822.1/01 | A | 8/8 (1) | 4/4 (1–2) | 11/15 | 2.5 | Yes (3.5) |
| Ck/KH/YU562/01 | B | 8/8 (1) | 4/4 (5–6) | 13/20 | 2.6 | No |
| Ph/HK/FY155/01 | C | 8/8 (1–2) | 4/4 (3–4) | 17/18 | 4.6 | Yes (4.3) |
| Ck/HK/FY150/01 | D | 8/8 (1–3) | 4/4 (4–5) | 8/13 | 3.8 | Yes (2.8) |
| Ck/HK/873.3/01 | E | 8/8 (1) | 4/4 (2–3) | 6/18 | 2.8 | Yes (3.5) |
The dose used was 105–106 egg infection dose (EID50). Routes of inoculation used were as follows: chicken, i.v.; quail, combination of oral, intranasal, and orbital routes; Balb/c mice, intranasal.
Mice observed for 10 days.
Mean of results from three mice.
In experimental infection, all five genotypes of H5N1 viruses have the ability to replicate well in mouse lung without prior adaptation. Genotypes A, C, D, and E spread to the brain, a feature not possessed by the Gs/Gd/96-like precursor viruses isolated in 1999. Genotype A, B, and C isolates were particularly lethal for mice, whereas the other genotypes and the Gs/Gd-like parental strains were less so (Table 2).
Discussion
Since the “bird flu” incident of 1997, this occasion is the first that H5N1 viruses have been detected in chickens and other terrestrial poultry in Hong Kong's retail, live poultry markets. Although no human H5N1 infection was detected in Hong Kong in association with the H5N1 outbreak in the retail poultry markets in May 2001, a number of observations justified public health concern. The parental Gs/Gd-like H5N1 viruses, hitherto established in geese, had undergone reassortment and generated multiple genotypes that had acquired the propensity to transmit from the aquatic avian reservoir to terrestrial poultry in nature. Some of these reassortants (genotypes B and C) had replaced the parental Gs/Gd virus from its reservoir in geese. Both of these genotypes were also isolated from terrestrial poultry—but only from one retail market in each case. All five genotypes were isolated from chickens and other terrestrial poultry in the retail live poultry markets. Genotype C viruses were isolated repeatedly from one market over a 4 month period. Given that this genotype was not isolated from any other market, it is likely that it was recirculating within the market. This genotype had reassorted further and acquired an NP gene from Dk/HK/Y280/97-like H9N2 viruses. The epidemiological data are consistent with this reassortment event having occurred within the retail market. Genotype E viruses were isolated on two occasions 3 weeks apart from the same market but were not found elsewhere.
In contrast, genotype A H5N1 viruses, first detected in late April, were found in many retail markets during May and were associated with increased chicken mortality in three markets. Although overt mortality of chicken within a retail market is influenced by many factors, including the rate of turnover of poultry within a stall, it is notable that genotype A was the only genotype with a deletion in the NA stalk region. Deletion in the stalk of the NA seems to be an adaptation associated with the transmission of aquatic avian influenza viruses to terrestrial poultry; such deletions are seen in H5N1/97 viruses (5, 22) as well as in H9N2 viruses (23) after interspecies transmission to chicken. It is possible that the NA deletion seen in the genotype A H5N1 viruses reflects their adaptation to terrestrial poultry and explains the widespread nature of this virus within chickens and other terrestrial poultry in the markets.
All H5N1 genotypes were highly pathogenic for chickens after i.v. inoculation, but genotypes A, B, and E were particularly so, killing all infected poultry within a day. The appearance of genotype A in the retail markets in May 2001 correlated with an overt increase in mortality in chickens in some markets. None of the viruses, including that isolated from a quail, had acquired the internal protein gene constellation of H5N1/97. However, the other putative precursors of H5N1/97, namely H9N2 (G1) and H6N1 (W312) (12, 13), were actively circulating in quail in the retail poultry markets (Fig. 3; refs. 17 and 18). Experimentally, all five H5N1 genotypes readily infect quail, increasing the possibility of reassortment leading to the generation of H5N1/97-like viruses.
