Abstract
1. Membrane potential was recorded intracellularly by micro-electrode in separated longitudinal muscle of guinea-pig ileum. Electrotonic potentials were evoked in longitudinal strips by passing current between large external electrodes in the partition chamber.
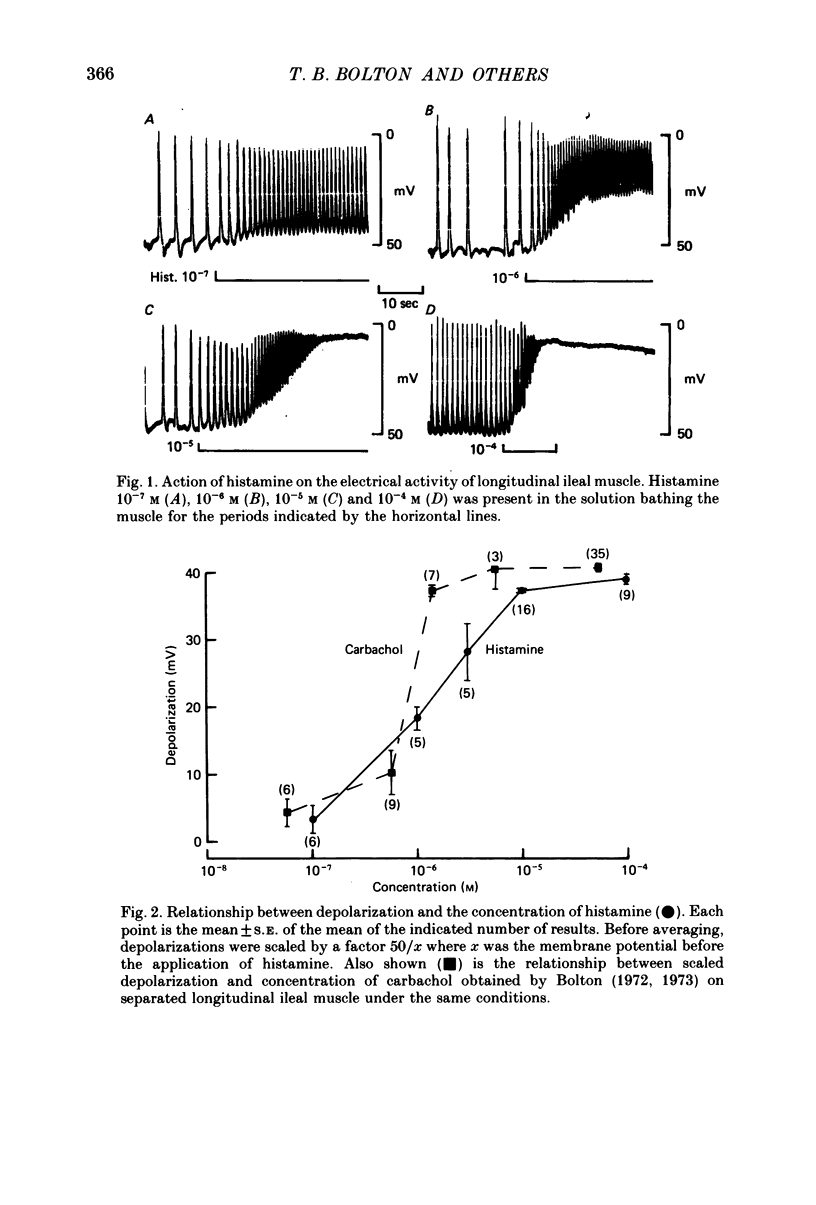
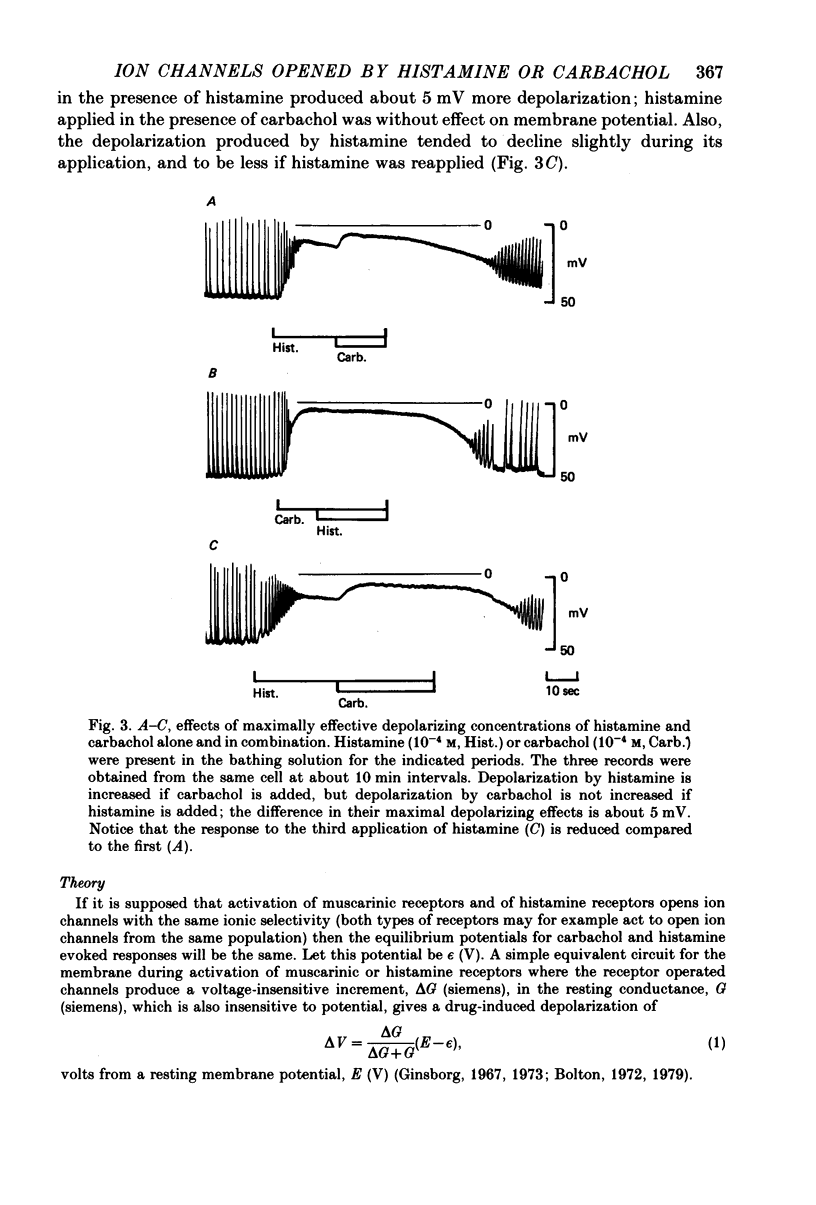
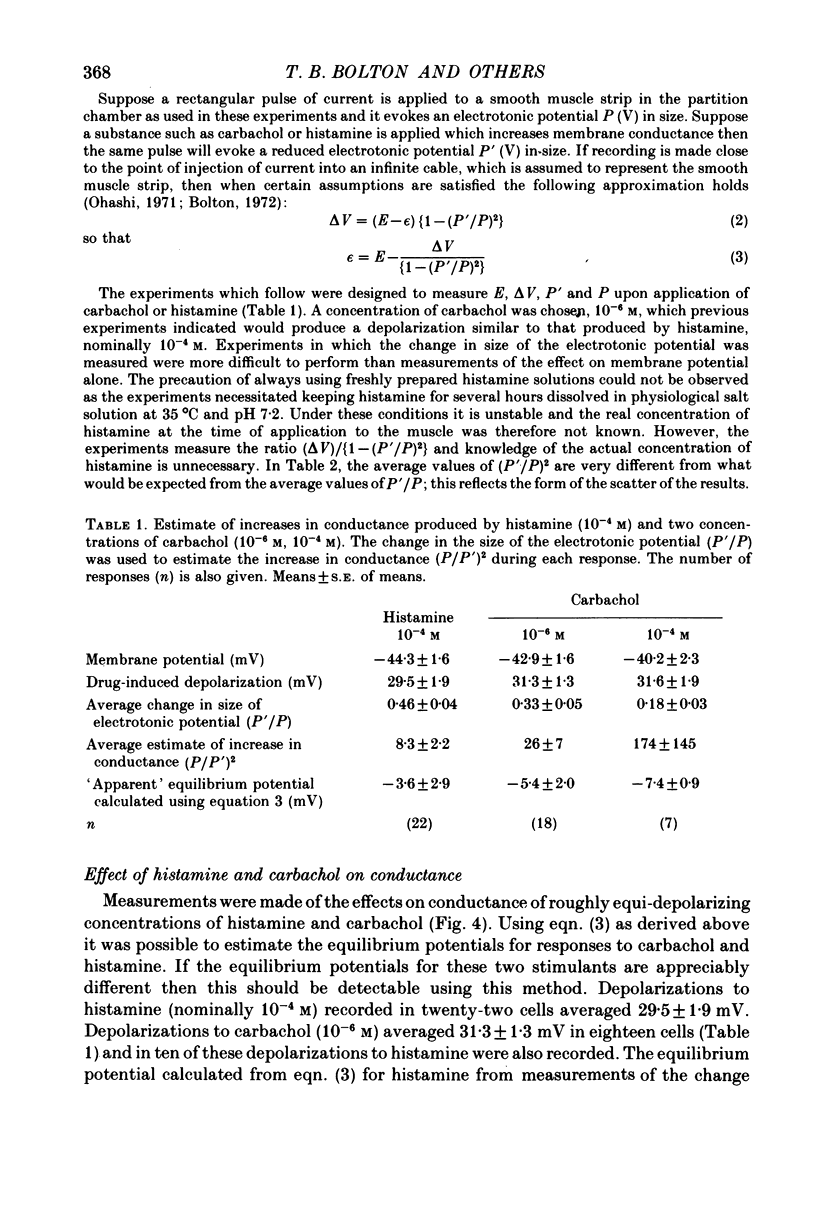
