Abstract
Mutations in α-synuclein (α-Syn) cause Parkinson's disease (PD) in a small number of pedigrees with familial PD. Moreover, α-Syn accumulates as a major component of Lewy bodies and Lewy neurites, intraneuronal inclusions that are neuropathological hallmarks of PD. To better understand the pathogenic relationship between alterations in the biology of α-Syn and PD-associated neurodegeneration, we generated multiple lines of transgenic mice expressing high levels of either wild-type or familial PD-linked Ala-30 → Pro (A30P) or Ala-53 → Thr (A53T) human α-Syns. The mice expressing the A53T human α-Syn, but not wild-type or the A30P variants, develop adult-onset neurodegenerative disease with a progressive motoric dysfunction leading to death. Pathologically, affected mice exhibit neuronal abnormalities (in perikarya and neurites) including pathological accumulations of α-Syn and ubiquitin. Consistent with abnormal neuronal accumulation of α-Syn, brain regions with pathology exhibit increases in detergent-insoluble α-Syn and α-Syn aggregates. Our results demonstrate that the A53T mutant α-Syn causes significantly greater in vivo neurotoxicity as compared with other α-Syn variants. Further, α-Syn-dependent neurodegeneration is associated with abnormal accumulation of detergent-insoluble α-Syn.
In the elderly population, Parkinson's disease (PD) is the second most common neurodegenerative disease after Alzheimer's disease (1). The illness is characterized by a variety of motoric dysfunctions including difficulties in initiating movements, slowed movements (bradykinesia), rigidity, and resting tremor (1). These signs result from reduction in the level of striatal dopamine, which accompanies progressive degeneration of dopaminergic neurons in the substantia nigra, pars compacta (SNpc) (1). Although the cause of most cases of PD are not known, genetic studies have identified two mutations, A30P and A53T, in α-synuclein (α-Syn) that cause PD in a number of pedigrees with autosomal dominant inheritance (2, 3). α-Syn is a highly conserved protein of 140 amino acids that is expressed predominantly in neurons and is particularly abundant in presynaptic terminals (4–8). It has been suggested that α-Syn may have a role in synaptic plasticity (4, 7, 9) and may modulate dopaminergic neurotransmission (10, 11). Significantly, in both sporadic and familial forms of PD, α-Syn is a major structural component of Lewy bodies (LBs) and Lewy neurites (LNs), intraneuronal inclusions that are neuropathological hallmarks of PD (12, 13). Additionally, α-Syn is implicated in the pathogenesis of several other neurodegenerative diseases including dementia with LBs and multiple systems atrophy (14–17). Thus, α-Syn abnormalities may be of significant pathogenic importance in the neurodegeneration associated with PD and other diseases that collectively are termed the α-synucleinopathies (14, 16, 17).
The significance of α-Syn abnormalities in the pathophysiology of these disorders is supported by recent results showing α-Syn-dependent neurodegeneration in transgenic flies (18), neuropathology in transgenic mice (19, 20), and dopaminergic degeneration in adenovirus-induced rats (21). However, it is not known whether α-Syn can cause progressive late-onset disease in mammals and whether mutant α-Syn variants exhibit differential in vivo neurotoxicity. To understand better how abnormalities in α-Syn biology cause neurodegeneration, we have examined the consequences of expressing wild type (WT) and familial PD-linked mutant human (Hu)α-Syns in transgenic mice in vivo. We report that expression of Huα-Syn(A53T) in transgenic mice leads to late-onset neurodegenerative disease characterized by motor dysfunction, progressing to death. In contrast, transgenic mice with comparably high levels of Huα-Syn(WT) or even higher levels of Huα-Syn(A30P) do not develop overt phenotype or pathology. The neuropathology in the Huα-Syn(A53T) mice is associated with neuronal accumulation of α-Syn, ubiquitin, and neurofilaments (NFs).
Methods
Huα-Syn Expression Vectors.
