Abstract
1. Inactivation of the Ca channels has been examined in isolated nerve cell bodies of Helix aspersa using the suction pipette method for voltage clamp and internal perfusion.
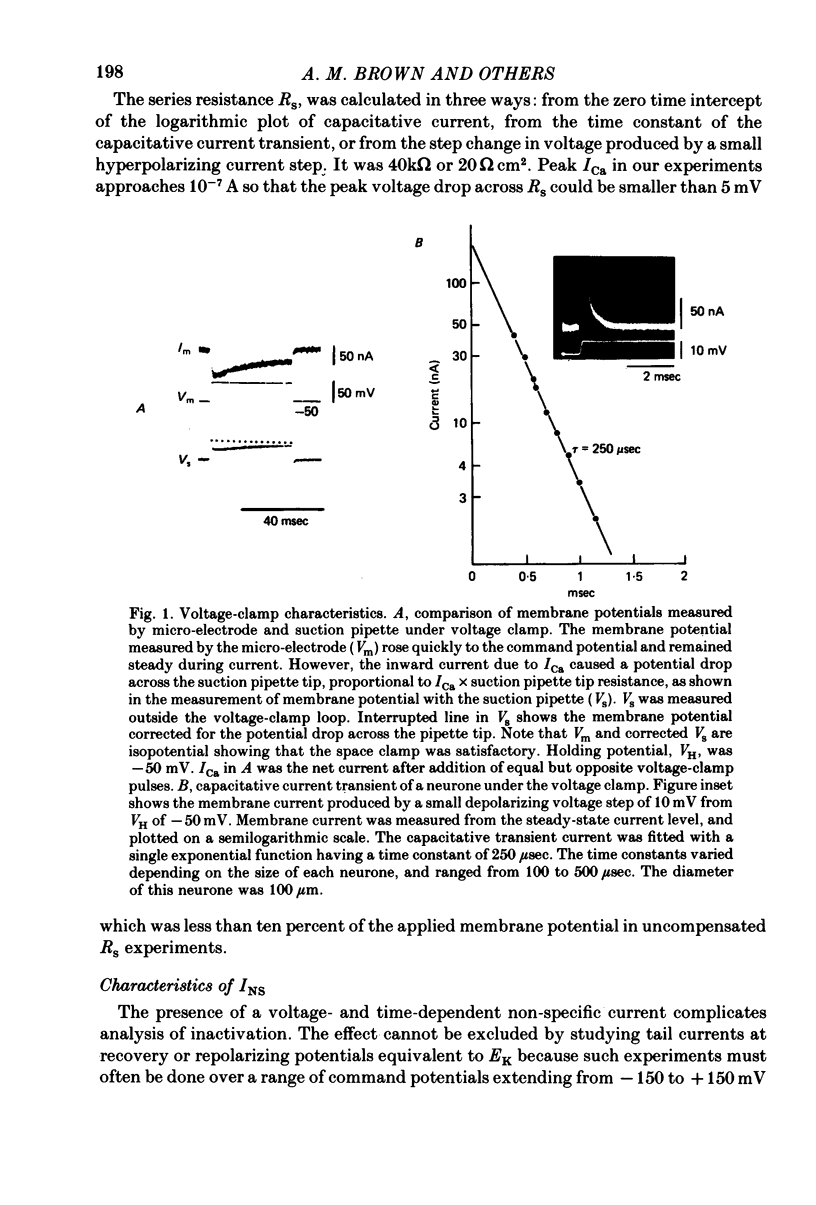
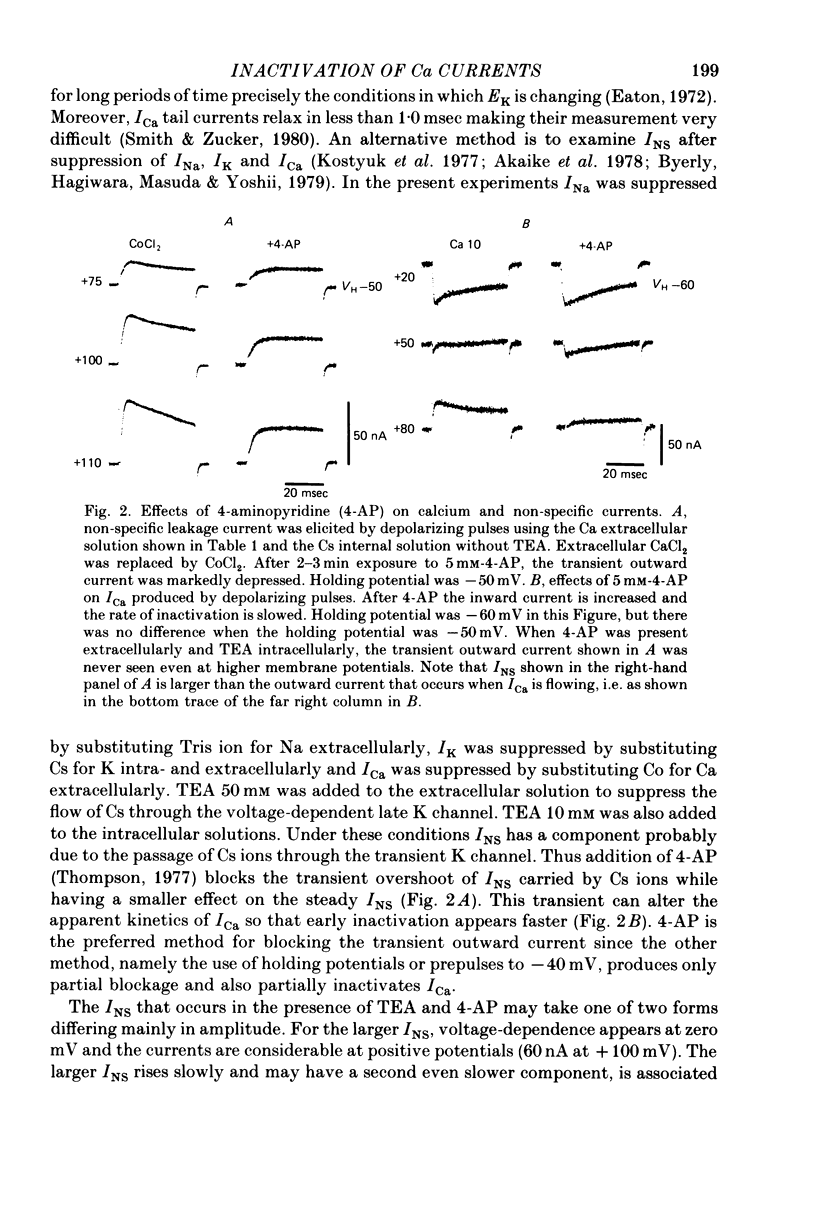
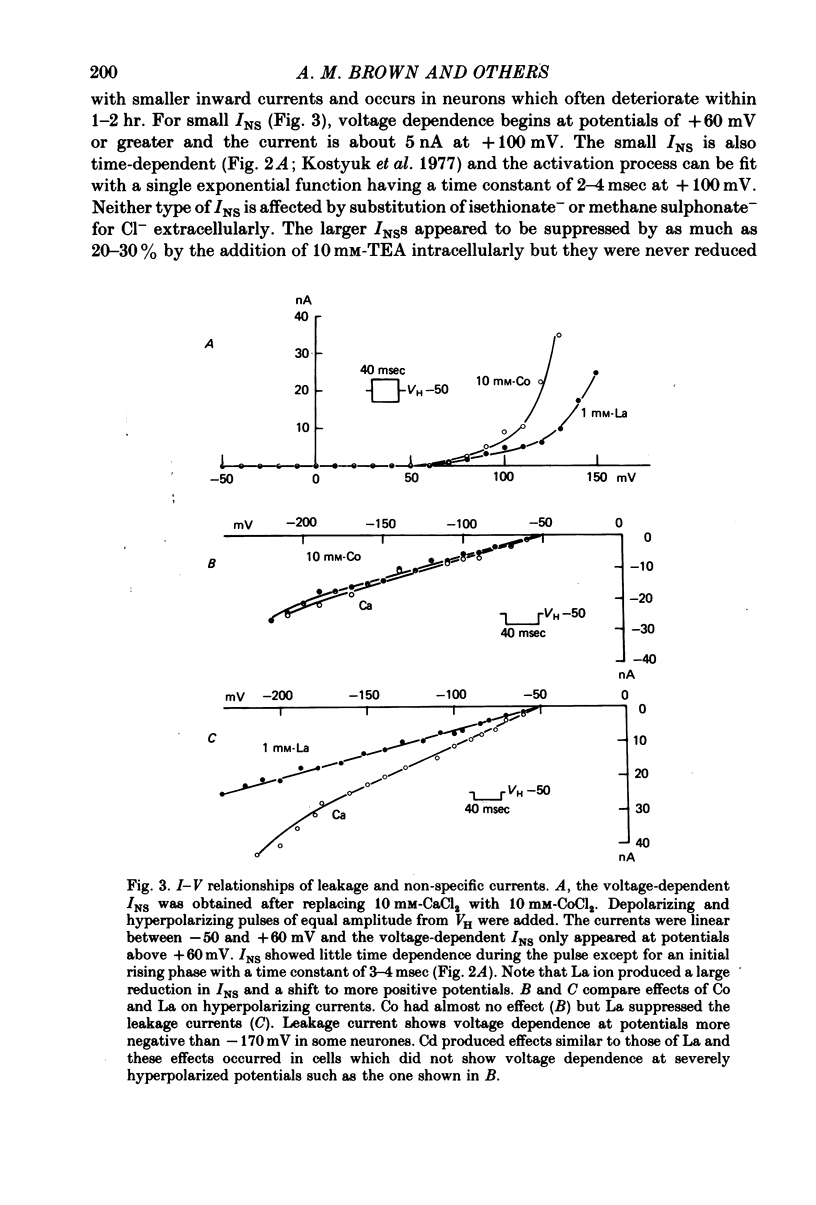
2. Satisfactory suppression of outward currents was essential. This was achieved over most of the voltage range by substitution of Cs ion for K ion and by the use of TEA intra- and extracellularly and 4-AP extracellularly. A small time- and voltage-dependent non-specific current remained at potentials above +60 mV.