2. Histamine increased the frequency of action potential discharge at low concentrations and depolarized the membrane. At higher concentrations it caused substantial depolarization and action potential discharge was abolished. Carbachol had similar actions but the maximal depolarization by carbachol (using 10-4 m) was some 4-5 mV greater than maximal depolarization by histamine (using 10-4 m).
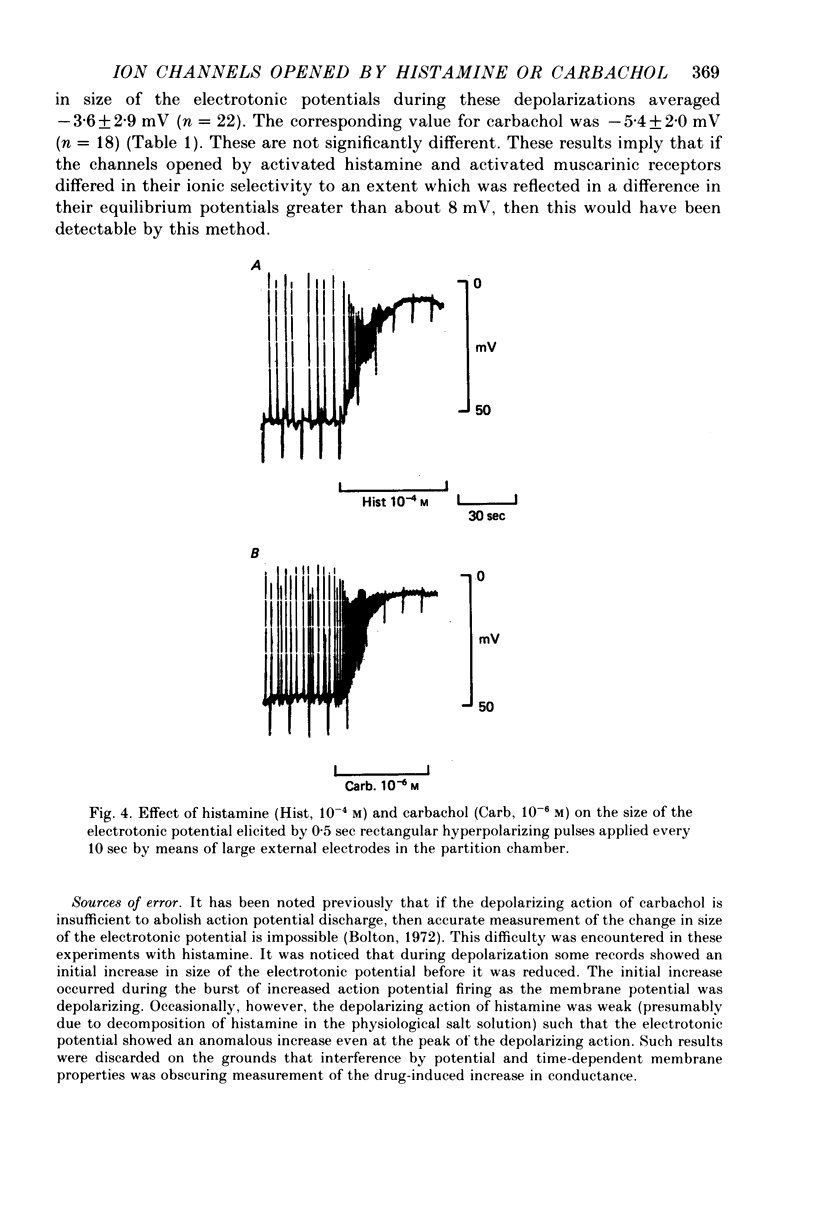
3. The change in size of evoked electrotonic potentials was used to estimate the effects of carbachol and histamine on the conductance of the smooth muscle membrane. The equilibrium potentials for histamine and carbachol depolarizations were estimated from their relative effects on potential and conductance and were found to