A cDNA encoding Huα-Syn was generated from human brain cDNA by PCR (22) with a sense primer spanning codons 1–6 of Huα-Syn (5′-gtcgaccatggatgtattcatgaaag-3′) and an antisense primer spanning codons 136–141 of Huα-Syn (5′-ctcgagcttaggcttcaggttcgtag-3′). The familial PD-linked missense mutations were introduced during the PCR by inclusion of a third primer encoding either the A53T (5′-catggtgtgacaacagtggctgag-3′) or A30P (5-gtggcagaagcaccaggaaagac-3′). The resulting 436-bp human cDNA was cloned into the pCRII-TA vector (Invitrogen). Appropriate clones were identified by DNA sequencing. The resulting WT and mutant Huα-Syn cDNAs were released from the pCRII vector by using SalI and XhoI and cloned into the XhoI-linearized mouse (Mo) prion protein promoter (PrP)-transgenic mouse expression vector (23), to generate MoPrP–Huα-Syn(WT), MoPrP–Huα-Syn(A30P), and MoPrP–Huα-Syn(A53T).
Generation of Huα-Syn Transgenic Mouse.
Transgenic mice were generated by using the MoPrP vector as described (23). Briefly, the NotI fragment of the MoPrP-Huα-Syn plasmid was purified by using the β-agardse method (New England Biolabs), and the transgene was introduced into the germ line of mouse by pronuclear injection of one-cell mouse embryos. Founders and subsequent transgenic progenies were identified by PCR analysis of tail DNA by using primers to detect both the endogenous MoPrP genomic sequences and transgene sequences (23). The primers used were a sense primer encoding codons 4–11 of Huα-Syn (5′-ttcatgaaaggactttcaaaggc-3′), a sense primer encoding codons 10–25 of MoPrP (5′-cctctttgtgactatgtggactgatgtcgg-3′), and an antisense primer complementary to a region of the MoPrP 3′-untranslated region (5′-gtggataccccctcccccagcctagacc-3′). All MoPrP–Huα-Syn transgenic mouse lines were established as C3H/HeJ × C57BL6/J hybrids and maintained by successively backcrossing into the C57Bl6/J strain.
Antibodies.
To follow the expression of Huα-Syn protein in transgenic mice, we generated anti-Huα-Syn antibodies (HuSyn1) that do not cross-react with rodent α-Syn by immunizing New Zealand white rabbits with the Keyhole limpet hemocyanin-conjugated synthetic peptides corresponding to amino acids 117–125 of Huα-Syn (NH-Pro-Val-Asp-Pro-Asp-Gln-Glu-Ala-Tyr-COOH). To detect both rodent and Huα-Syn, an anti-α-Syn mouse mAb (Syn-1, S63320, Transduction Laboratories, Lexington, KY) was used. We also used other antibodies as follows: rabbit anti-β-Syn antisera (AB5086, Chemicon); rabbit anti-tyrosine hydroxylase antisera (Pel-Freez Biologicals); rabbit antiubiquitin antisera (Dako); rabbit antiglial fibrillary acid protein (GFAP) antisera (Dako); and mouse antiphosphorylated NF-H subunit (SMI-31, Sternberger, Baltimore, MD).
Analysis of Transgene Expression.
For Northern blot and reverse transcription–PCR analysis of mRNA, total RNA was isolated from brain regions of transgenic or nontransgenic mice by using Trizol reagent (GIBCO/BRL). The expression of α-Syn mRNA was determined by Northern blot analysis (22, 24, 25) with 32P-labeled full-length Huα-Syn cDNA probe. Analysis of α-Syn mRNA expression by in situ hybridization (22) was performed by using a 33P-labeled cRNA probe generated from Huα-Syn cDNA.
Expression of α-Syn and other proteins were determined by immunoblot analysis of SDS/PAGE-separated total SDS-soluble proteins (22, 24). Quantitative immunoblot analysis was achieved by using 125I-conjugated protein A as described (22, 24). Nonionic detergent-soluble and -insoluble fractions were prepared by homogenization of tissue in TNE buffer (10 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5 mM EDTA) containing protease inhibitors (5 mM PMSF/10 μg/ml aprotinin/10 μg/ml leupeptin/10 μg/ml pepstatin) and detergents (0.5% Nonidet P-40). The homogenate was centrifuged (5 min at 100,000 × g), and the resulting pellet (P1) and supernatant (S1, soluble) fractions were collected. The P1 was washed once in TNE containing nonionic detergents, and the resulting pellet (P2, nonionic detergent-insoluble) was solubilized in TNE buffer containing 1% SDS.