Without prior adaptation, it is unusual for human influenza viruses to be lethal for mice after intranasal inoculation (24). Data for avian viruses are more limited, but the H5N1/97 virus and one of its precursors (H9N2, Qa/HK/G1/97) cause lethal infection of mice and spread to the brain, whereas other H9N2 and H5 subtype viruses do not (23, 25). Therefore, it is relevant that these new reassortant H5N1 viruses were lethal for mice without the need for prior adaptation, and that genotypes A, C, D, and E were able to spread to the brain. This finding raises the question whether pathogenicity and neurotropism for mice are markers of pathogenicity for mammals (including humans). If so, these viruses may have greater potential for transmission to mammals, and possibly, to man. The molecular correlates associated with the mouse neurotropism also deserve examination. The gene segments common to all genotypes but absent in the precursor Gs/Gd/96-like viruses isolated in 1999 are the PB2 and NS genes.
The reassortment events in 2001 provide an insight to early events that may have taken place in the genesis of H5N1/97. Although H5N1 Gs/Gd persisted in geese at least since 1996 onwards, and these viruses can infect chickens in an experimental setting (15), in nature, they were only able to cause widespread outbreaks in chickens after reassortment. Further, the dominant H5N1 genotype in Hong Kong's retail markets in 2001 (genotype A) had acquired a deletion in the stalk of the NA similar although not identical to that found in H5N1/97. It is reasonable to speculate that a sequence of events similar to those recorded here, albeit then undetected, occurred in the run-up to the H5N1 “bird flu” incident in 1997; i.e., that a reassortant H5N1 Gs/Gd-like virus crossed from its reservoir in geese to terrestrial poultry (especially chickens) before acquiring their internal protein genes through further reassortment with H9N2 (G1) and/or H6N1 (W312) viruses found in quail. In 1997, these viruses then amplified in Hong Kong's retail markets, leading to human infection and disease.
The segregation measures taken since 1998 to prevent introduction of live aquatic poultry into Hong Kong's live poultry retail markets and the serological screening of imported poultry successfully kept H5N1 Gs/Gd-like viruses out of the retail markets until 2001. But these new reassortants, possibly because of their wider host range and/or more efficient transmissibility, seem to pose a greater challenge. After the H5N1 outbreak in May 2001, a number of additional steps were taken to reduce further the risk of an H5N1/97-like virus reemerging. As quail are the main reservoir of the other precursors of H5N1/97, namely, Quail/HK/G1/97 and Teal/HK/W312/97-like viruses (17, 18), the sale of live quail together with other live poultry in Hong Kong's live poultry markets was prohibited by legislation, with effect from February 2002. In an effort to interrupt the amplification of viruses that might gain access to the retail markets, a once-a-month “rest-day” was introduced where the retail markets are completely emptied of poultry (any remaining poultry are slaughtered for sale as chilled carcasses); the markets are thoroughly cleaned and restocked the next day with freshly imported poultry.
Hong Kong, with its comprehensive avian influenza surveillance system, acts as a sentinel post for the wider region (10). It is likely that these H5N1 viruses are now widespread in the region. A third incursion of H5N1 reassortants into Hong Kong's retail markets and farms in 2002 (unpublished data) supports this assertion. If so, the multiplicity of H5N1 genotypes now circulating in terrestrial poultry in the wider region increases the opportunity for the emergence of potential pandemic strains through further reassortment, provided they develop the ability to infect humans with efficient human-to-human transmission. Although it is not direct proof of the ability to cause disease in humans, the capability of these avian reassortants, without prior adaptation, to cause lethal infections in mice and spread to the brain is unusual, suggesting that they may have an extended mammalian host range, an additional cause for concern.
In 2001, prospective surveillance provided early warning and allowed preemptive intervention, something not possible in 1997. This occasion is the first where control measures have been taken to prevent the reemergence of an influenza virus potentially hazardous to humans—a significant step in pandemic preparedness and probably the ultimate level of prevention of a zoonotic disease. However, measures in Hong Kong are not likely to reduce the possibility of such a virus arising in the wider region of East Asia including southern China that has been recognized as an influenza epicenter (4). The recent reassortment events documented in H5N1 viruses, therefore, justify renewed pandemic concern.