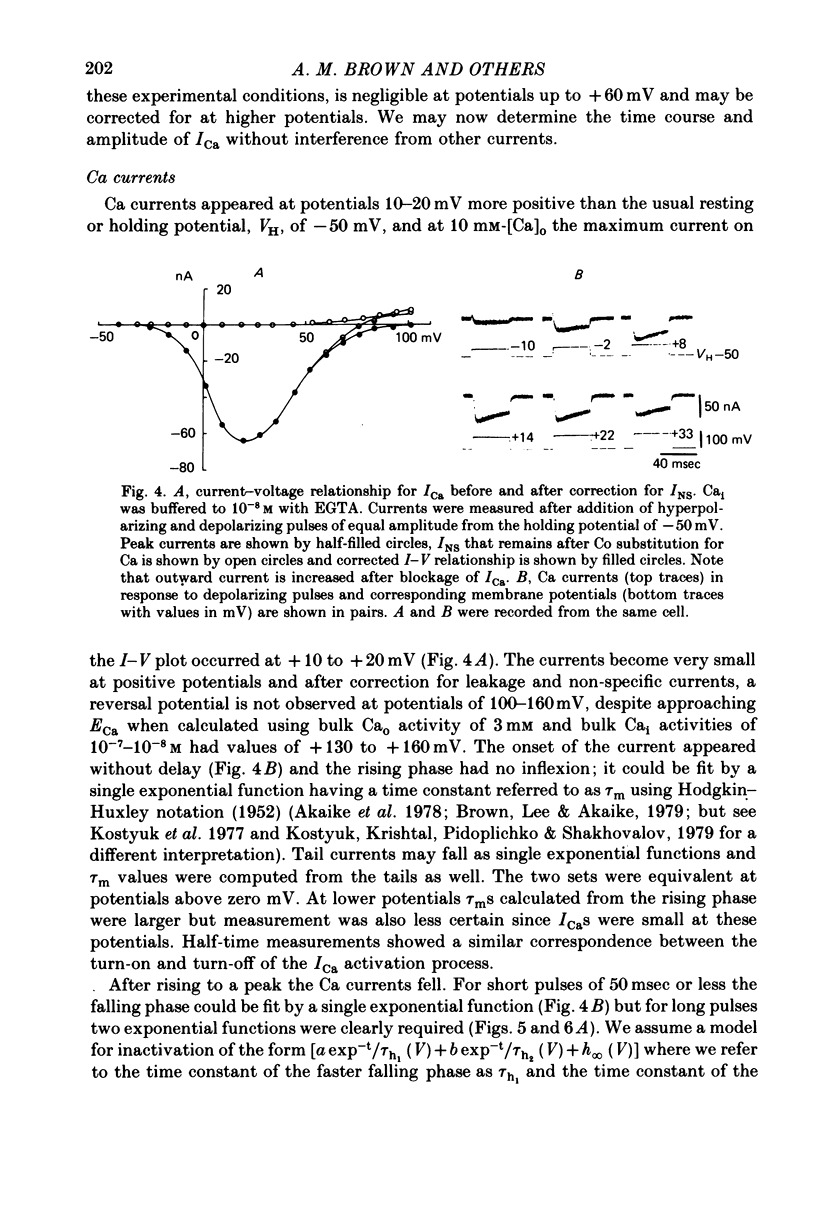
3. In these solutions, Ca current approaches ECa but cannot be detected in the outward direction. The Ca channel appears to be impermeable to Cs and Tris ions.
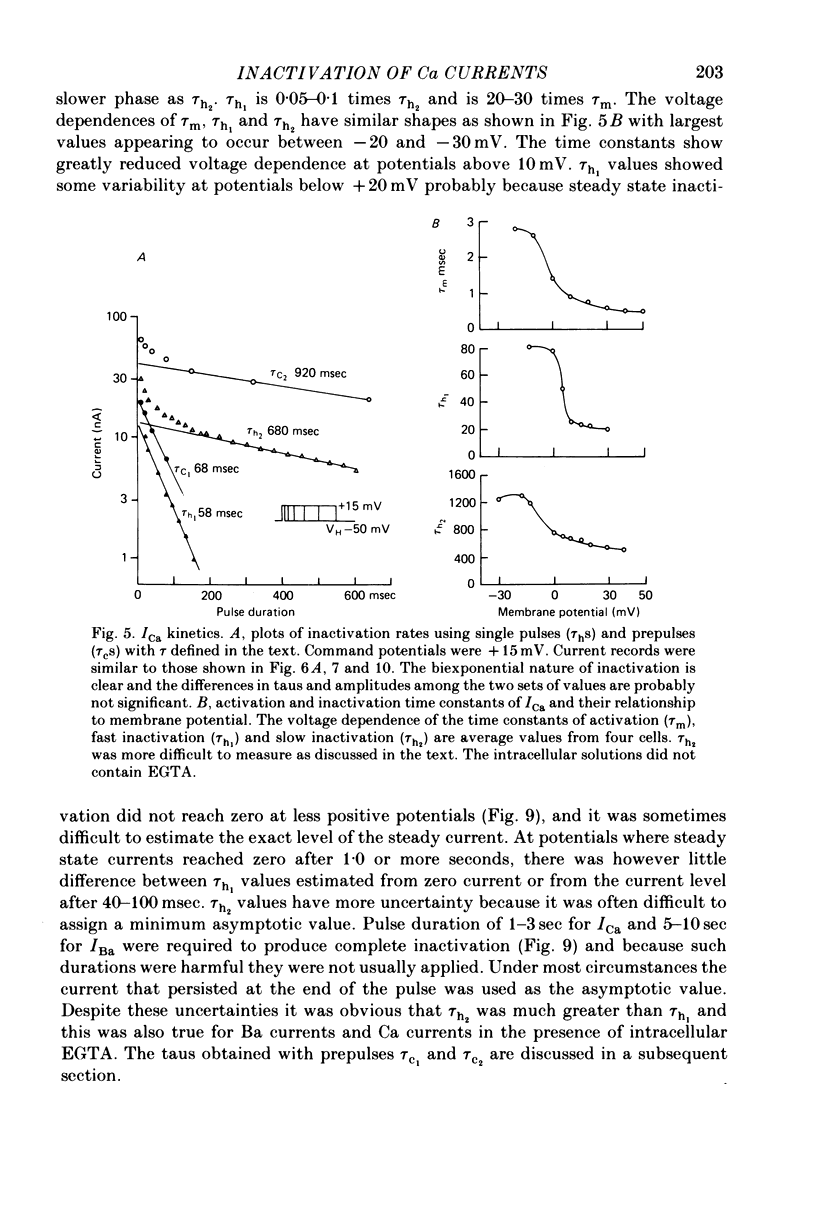
4. Inactivation of Ca currents occurs as a bi-exponential process. The faster rate is 10-20 times the slower rate and is about one twentieth the rate of activation. The development of inactivation during a single voltage-clamp step and the onset of inactivation produced by prepulses followed after brief intervals by a test pulse, have roughly similar time courses.
