Abstract
1. The effect of ouabain on cellular volume recovery in rabbit, guinea-pig and rat renal cortical slices was studied. A concentration of ouabain that is maximally effective in inhibiting slice potassium accumulation was determined for each species. Slices from each species were either freshly prepared and then incubated, or leached and then incubated, or preincubated in oxygenated ordinary medium (equilibrated), leached and then reincubated in media with and without this concentration of ouabain. All incubations were at 25 °C.
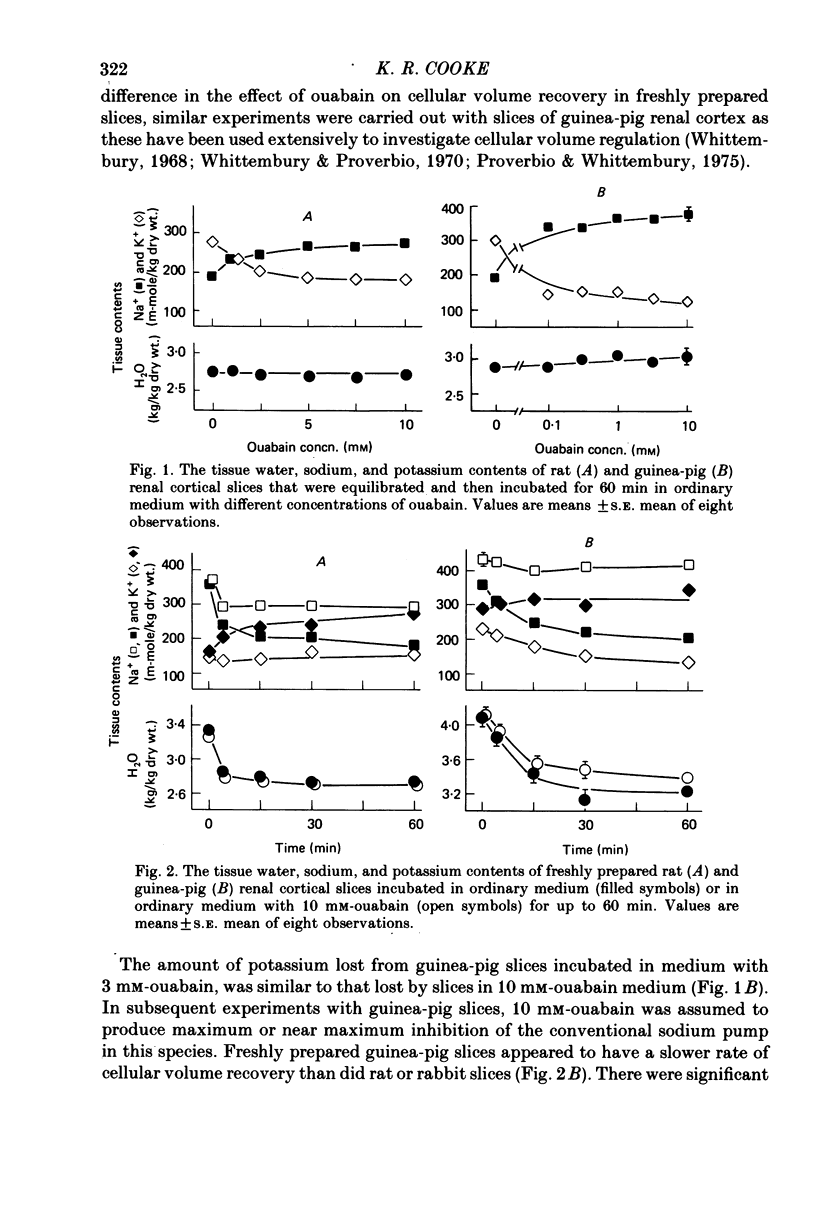
2. Potassium loss produced by ouabain was greater in rabbit and guinea-pig slices than in rat slices.
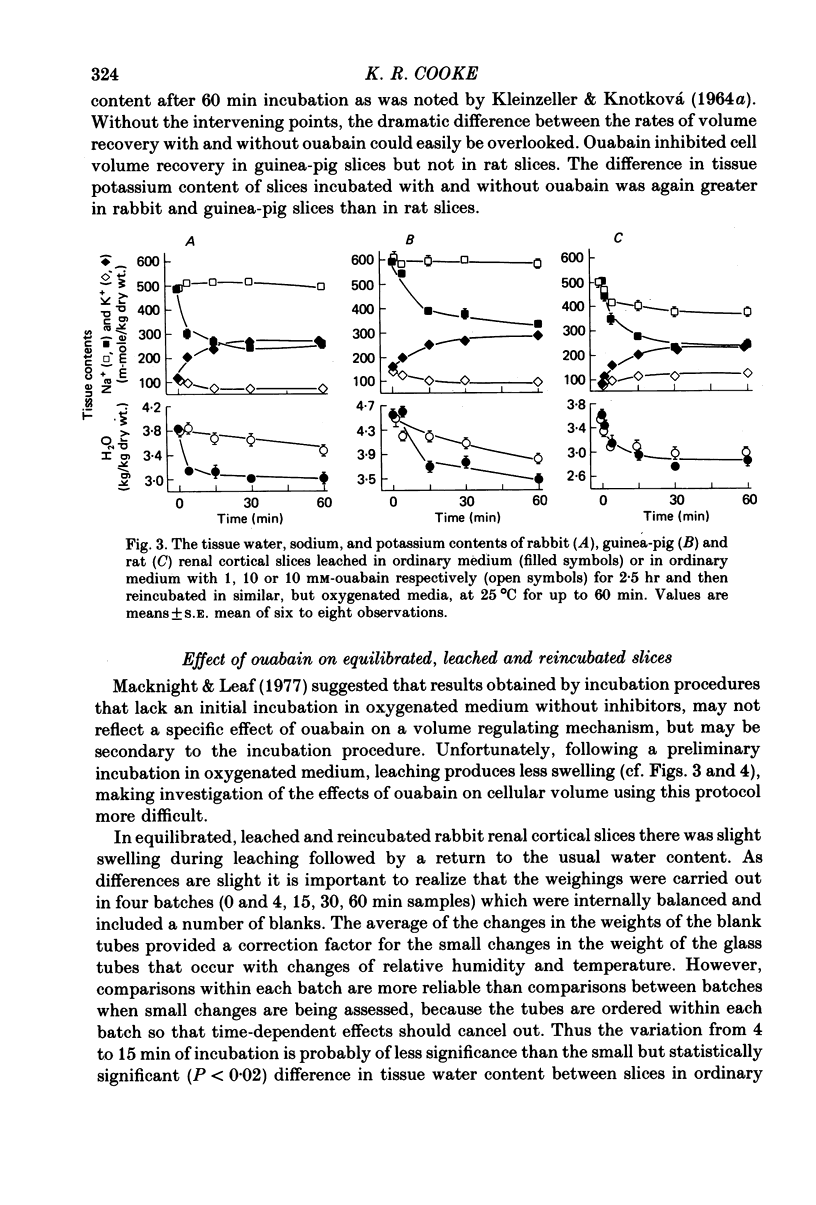
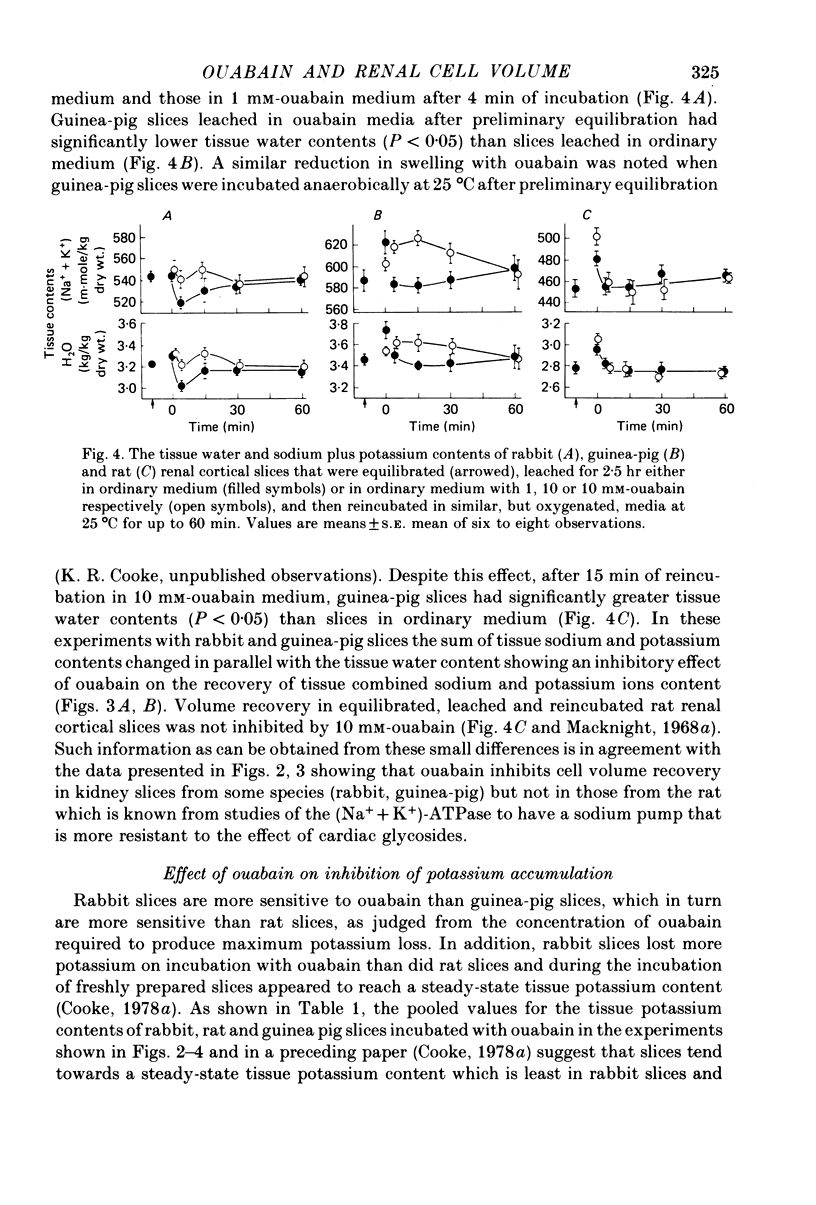
3. With slices that were freshly prepared and then incubated, and with slices that were leached and then incubated, cellular volume recovery was