For immunohistochemical analysis, mice were perfused intra-aortically with PBS followed by 4% paraformaldehyde. Brains were processed for either frozen (22) or paraffin-embedded (26) sections. Both frozen and paraffin sections were processed for a variety of histochemical and immunocytochemical analyses (22, 26).
Results
Expression of A53T α-Syn Causes Late-Onset, Rapidly Progressive Neurological Abnormalities.
To achieve high levels of transgene expression in mice, the transgene expression (cDNAs encoding human WT, A30P, or A53T mutant α-Syn) was controlled by the murine prion promotor (MoPrP–Huα-Syn; ref. 23). For each transgene construct, between 12 and 23 founders were identified, and progenies were generated from at least six founders with the highest transgene copy numbers. After initial analysis of transgene expression from the progenies, lines were established from founders with high, medium, and low levels of transgene expression in the brain.
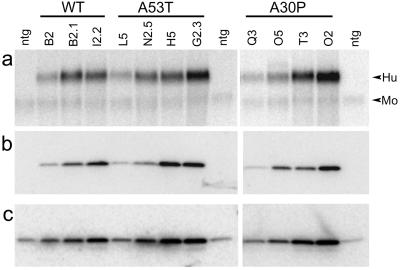
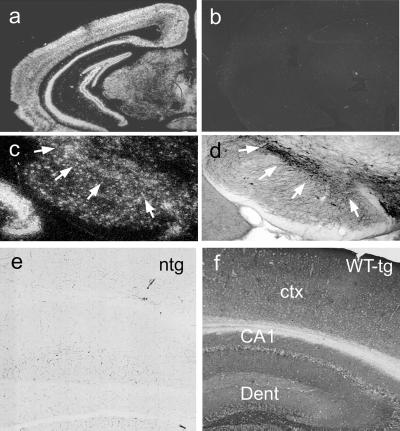
Analysis of transgene-derived mRNA (Fig. 1a) and protein (Fig. 1 b and c) show high levels of Huα-Syn expression in the brains of transgenic mice. The levels of total α-Syn protein (human and mouse combined) in the brains of MoPrP–Huα-Syn transgenic mice were 4–15 times higher than in the nontransgenic mice (Fig. 1 a and b). In situ hybridization analysis shows widespread expression of transgene-derived Huα-Syn mRNA in all brain regions (Fig. 2 a–d). Although some expression was observed in glial cells, the predominant expression was associated with neurons including neurons in the SNpc (Fig. 2 c and d). Immunocytochemical analysis using HuSyn-1 rabbit polyclonal Ab shows that all three Huα-Syn variants appear in punctate structures in the neuropil, a finding consistent with enrichment of the protein at presynaptic terminals (Fig. 2 e and f).
Figure 1.
Expression of Huα-Syn in transgenic mice. (a) Northern blot analysis of total brain RNA from nontransgenic (ntg) littermates, MoPrP–Huα-Syn(WT) transgenic (lines B2-1, B2, and I2-2), MoPrP–Huα-Syn(A53T) transgenic (lines L5, N2-5, H5, and G2-3), and MoPrP–Huα-Syn(A30P) transgenic (lines Q3, O5, T3, O2) mice by using Huα-Syn cDNA probe. The autoradiogram shows high-level expression of Huα-Syn mRNAs in the brains of transgenic mice. Because of sequence homology between Hu- and Moα-Syn, the cRNA probe also detects endogenous Moα-Syn mRNA (Mo). (b and c) Immunoblot analysis of α-Syn polypeptides in MoPrP–Huα-Syn transgenic mice. Total brain protein from nontransgenic and transgenic mice (lanes are the same as described for a) were subjected to immunoblot analysis by using HuSyn-1 rabbit polyclonal Ab (b) or Syn-1 (c), an anti-pan α-Syn mAb.
Figure 2.