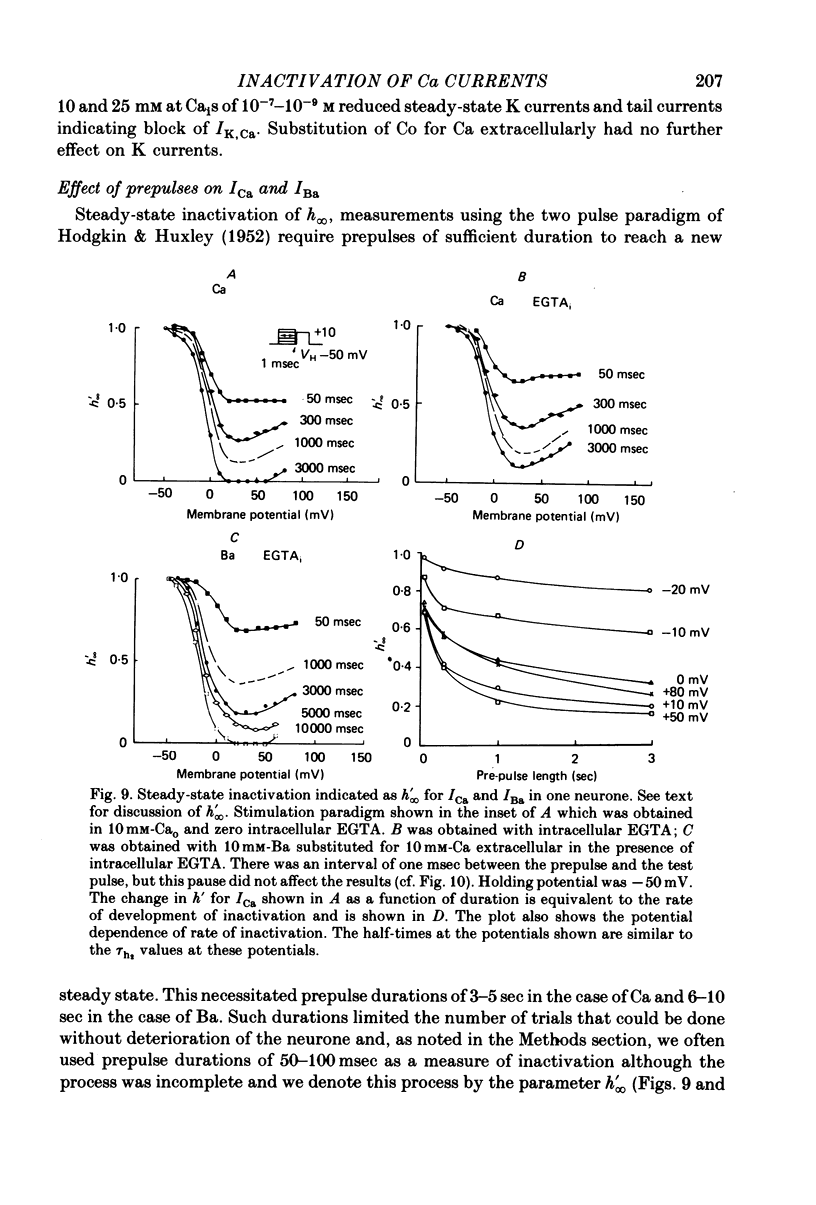
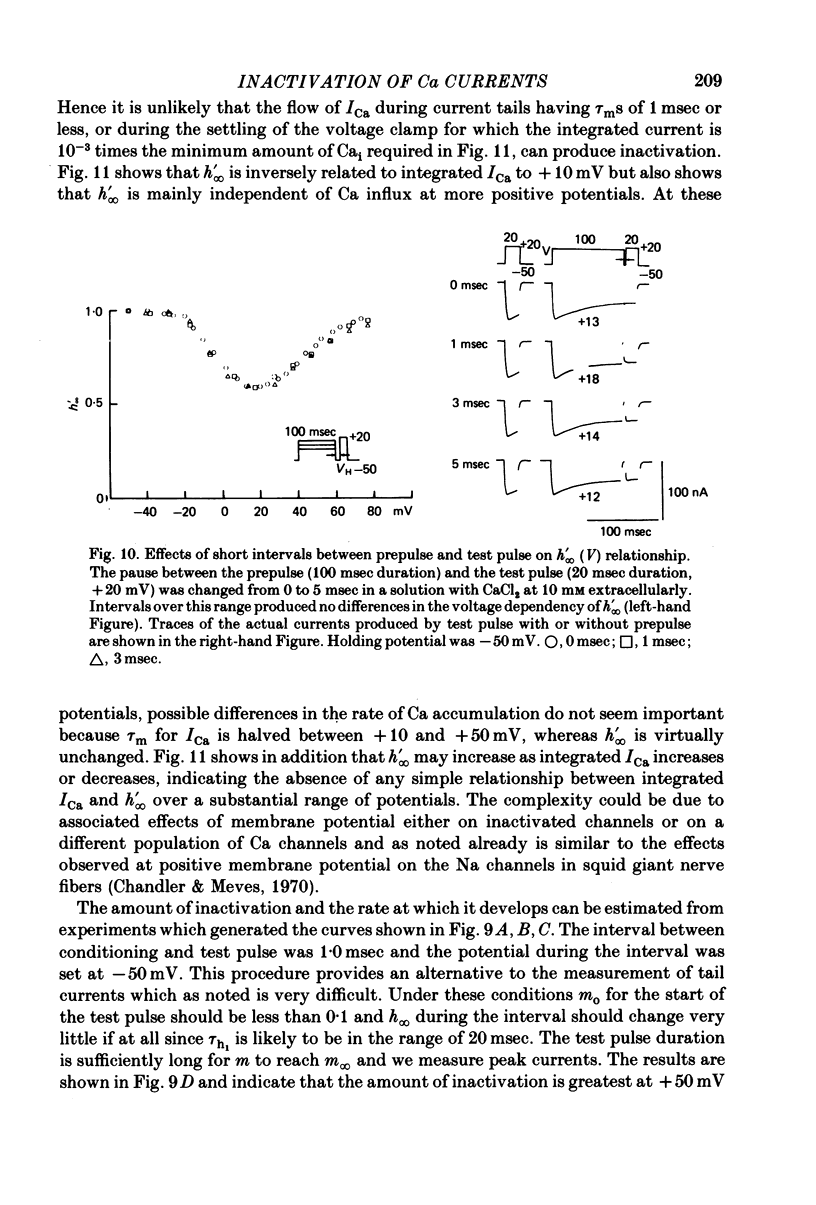
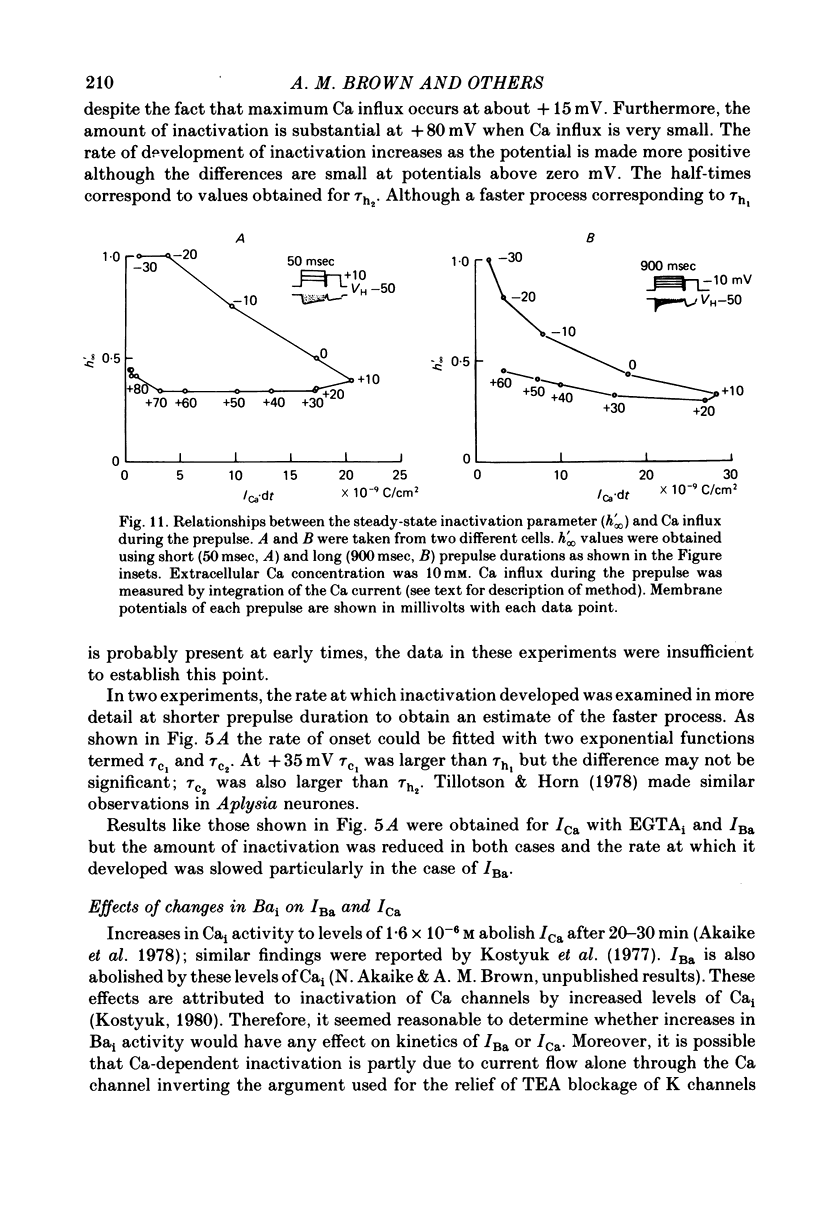
5. The rates of inactivation increase monotonically at potentials more positive than about -25 mV. The amount of steady-state inactivation increases with membrane depolarizations to potentials of about +50 mV. At more positive potentials, steady-state inactivation is reduced.