inhibited by ouabain in rabbit and guinea-pig slices, but not in rat slices.
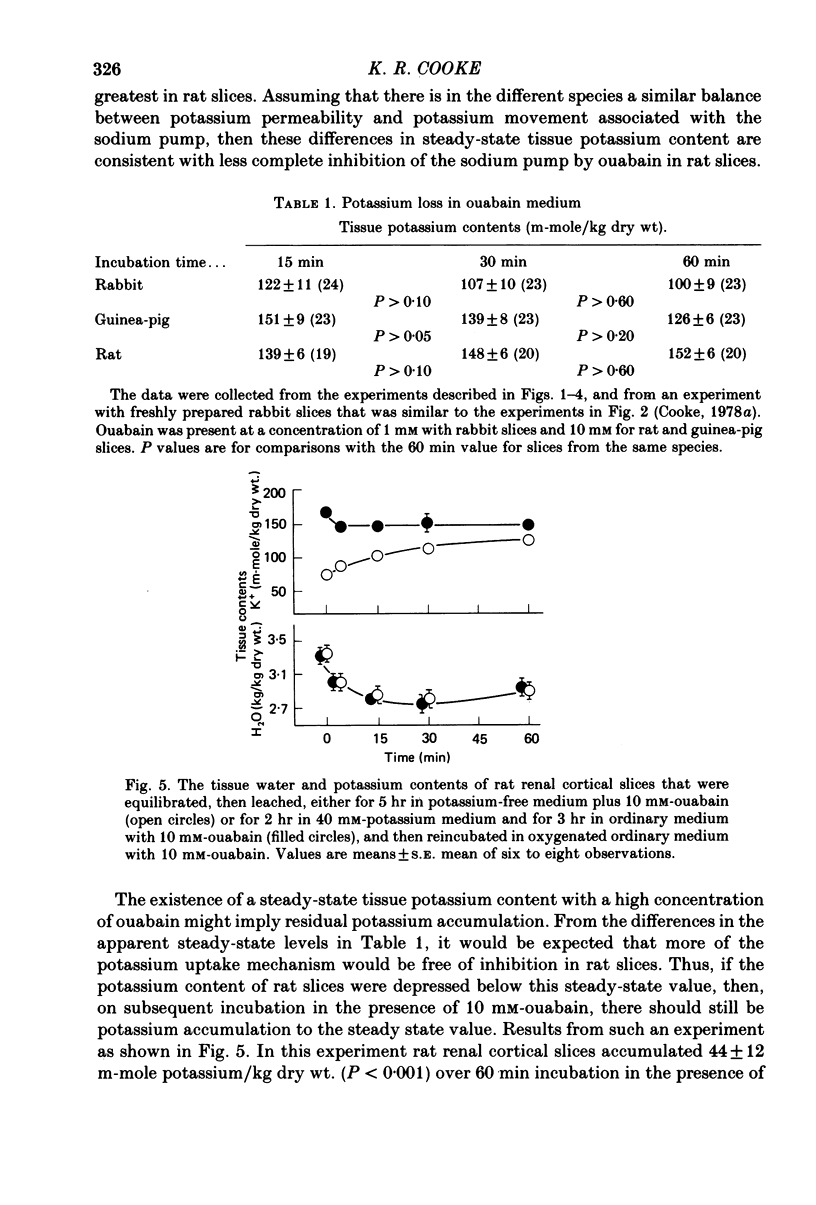
4. After equilibration, swelling during leaching was less, especially in rabbit and guinea-pig slices. However, on subsequent reincubation, significant differences in tissue water and cation contents that were consistent with inhibition of cellular volume recovery by ouabain, were seen in slices from these two species, but not in rat slices.