Cellular localization of Huα-Syn expression in transgenic (tg) mice. (a and b) In situ hybridization analysis of Huα-Syn mRNA expression in brains of Huα-Syn transgenic mice. The antisense (a) and control sense (b) probe to Huα-Syn mRNA shows high-level, widespread expression of Huα-Syn in most brain regions. (c and d) To determine that Huα-Syn mRNA is expressed in the SNpc of transgenic mice, adjacent sections were processed for in situ hybridization (c) and tyrosine hydroxylase immunocytochemistry (d). The arrows outline the location of dopaminergic neurons in the SNpc. (e and f) Immunocytochemical analysis of Huα-Syn polypeptides in MoPrP–Huα-Syn transgenic mice brains. Coronal brain sections from nontransgenic mouse (e) and Huα-Syn(WT) transgenic mouse from I2-2 (f) were immunoreacted with HuSyn-1 antisera. Punctate, neuropil localizations of the Huα-Syn polypeptides are consistent with enrichment of α-Syn at the synaptic terminals. Immunocytochemical analysis of Huα-Syn(A53T) and Huα-Syn(A30P) showed results similar to the WT protein. ctx, cortex; Dent, dentate.
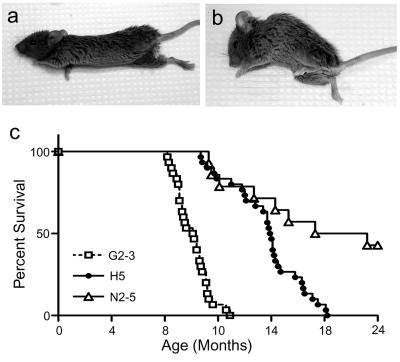
Once multiple lines of Huα-Syn transgenic mice were established, the mice were followed for signs of neurological abnormalities. As the animals aged, mice from three of four established Huα-Syn(A53T) lines (G2-3, H5, and N2-5) developed motor signs characterized by sustained posturing, reduced amplitude, and abundance of spontaneous activity. In many instances, the affected mice seem to exhibit bradykinesia, mild ataxia, and dystonia. The mice eventually develop progressive loss of righting reflex and paralysis (Fig. 3 a and b; Fig. 7 and Movie 1, which are published as supporting information on the PNAS web site, www.pnas.org). In most of the affected animals, the disease rapidly progressed to death, potentially because of the inability to feed and dehydration, within 14–21 days after the initial onset (Fig. 3b). The average age of onset for the clinical abnormalities in the first 30 affected mice were 10.1 ± 1.2 months for line G2-3(A53T) and 15.7 ± 2.7 months for line H5(A53T) (Fig. 3c). The average age of onset for affected mice in line N2-5(A53T) was 15.7 ± 4.2 months, but ≈50% of mice failed to develop disease at 24 months of age (Fig. 3c). Thus, higher levels of A53T α-Syn (G2-3 > H5 > N2-5; Fig. 1) were associated with earlier ages of disease onset and a greater penetrance of the disease phenotype. In the remaining A53T α-Syn line (L5), expression of the transgene at levels comparable with the endogenous protein was not sufficient to cause disease.
Figure 3.
Expression of Huα-Syn(A53T) lead to neurological abnormalities and shortened lifespan of the transgenic mice. (a and b) G2-3(AT) transgenic mice at the middle (a) and late (b) stage of the neurological dysfunction. (c) Survival curves for the Huα-Syn(A53T) transgenic lines. The higher expression of transgene is associated with earlier disease onset and death.
Despite the high levels of transgene expression comparable with those of A53T lines, the mice with WT or A30P transgenes did not develop any overt neurological abnormalities even at 24 months of age [n = 14 for I2-2(WT), n = 28 for O2(A30P), and n = 16 for T3(A30P)]. The levels of transgene expression achieved in WT and A30P lines would have been sufficient to cause disease with the A53T transgene, because similar or lower levels of αSyn(A53T) transgene expression lead to disease in these transgenic mice (see Fig. 1). Specifically, the levels of Huα-Syn in the I2-2(WT) and T3(A30P) lines were comparable with that observed with the H5(A53T) and N2-5(A53T) lines. However, none of the mice from I2-2(WT) or T3(A30P) lines developed neurological abnormalities at any age. Further, despite the fact that the level of Hα-Syn expression in line O2(A30P) is higher than any of the other MoPrP–Huα-Syn line including line G2-3(A53T) (Fig. 1 b and c), the mice from line O2(A30P) do not develop neurological abnormalities. Thus, both WT and A30P Huα-Syn variants are significantly less pathogenic to neurons in vivo than the A53T mutant Huα-Syn.