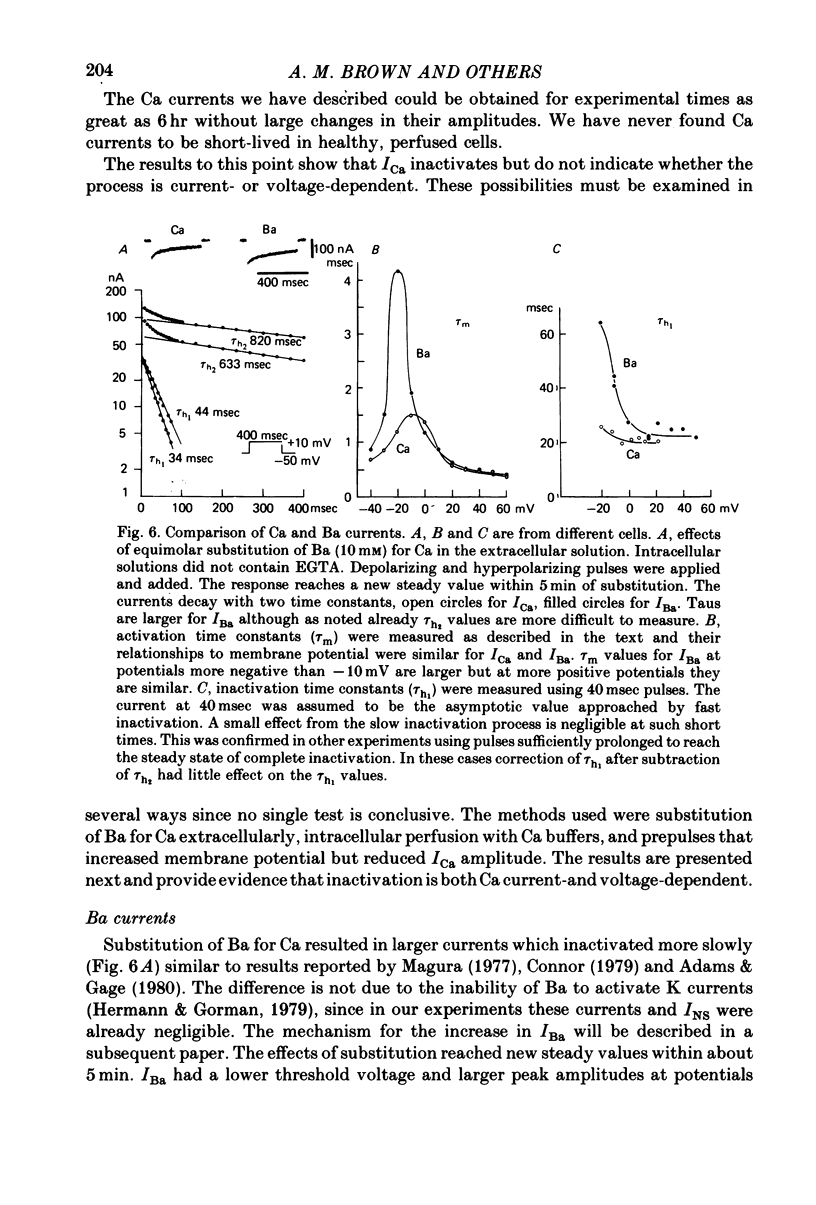
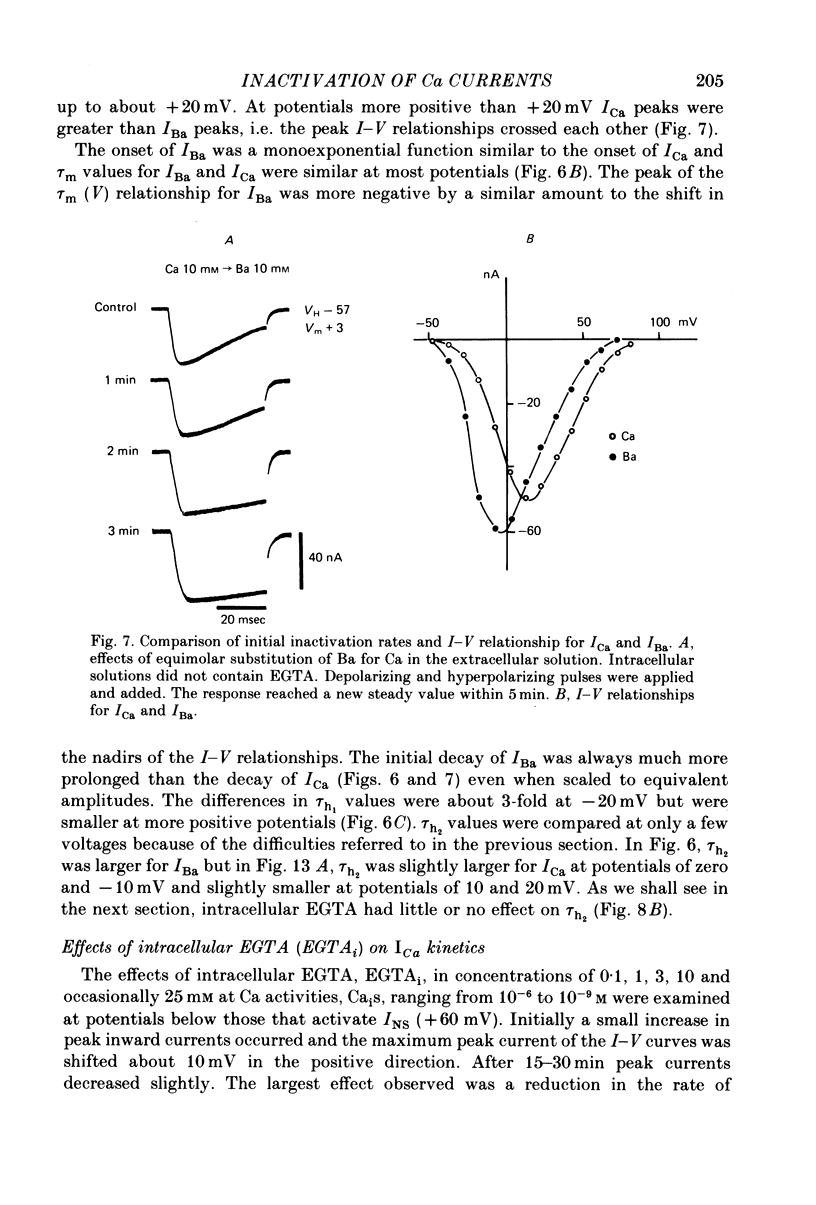
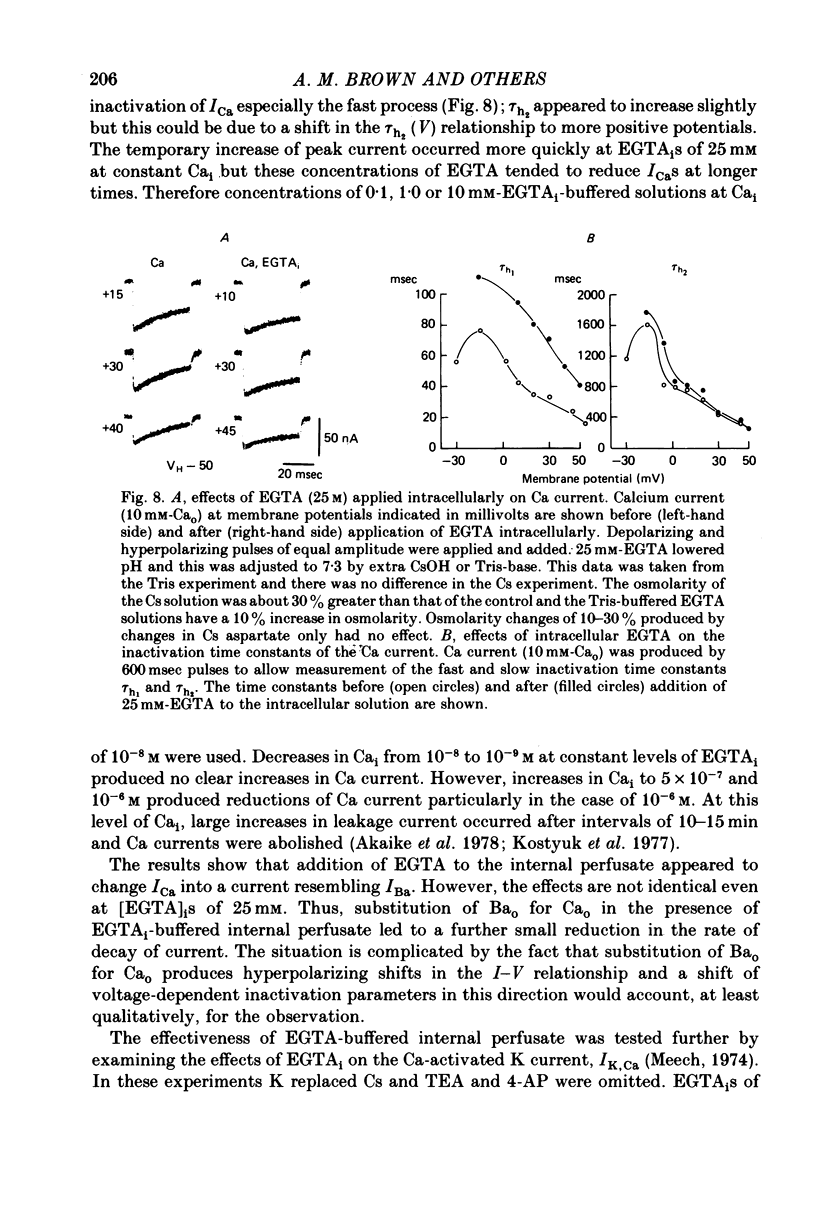
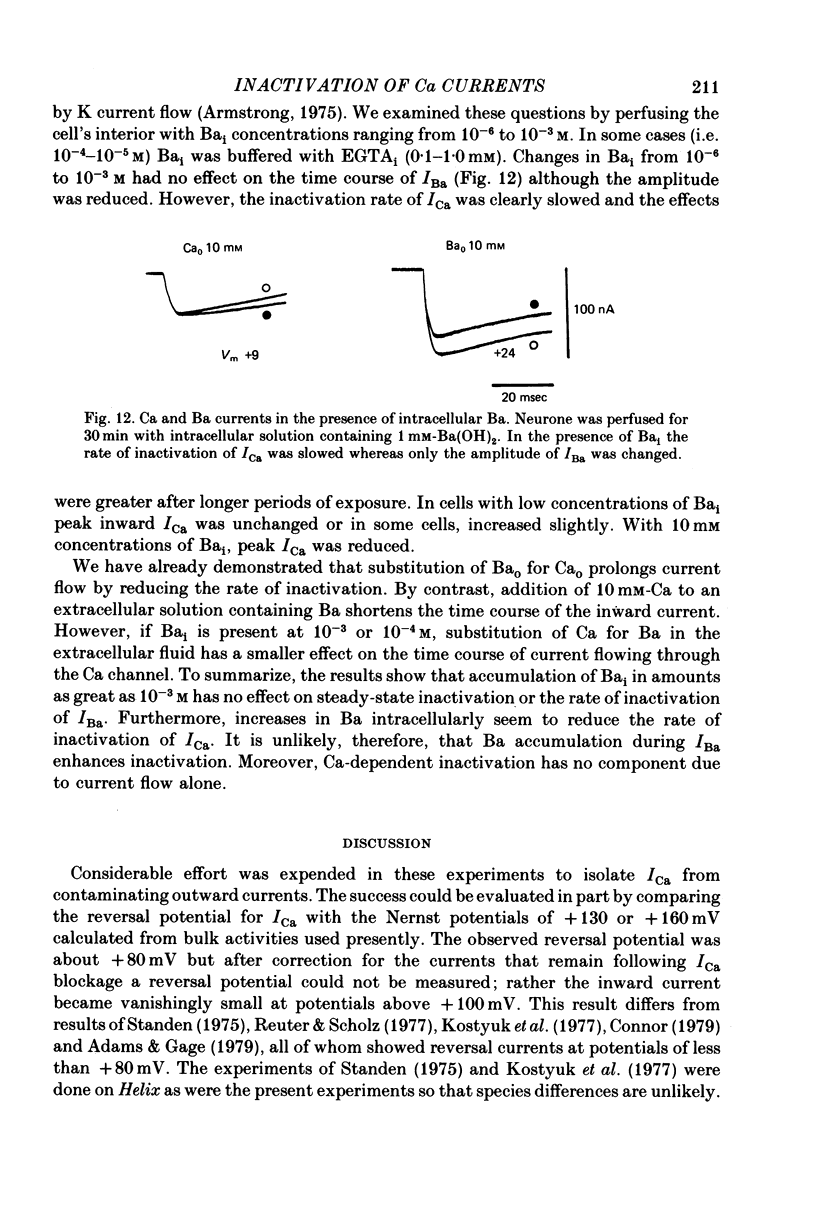
6. Intracellular EGTA slows the faster rate of inactivation of ICa and reduces the amount of steady-state inactivation measured with a standard two pulse protocol. The effect is specifically related to Ca chelation and hydrogen ions are not involved. This component of inactivation is referred to as Ca current-dependent inactivation and is consistent with observations that increased Cai inactivates the Ca channel. The process does not depend upon current flow alone since Ba currents of comparable or greater magnitude have smaller initial rates of inactivation. Furthermore, application of Ba ion intracellularly in large concentrations has no effect on steady-state inactivation.
7. The bi-exponential inactivation process that persists in the presence of EGTAi is similar to that occurring when extracellular Ba ion carries current through the Ca channel. Steady-state inactivation also persists and is similar in the two cases. Therefore it is concluded that inactivation is voltage-dependent as well as Ca current-dependent.
8. Diffusion models that included reasonable values for the effect of binding on diffusion, even when combined with declining influxes, did not account for this `mixed' form of calcium- and voltage-dependent inactivation. A compartmental model in which the particular kinetic model of voltage-dependent inactivation was not critical described the Ca current-dependent inactivation.