5. Slices from all three species, when incubated with concentrations of ouabain that were maximally effective in inhibiting potassium accumulation, appeared to approach a steady-state tissue potassium content that was greatest in rat slices and least in rabbit slices. Rat slices, previously depleted of potassium, reaccumulated potassium in the presence of 10 mm-ouabain to reach this steady-state potassium content.
6. Despite superficial appearance to the contrary (especially in the case of rat slices) these results are consistent with a major role for the conventional pump in controlling cortical cell volume. They do not provide evidence for the postulate that renal cortical cells possess a separate, ouabain-insensitive mechanism regulating cell volume.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Brody T. M., So R. H., Tobin T., Baskin S. I. Factors and agents that influence cardiac glycoside-Na+, K+-ATPase interaction. Ann N Y Acad Sci. 1974;242(0):617–634. doi: 10.1111/j.1749-6632.1974.tb19121.x. [DOI] [PubMed] [Google Scholar]
- Akera T., Brody T. M. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977 Sep;29(3):187–220. [PubMed] [Google Scholar]
- Allen J. C., Schwartz A. A possible biochemical explanation for the insensitivity of the rat to cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):42–46. [PubMed] [Google Scholar]
- Boulpaep E. L. Electrical phenomena in the nephron. Kidney Int. 1976 Feb;9(2):88–102. doi: 10.1038/ki.1976.14. [DOI] [PubMed] [Google Scholar]
- Cooke K. R. Effect of the CO-2-bicarbonate buffer system on the water and ion contents of rat renal cortical slices. Biochim Biophys Acta. 1976 Jun 23;437(1):280–288. doi: 10.1016/0304-4165(76)90371-8. [DOI] [PubMed] [Google Scholar]
- Cooke K. R. Ouabain and regulation of cellular volume in freshly prepared slices of rabbit renal cortex. J Physiol. 1978 Jun;279:361–374. doi: 10.1113/jphysiol.1978.sp012349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler D. K. Comparative pharmacology of cardiac glycosides. Fed Proc. 1967 Jul-Aug;26(4):1119–1124. [PubMed] [Google Scholar]
- Erdmann E., Schoner W. Ouabain-receptor interactions in (Na + +K + )-ATPase preparations from different tissues and species. Determination of kinetic constants and dissociation constants. Biochim Biophys Acta. 1973 May 11;307(2):386–398. doi: 10.1016/0005-2736(73)90104-1. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Graf J., Giebisch G. Intracellular sodium activity and sodium transport in necturus gallbladder epithelium. J Membr Biol. 1979 Jun 7;47(4):327–355. doi: 10.1007/BF01869743. [DOI] [PubMed] [Google Scholar]
- Györy A. Z., Brendel U., Kinne R. Effect of cardiac glycosides and sodium ethacrynate on transepithelial sodium transport in in vivo micropuncture experiments and on isolated plasma membrane Na-K ATPase in vitro of the rat. Pflugers Arch. 1972;335(4):287–296. doi: 10.1007/BF00586219. [DOI] [PubMed] [Google Scholar]
- Hughes P. M., Macknight D. C. The regulation of cellular volume in renal cortical slices incubated in hyposmotic medium. J Physiol. 1976 May;257(1):137–154. doi: 10.1113/jphysiol.1976.sp011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. ELECTROLYTE TRANSPORT IN RAT DIAPHRAGM. Physiol Bohemoslov. 1964;13:317–326. [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. THE EFFECT OF OUABAIN ON THE ELECTROLYTE AND WATER TRANSPORT IN KIDNEY CORTEX AND LIVER SLICES. J Physiol. 1964 Dec;175:172–192. doi: 10.1113/jphysiol.1964.sp007510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Spring K. R. Luminal Na+ entry into Necturus proximal tubule cells. Am J Physiol. 1979 Mar;236(3):F295–F301. doi: 10.1152/ajprenal.1979.236.3.F295. [DOI] [PubMed] [Google Scholar]
- LITTLE J. R. DETERMINATION OF WATER AND ELECTROLYTES IN TISSUE SLICES. Anal Biochem. 1964 Jan;7:87–95. doi: 10.1016/0003-2697(64)90122-8. [DOI] [PubMed] [Google Scholar]