Previously, other investigators have shown that either expression of the WT Huα-Syn (19) or the A53T Huα-Syn (20) lead to progressive deficits in the Rota-rod performance without obvious overt neurological symptoms or premature lethality. These early deficits in Rota-rod performance were linked to defects in lower motor neurons (20). In contrast to these earlier α-Syn transgenic mice, our α-Syn transgenic mice do not show deficits in Rota-rod performance before the onset of neurological symptoms (Fig. 8 and Supporting Methods, which are published as supporting information on the PNAS web site).
Neurological Abnormalities in A53T α-Syn Transgenic Mice Are Accompanied by Neuropathology.
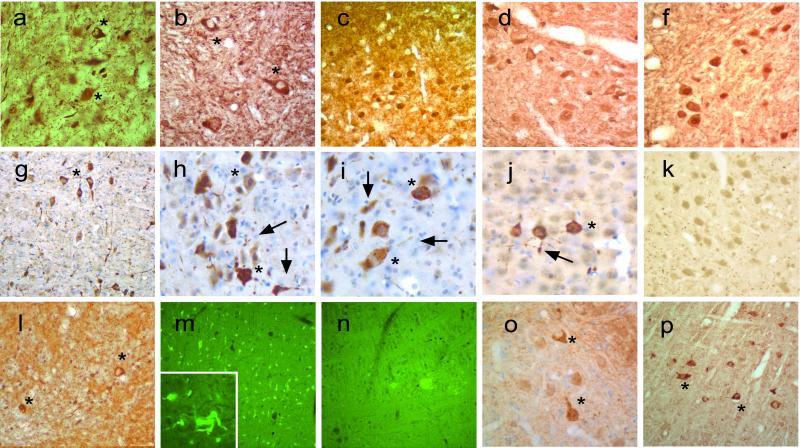
To determine the cytopathology associated with the neurological abnormalities in the α-Syn transgenic mice, the brains and the spinal cord from the A53T, A30P, and WT α-Syn transgenic mice were examined by using a variety of approaches. Because LBs and LNs are associated traditionally with abnormal accumulations of α-Syn, ubiquitin, and NF subunits (12, 13, 27–29), we asked whether abnormalities in the distributions of these proteins were present in the Huα-Syn transgenic mice. In neurologically affected Huα-Syn(A53T) mice, immunostaining for α-Syn revealed pathological accumulations of α-Syn in neuronal cell bodies and neurites (Fig. 4). In general, aberrant α-Syn accumulations were limited to the neuronal populations within the midbrain (data not shown), cerebellum (Fig. 4 a and o), brainstem (Fig. 4b), and spinal cord (data not shown). As in Huα-synucleinopathologies, abnormal distribution of α-Syn was associated with parallel accumulation of ubiquitin (Fig. 4 g–k) and phosphorylated NF-H (Fig. 4l) in perikarya and neurites. These neuritic accumulations of α-Syn and ubiquitin are highly reminiscent of abnormalities seen in Hu α-synucleinopathies. Although the inclusions did not resemble the compact, spherical morphology of LBs, thioflavin-S staining showed that many of the neurons in the affected regions contain fibrillar inclusions and that the rapid course of the disease may have precluded formation of compact inclusions (Fig. 4 m and n).
Figure 4.