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. J., Gage P. W. Divalent ion currents and the delayed potassium conductance in an Aplysia neurone. J Physiol. 1980 Jul;304:297–313. doi: 10.1113/jphysiol.1980.sp013325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen M. C., Brown A. M., Yasui S. The role of diffusion in the photoresponse of an extraretinal photoreceptor of Aplysia. J Physiol. 1979 Feb;287:283–301. doi: 10.1113/jphysiol.1979.sp012659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Potassium pores of nerve and muscle membranes. Membranes. 1975;3:325–358. [PubMed] [Google Scholar]
- Ashcroft F. M., Standen N. B., Stanfield P. R. Calcium currents in insect muscle [proceedings]. J Physiol. 1979 Jun;291:51P–52P. [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R., Tillotson D. Calcium-mediated inactivation of calcium current in Paramecium. J Physiol. 1980 Sep;306:193–203. doi: 10.1113/jphysiol.1980.sp013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Akaike N., Tsuda Y., Morimoto K. Ion migration and inactivation in the calcium channel. J Physiol (Paris) 1980 Sep;76(5):395–402. [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Time course separation of two inward currents in molluscan neurons. Brain Res. 1977 Jan 7;119(2):487–492. doi: 10.1016/0006-8993(77)90330-4. [DOI] [PubMed] [Google Scholar]