be not significantly different; measurements of the effects on conductance showed that 10-4 m-histamine increased conductance about 8-fold whilst 10-4 m-carbachol had a much greater effect on conductance. This difference could explain the differing maximal depolarizing effects of these agents if both were assumed to open channels having the same ionic selectivity (i.e. equilibrium potential).
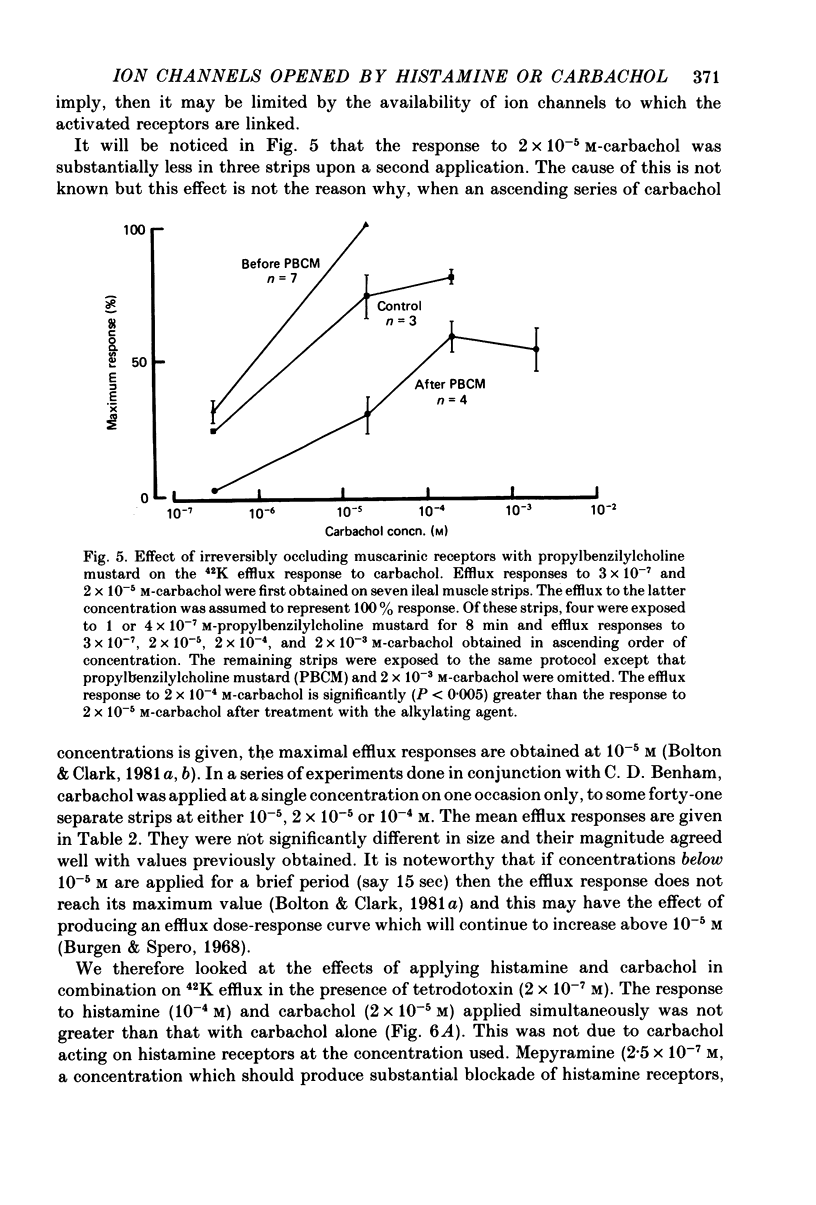
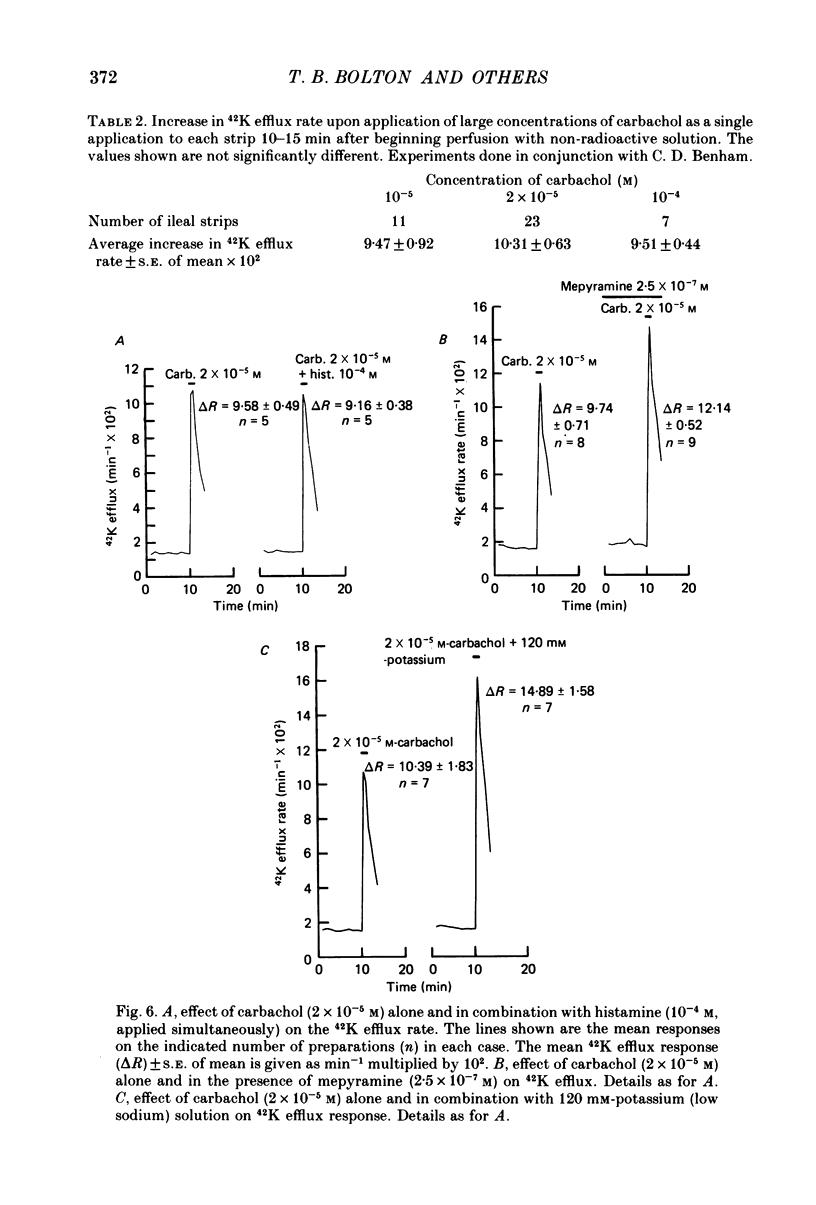
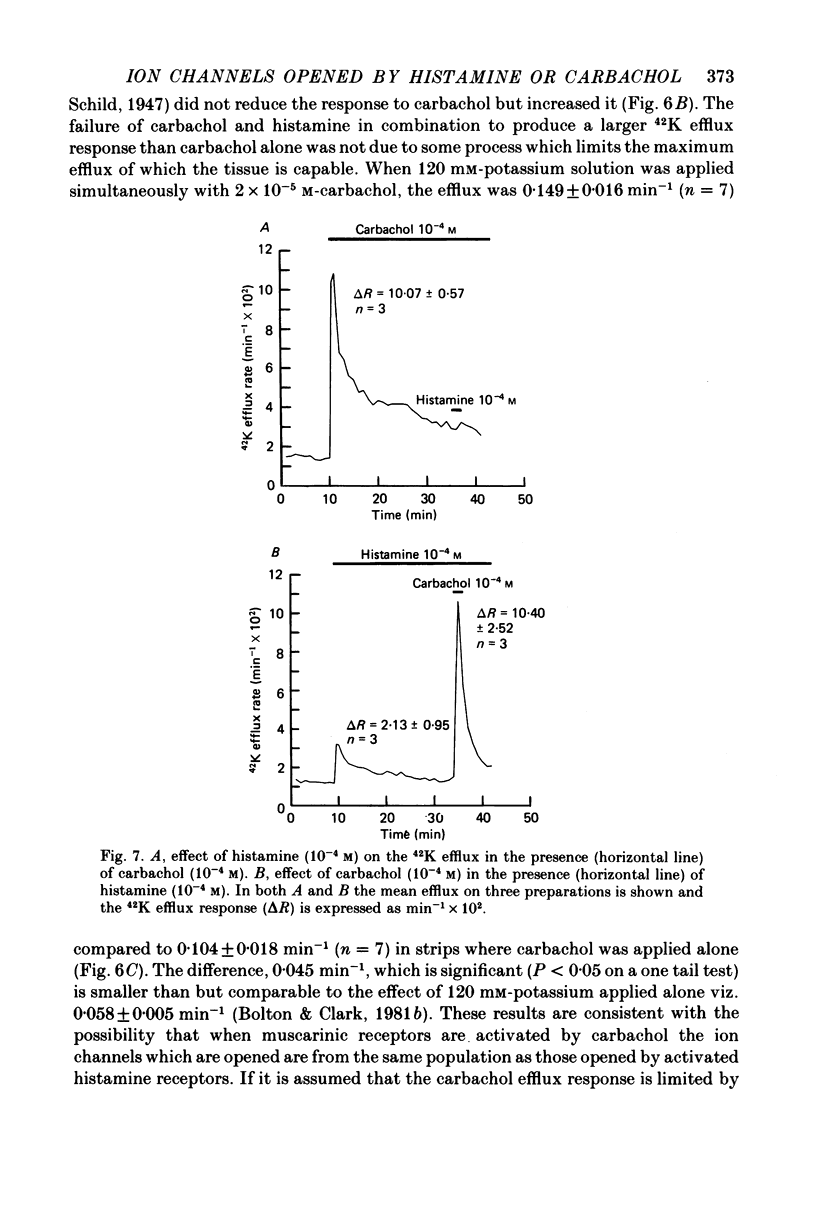
4. The efflux of 42K was studied in separated strips of longitudinal ileal muscle from guinea-pig. In the presence of a concentration of carbachol (2 × 10-5 m or 10-4 m) having a maximal effect on 42K efflux rate, histamine (10-4 m) did not increase efflux further although 120 mm-potassium did so. Experiments with the irreversible muscarinic receptor blocker, propylbenzilylcholine mustard, indicated that the number of muscarinic receptors did not limit the 42K efflux response to carbachol and it was suggested that the response was limited by the availability of ion channels which could be opened by activated muscarinic receptors.