Neuropathology in Huα-Syn transgenic mice. (a–c) Pathological neuronal accumulation of α-Syn in neurons from sick G2-3(A53T) transgenic mice. Abnormal α-Syn accumulations in neurons within deep cerebellar nuclei (a), near reticulo-pontine nuclei (b), and the neocortex (c). (d and f) Increased accumulation of α-Syn in neurons within the a deep cerebellar nuclei of O2(A30P) monitored with HuSyn-1 (a) and Syn-1 (b). Note a more uniform neuronal distribution of α-Syn accumulation. (g–j) Ubiquitin pathology in sick G2-3(A53T) mice. Pathological somal and neuritic accumulation of ubiquitin was prominent in pons (g and h) and cerebellum (i). Occasional cortical neurons (j) also exhibit ubiquitin pathology. (k) Despite α-Syn accumulation in mice from line O2(A30P) (shown in d and f), these mice do not develop ubiquitin pathology. Additional pathology in sick A53T mice includes accumulation of phosphorylated NF-H in deep cerebellar nuclei (l) and thioflavin-S-positive structures (m and Inset). (n) Thioflavin-S-treated nontransgenic mice are shown as negative control for m. (o and p) α-Syn (o) and ubiquitin (p) pathology in the Pons from preclinical H5(A53T) transgenic mice. The asterisks mark examples of perikaryal pathology, and the arrows mark the neuritic pathology.
Examination of presymptomatic mice from Huα-Syn(A53T) lines at ages close to the age of onset for clinical signs showed abnormal neuronal accumulations of α-Syn (Fig. 4o) and ubiquitin (Fig. 4p). These abnormalities were similar in distribution and appearance to those in clinically affected mice, but neuronal abnormalities were quantitatively and qualitatively less severe in the presymptomatic mice. Abnormal neuronal accumulations of α-Syn and ubiquitin were not obvious in younger (2–4-month old) Huα-Syn(A53T) mice (data not shown). Thus, the neuropathological abnormalities develop before clinical expression of motoric dysfunction, and the pathology worsens with disease progression in Huα-Syn(A53T) mice. Consistent with the lack of clinical phenotype, mice expressing Huα-Syn(WT) did not show obvious abnormal accumulation of α-Syn or ubiquitin (data not shown). Mice from line O2(A30P), which expresses very high levels of A30P α-Syn (see Fig. 1), show significant somal accumulation of α-Syn (Fig. 4 d and f). However, the accumulated α-Syn appears more evenly distributed, and the A30P α-Syn expression in mice, even at advanced age, does not lead to accumulation of ubiquitin in neurons (Fig. 4k).
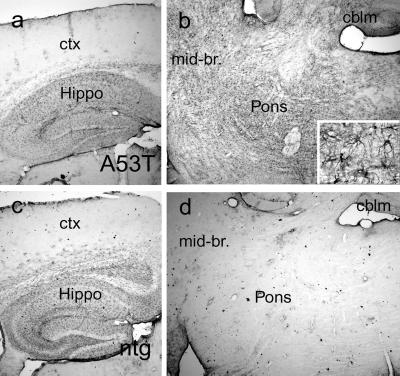
Because astroglial reaction is a reliable indicator of neurodegenerative changes, we examined brain sections of neurologically affected Huα-Syn(A53T) mice for GFAP immunoreactivity (Fig. 5). A comprehensive survey of GFAP expression indicated that the astroglial reaction is prominent in affected brain regions of the Huα-Syn(A53T) transgenic mice including dorsal midbrain, deep cerebellar nuclei, brainstem, and spinal cord (Fig. 5b). In contrast, cortex, hippocampus, thalamus, and caudate/putamen did not show increased GFAP expression (Fig. 5a). These results indicate that the neurological abnormalities in Huα-Syn(A53T) mice are associated with regionally selective involvement of neurons within midbrain, cerebellum, brainstem, and spinal cord. Specifically, the pathological changes in spinal cord include a significant astrocytic response as well as ubiquitin and α-Syn accumulation in ventral horn motor neurons. Other brain regions such as cortex and hippocampus were free of significant pathology. Parallel with the lack of neurological dysfunction in A30P and WT lines, GFAP staining in the brains of mice from WT and A30P lines were similar to the age-matched controls (data not shown).
Figure 5.
Astroglial reaction is limited to brain regions showing extensive neuropathology. Sagittal mouse brain sections from a clinically affected G2-3(A53T) transgenic mouse (a, b, and Inset) and an age-matched littermate mouse (c and d) were processed for GFAP immunocytochemistry. The GFAP immunoreaction in the cortex (ctx) and hippocampus (Hippo) of G2-3 transgenic mice (a) shows very little pathology and is almost identical to that of nontransgenic (ntg) littermates (c). However, the midbrain (mid-br) and brainstem areas (b) of affected G2-3 transgenic mice show obvious increases in GFAP-immunostained astroglial cells compared with the nontransgenic littermate (d). cblm, cerebellum.