- Eaton D. C. Potassium ion accumulation near a pace-making cell of Aplysia. J Physiol. 1972 Jul;224(2):421–440. doi: 10.1113/jphysiol.1972.sp009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. Potassium activation associated with intraneuronal free calcium. Science. 1978 Apr 28;200(4340):437–439. doi: 10.1126/science.644308. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M. P., Reese T. S., Brinley F. J., Jr Endoplasmic reticulum sequesters calcium in the squid giant axon. Science. 1978 Dec 22;202(4374):1300–1303. doi: 10.1126/science.725607. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Lambert J. D., Gayton R. J., Loker J. E., Walker R. J. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A Comp Physiol. 1975 Jan 1;50(1A):1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium ionic channels in electrically excitable membrane. Neuroscience. 1980;5(6):945–959. doi: 10.1016/0306-4522(80)90178-5. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I., Shakhovalov YuA Kinetics of calcium inward current activation. J Gen Physiol. 1979 May;73(5):675–680. doi: 10.1085/jgp.73.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Magura I. S. Long-lasting inward current in snail neurons in barium solutions in voltage-clamp conditions. J Membr Biol. 1977 Jul 14;35(3):239–256. doi: 10.1007/BF01869952. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. Voltage-clamp studies of the calcium inward current in an identified snail neurone: comparison with the sodium inward current. J Physiol. 1975 Jul;249(2):253–268. doi: 10.1113/jphysiol.1975.sp011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]