Supplementary Material
Acknowledgments
We acknowledge the assistance of P. Ghose, N. Kung, and J. M. Lou at the University of Hong Kong; S. M. Li, C. H. Li, W. M. Poon, and C. H. Chow at the Agriculture, Fisheries, and Conservation Department, Hong Kong SAR for assisting with the virus isolation and epidemiology; and J. Humberd and P. Seiler at St. Jude Children's Research Hospital, Memphis, TN for assistance with the animal pathogenicity studies. These studies were supported by Public Health Research Grant AI95357 from the National Institute of Allergy and Infectious Diseases, Wellcome Trust Grant 057476/Z/99/Z, and Grant HKU/7334/01M from the Research Grants Council of Hong Kong.
Abbreviations
- HA
hemagglutinin
- NP
nucleoprotein
- NA
neuraminidase
- NS
nonstructural
- Gs
goose
- Ck
chicken
- Dk
duck
- Qa
quail
- Ph
pheasant
- Pg
pigeon
- HK
Hong Kong
Footnotes
References
- 1.Kilbourne E D. J Infect Dis. 1973;127:478–487. doi: 10.1093/infdis/127.4.478. [DOI] [PubMed] [Google Scholar]
- 2.Webster R G, Bean W J, Gorman O T, Kawaoka Y. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shortridge K F. Semin Respir Infect. 1992;7:11–25. [PubMed] [Google Scholar]
- 4.Shortridge K F, Stuart-Harris C H. Lancet. 1982;ii:812–813. doi: 10.1016/s0140-6736(82)92693-9. [DOI] [PubMed] [Google Scholar]
- 5.Claas E C J, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 6.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 7.Yuen K Y, Chan P K S, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, et al. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 8.Hatta M, Gao P, Halfmann P, Kawaoka Y. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- 9.Webster R G. Science. 2001;293:1773–1775. doi: 10.1126/science.1065206. [DOI] [PubMed] [Google Scholar]
- 10.Shortridge K F, Gao P, Guan Y, Ito T, Kawaoka Y, Markwell D, Takeda A, Webster R G. Vet Microbiol. 2000;74:141–147. doi: 10.1016/s0378-1135(00)00174-7. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Subbarao K, Cox N, Guo Y. Virology. 1999;261:15–19. doi: 10.1006/viro.1999.9820. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Shortridge K F, Krauss S, Webster R G. Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann E, Stech J, Leneva I, Krauss S, Scholtissek C, Chin P S, Peiris M, Shortridge K F, Webster R G. J Virol. 2000;74:6309–6315. doi: 10.1128/jvi.74.14.6309-6315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauthen A N, Swayne D E, Shultz-Cherry S, Perdue M L, Suarez D L. J Virol. 2000;74:6592–6599. doi: 10.1128/jvi.74.14.6592-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster R G, Guan Y, Peiris M, Walker D, Krauss D, Zhou N N, Govorkova E A, Ellis T M, Dyrting K C, Sit T, et al. J Virol. 2002;76:118–126. doi: 10.1128/JVI.76.1.118-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Y, Peiris M, Kong K F, Dyrting K C, Ellis T M, Sit T, Zhang L J, Shortridge K F. Virology. 2002;292:16–23. doi: 10.1006/viro.2001.1207. [DOI] [PubMed] [Google Scholar]
- 17.Guan Y, Shortridge K F, Krauss S, Chin P S, Dyrting K C, Ellis T M, Webster R G, Peiris M. J Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin P S, Hoffmann E, Webby R, Webster R G, Guan Y, Peiris M, Shortridge K F. J Virol. 2002;76:507–516. doi: 10.1128/JVI.76.2.507-516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senne D A, Panigrahy B, Kawaoka Y, Pearson J E, Suss J, Lipkind M, Kida H, Webster R G. Avian Dis. 1996;40:425–437. [PubMed] [Google Scholar]
- 20.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, et al. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 21.Dowdle W R, Schild G C. In: The Influenza Viruses and Influenza. Kilbourne E D, editor. New York: Academic; 1975. pp. 243–268. [Google Scholar]
- 22.Dybing J K, Schultz-Cherry S, Swayne D, Suarez D L, Perdue M L. J Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y J, Krauss S, Senne D A, Mo I P, Lo K S, Xiong X P, Norwood M, Shortridge K F, Webster R G, Guan Y. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- 24.Bosch F X, Orlich M, Klenk H D, Rott R. Virology. 1979;95:197–207. doi: 10.1016/0042-6822(79)90414-8. [DOI] [PubMed] [Google Scholar]
- 25.Matrosovich M, Zhou N, Kawaoka Y, Webster R. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.