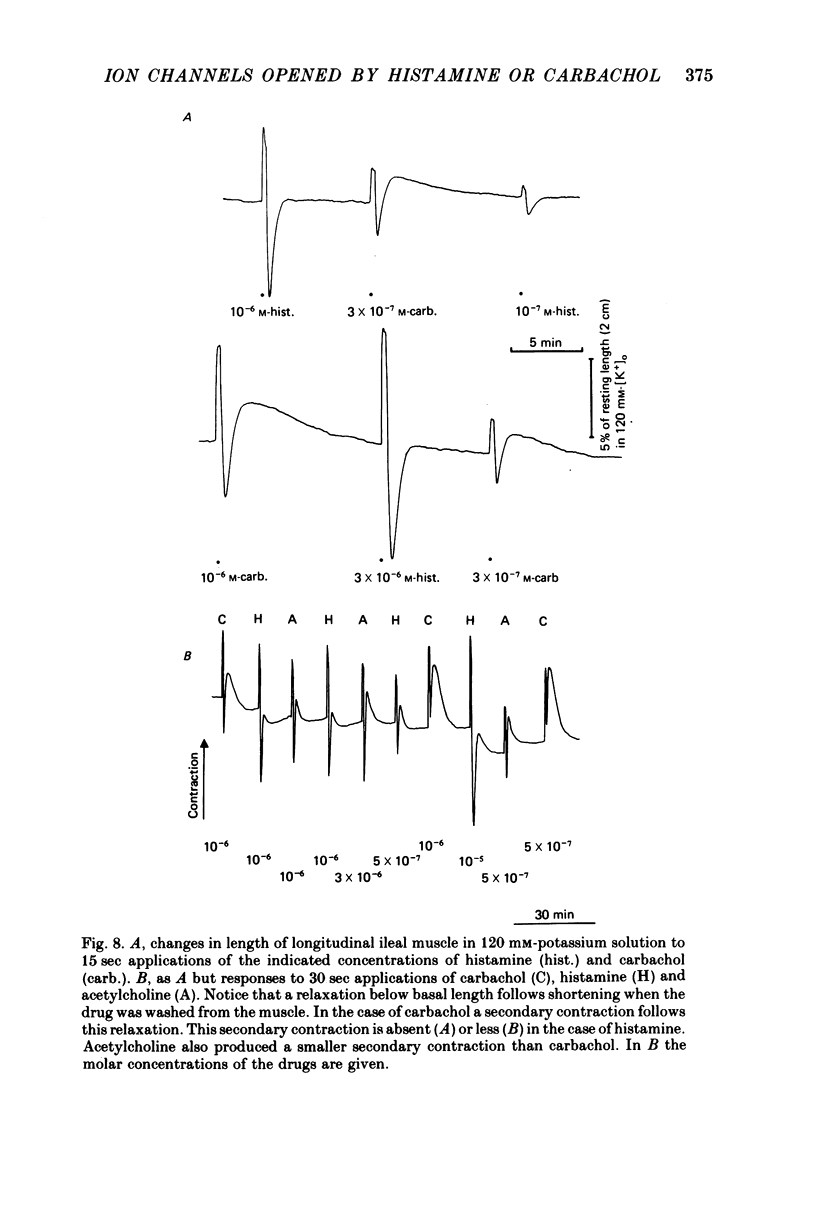
5. Contractions to histamine and carbachol in 120 mm-potassium depolarizing solution were followed upon washing by a relaxation below basal tension. Carbachol, but not histamine, showed a pronounced and long lasting secondary contraction following this relaxation.