α-Syn-Dependent Neurodegeneration in Huα-Syn(A53T) Transgenic Mice Is Associated with Accumulation of Detergent-Insoluble α-Syn.
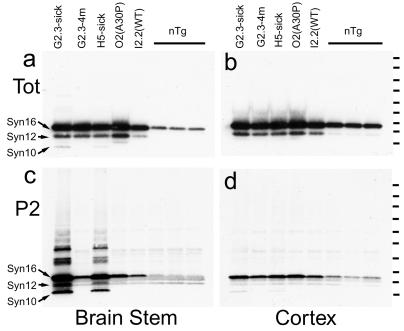
To determine whether abnormal accumulation of α-Syn is associated with changes in the biochemical properties of α-Syn, pathologically affected (brainstem) and unaffected (cortex) brain regions from Huα-Syn transgenic mice and nontransgenic littermate mice were fractionated into nonionic detergent-soluble (S1) and -insoluble (P2) fractions. The immunoblot analyses of total SDS-soluble proteins using Syn-1 mAb showed two major α-Syn polypeptides corresponding to the full-length α-Syn polypeptide (α-Syn16) and a truncated (α-Syn12) α-Syn polypeptide, which are present in all the samples from the Huα-Syn transgenic mice (Fig. 6 a and b). Analysis of S1 (data not shown) and P2 (Fig. 6 c and d) fractions demonstrated that the majority of α-Syn fractionates with the S1 fraction (data not shown), an observation consistent with α-Syn being a cytosolic protein (5–8, 30). However, α-Syn in the P2 fractions from brainstems of clinically affected Huα-Syn(A53T) transgenic mice were different quantitatively and qualitatively from α-Syn polypeptides in the other P2 fractions (Fig. 6c). Specifically, the brainstem P2 fraction from the affected Huα-Syn(A53T) contained significantly more detergent-insoluble full-length (≈16-kDa) α-Syn. Moreover, the P2 fraction also enriched for α-Syn with a lower molecular mass α-Syn (α-Syn10) and a series of higher molecular mass aggregates of α-Syn. These biochemical changes in α-Syn are linked directly to the presence of neuropathology, because they are not found in cortex (Fig. 6d), which is relatively free from lesions in these mice. Moreover, in younger Huα-Syn(A53T) mice, α-Syn are not identified with abnormal biochemical alterations (Fig. 6c). Finally, parallel analysis of Huα-Syn(WT) and Huα-Syn(A30P) mice show that the α-Syn species enriched in the P2 fraction is specific to the Huα-Syn(A53T) expression (Fig. 6c). Although the clarification of the proteolytic processing and the nature of the α-Syn aggregates will require further study, it is clear that proteolytic processing of α-Syn and formation of stable aggregates are significant components in the pathogenic mechanisms of α-Syn-dependent neuropathology, which also is reflected in the clinical manifestation occurring in our mice.
Figure 6.
Accumulation of nonionic detergent-insoluble α-Syn polypeptides in neurologically affected Huα-Syn(AT) transgenic mice. (a and b) Immunoblot analysis, using Syn-1 mAb, of total SDS-soluble brainstem (a) and cortical extracts (b) from Huα-Syn transgenic mice and nontransgenic (nTg) litter mates. In extracts from the transgenic mice, full-length α-Syn at ≈16 kDa (α-Syn16) and a truncated α-Syn at 12 kDa (α-Syn12) are obvious. (c and d) Immunoblot analysis, using Syn-1 mAb, of nonionic detergent-insoluble (P2) fractions from brainstem (c) and cortex (d). (d) Neuropathological changes in the brainstem of clinically affected mice (G2-3-Sick and H5-Sick) are associated with the increased accumulations of full-length α-Syn (α-Syn16), truncated α-Syns (α-Syn12 and α-Syn 10), and high molecular mass α-Syn polypeptides. These biochemical changes of α-Syn were not seen in brainstems of mice without pathology. (d) Consistent with lack of significant pathology in cortex, none of the cortical P2 fractions show abnormal α-Syn polypeptides. The molecular mass standards (tick to the right of the gels) are 9, 13, 20, 35, 50, 60, 80, 111, and 173 kDa.