- Linshaw M. A., Stapleton F. B. Effect of ouabain and colloid osmotic pressure on renal tubule cell volume. Am J Physiol. 1978 Nov;235(5):F480–F491. doi: 10.1152/ajprenal.1978.235.5.F480. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Pilgrim J. P., Robinson B. A. The regulation of cellular volume in liver slices. J Physiol. 1974 Apr;238(2):279–294. doi: 10.1113/jphysiol.1974.sp010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D. The effects of ethacrynic acid on the electrolyte and water contents of rat renal cortical slices. Biochim Biophys Acta. 1969 Mar 11;173(2):223–233. doi: 10.1016/0005-2736(69)90106-0. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. The extracellular space in rat renal cortical slices incubated at 0.5 degrees and 25 degrees. Biochim Biophys Acta. 1968 Aug;163(1):85–92. doi: 10.1016/0005-2736(68)90035-7. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Water and electrolyte contents of rat renal cortical slices incubated in potassium-free media and media containing ouabain. Biochim Biophys Acta. 1968 Mar 1;150(2):263–270. doi: 10.1016/0005-2736(68)90169-7. [DOI] [PubMed] [Google Scholar]
- Maude D. L. Stop-flow microperfusion of proximal tubules in rat kidney cortex slices. Am J Physiol. 1968 Jun;214(6):1315–1321. doi: 10.1152/ajplegacy.1968.214.6.1315. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver D. J., Raine A. E. The influence of electrolytes on the volume of non-metabolizing renal cortical cells. J Physiol. 1972 Sep;225(3):555–564. doi: 10.1113/jphysiol.1972.sp009955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio F., Whittembury G. Cell electrical potentials during enhanced sodium extrusion in guinea-pig kidney cortex slices. J Physiol. 1975 Sep;250(3):559–578. doi: 10.1113/jphysiol.1975.sp011070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari P. K., Daniel E. E., Paton D. M. Regulation of cellular volume in rat myometrium. Biochim Biophys Acta. 1973 Oct 11;323(2):297–308. doi: 10.1016/0005-2736(73)90153-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. W. The difference in sensitivity to cardiac steroids of (Na++K+)-stimulated ATPase and amino acid transport in the intestinal mucosa of the rat and other species. J Physiol. 1970 Jan;206(1):41–60. doi: 10.1113/jphysiol.1970.sp008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Frizzell R. A., Nellans H. N. ION transport by mammalian small intestine. Annu Rev Physiol. 1974;36:51–91. doi: 10.1146/annurev.ph.36.030174.000411. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Giebisch G. Kinetics of Na+ transport in Necturus proximal tubule. J Gen Physiol. 1977 Sep;70(3):307–328. doi: 10.1085/jgp.70.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin T., Brody T. M. Rates of dissociation of enzyme-ouabain complexes and K 0.5 values in (Na + + K + ) adenosine triphosphatase from different species. Biochem Pharmacol. 1972 Jun 1;21(11):1553–1560. doi: 10.1016/0006-2952(72)90305-x. [DOI] [PubMed] [Google Scholar]
- Tobin T., Henderson R., Sen A. K. Species and tissue differences in the rate of dissociation of ouabain from (Na+ + K+)-ATPase. Biochim Biophys Acta. 1972 Aug 9;274(2):551–555. doi: 10.1016/0005-2736(72)90201-5. [DOI] [PubMed] [Google Scholar]
- Van Os C. H., Slegers J. F. Characteristics of Naplus-Kplus-stimulated ATPase in rabbit gall bladder epithelium. Pflugers Arch. 1970;319(1):49–56. doi: 10.1007/BF00586427. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WILLIS J. S. ION MOVEMENTS AND OXYGEN CONSUMPTION IN KIDNEY CORTEX SLICES. J Physiol. 1963 Aug;168:158–177. doi: 10.1113/jphysiol.1963.sp007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTEMBURY G. SODIUM EXTRUSION AND POTASSIUM UPTAKE IN GUINEA PIG KIDNEY CORTEX SLICES. J Gen Physiol. 1965 Mar;48:699–717. doi: 10.1085/jgp.48.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittembury G., Grantham J. J. Cellular aspects of renal sodium transport and cell volume regulation. Kidney Int. 1976 Feb;9(2):103–120. doi: 10.1038/ki.1976.15. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]
- Whittembury G. Sodium and water transport in kidney proximal tubular cells. J Gen Physiol. 1968 May;51(5 Suppl):303S+–303S+. [PubMed] [Google Scholar]