6. These results are consistent with the idea that activated histamine and activated muscarinic receptors open the same ion channels in the smooth muscle membrane to produce depolarization, increased action potential discharge and contraction, although muscarinic receptors can open more of these. However, there was evidence that the opening of these channels is not the only pathway between receptor activation and contraction.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANERJEE A. K., LEWIS J. J. EFFECTS OF SMOOTH MUSCLE STIMULANTS AND THEIR ANATAGONISTS UPON POTASSIUM ION UPTAKE AND RELEASE IN STRIPS OF GUINEA-PIG ILEUM. J Pharm Pharmacol. 1964 Feb;16:134–136. doi: 10.1111/j.2042-7158.1964.tb07436.x. [DOI] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The movement of potassium between smooth muscle and the surrounding fluid. J Physiol. 1956 Mar 28;131(3):690–703. doi: 10.1113/jphysiol.1956.sp005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Changes in configuration of spontaneously discharged spike potentials from smooth muscle of the guinea-pig's taenia coli; the effect of electrotonic currents and of adrenaline, acetylcholine and histamine. J Physiol. 1957 Feb 15;135(2):412–425. doi: 10.1113/jphysiol.1957.sp005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K. The influence of drugs upon 42 K + fluxes in guinea-pig ileum in vitro. Arch Int Pharmacodyn Ther. 1972;198(1):173–186. [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P. Actions of various muscarinic agonists on membrane potential, potassium efflux, and contraction of longitudinal muscle of guinea-pig intestine. Br J Pharmacol. 1981 Feb;72(2):319–334. doi: 10.1111/j.1476-5381.1981.tb09131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P. Effects of histamine, high potassium and carbachol on 42K efflux from longitudinal muscle of guinea-pig intestine. J Physiol. 1981 Nov;320:347–361. doi: 10.1113/jphysiol.1981.sp013954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Effects of stimulating the acetylcholine receptor on the current-voltage relationships of the smooth muscle membrane studied by voltage clamp of potential recorded by micro-electrode. J Physiol. 1975 Aug;250(1):175–202. doi: 10.1113/jphysiol.1975.sp011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgen A. S., Hiley C. R., Young J. M. The binding of (3H)-propylbenzilycholine mustard by longitudinal muscle strips from guinea-pig small intestine. Br J Pharmacol. 1974 Jan;50(1):145–151. doi: 10.1111/j.1476-5381.1974.tb09602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill E. W., Rang H. P. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Mol Pharmacol. 1966 Jul;2(4):284–297. [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Hill S. J., Young J. M., Marrian D. H. Specific binding of 3H-mepyramine to histamine H1 receptors in intestinal smooth muscle. Nature. 1977 Nov 24;270(5635):361–363. doi: 10.1038/270361a0. [DOI] [PubMed] [Google Scholar]
- Ohashi H. The relative contribution of K and Cl to the total increase of membrane conductance produced by adrenaline on the smooth muscle of guinea-pig Taenia coli. J Physiol. 1971 Jan;212(2):561–575. doi: 10.1113/jphysiol.1971.sp009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Taylor I. K., Cuthbert A. W., Young M. Muscarinic receptors in rat intestinal muscle: comparison with the guinea pig. Eur J Pharmacol. 1975 Apr;31(2):319–326. doi: 10.1016/0014-2999(75)90055-2. [DOI] [PubMed] [Google Scholar]