Discussion
In summary, we have generated several transgenic mouse lines expressing high levels of WT and familial PD-linked mutant (A30P and A53T) Huα-Syn. Expression of Huα-Syn(A53T) leads to an adult-onset neurodegenerative disease characterized by motoric dysfunction that rapidly progresses to death. However, despite the high levels of transgene expression, mice from the Huα-Syn(WT) and Huα-Syn(A30P) lines do not develop neurological dysfunction. Although other studies have shown that the expression of Huα-Syn(A53T) or Huα-Syn(WT) is associated with neuropathology in transgenic mice (19, 20) and in flies (18), our studies clearly document that Huα-Syn(A53T) leads to significantly enhanced in vivo neurotoxicity in mammals compared with either the A30P or WT Huα-Syn variants. Further, expression of Huα-Syn(A53T) causes progressive neurodegeneration leading to death. Although increased in vivo toxicity of the A53T mutation is consistent with the A53T mutation being associated with a more aggressive disease course in humans than the A30P mutation (2, 3), the lack of significant neuropathology in mice expressing Huα-Syn(A30P) is unexpected. Although the A30P mutation is found in a small pedigree with only two affected members (3), toxicity associated with A30P mutation was believed to be greater (31, 32), because A30P is a very nonconservative change, whereas the A53T mutation is actually a normal sequence in rodents (2, 3). Thus, it is possible that the two mutations in Huα-Syn initiate disease in different ways, or A30P could be a rare polymorphism.
In addition to the pathological specificity of the mutant α-Syn, the cellular specificity of the Huα-Syn(A53T)-dependent pathology is remarkable. Although the transgene is highly expressed in all brain regions (ref. 23; Fig. 2), only a subset of neuronal populations are affected pathologically. Thus, it is clear that even in transgenic mice where very high levels of mutant α-Syn protein can be achieved, specific cell populations are more vulnerable to α-Syn-dependent toxicity. Significantly, although our in situ hybridization studies demonstrate that the transgene is expressed in the SNpc, we do not observe a significant loss of striatal dopamine (Supporting Methods and Fig. 9, which are published as supporting information on the PNAS web site). Similarly, immunocytochemical analysis did not reveal any obvious qualitative changes in tyrosine hydroxylase or dopamine transporter expression in the striatum and SNpc (data not shown). Moreover, the dopaminergic neurons in the SNpc are free of obvious neuropathological changes (data not shown). The lack of pathology in SNpc may indicate that rodent SNpc neurons may be more resistant to α-Syn toxicity than human SNpc neurons. This possibility is supported also by the lack of pathology in transgenic mice where α-Syn expression was directed to in the dopaminergic neurons by using the tyrosine hydroxylase promotor (33). Human and primate nigral neurons are orders of magnitude more sensitive then the rodent dopaminergic neurons to the Parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; ref. 34). Thus, there is a clear precedence for differential vulnerability of human versus rodent dopaminergic neurons.
Depite the lack of obvious pathology in SNpc neurons, the affected regions (red nuclei, brainstem, cerebellum, and spinal motor neurons) are known to impact motor behavior in mice. Thus, dramatic motor dysfunction in the α-Syn mice is predictable. Moreover, pathologically affected neurons in the MoPrP–Huα-Syn(A53T) transgenic mice exhibit cellular abnormalities that are remarkably similar to those observed in Hu α-synucleinopathies. Specifically, the neurological dysfunction in Huα-Syn(A53T) transgenic mice is associated with abnormal accumulations of α-Syn, ubiquitin, and NFs. Remarkably, examination of Huα-Syn(A30P) mice from line O2 shows neuronal α-Syn accumulation without ubiquitin accumulations (Fig. 4 d, f, and k) and without abnormal fragmentation of α-Syn (Fig. 6c). Thus, in transgenic mice, neuronal accumulation of α-Syn does not always lead to neuronal ubiquitin abnormalities and abnormal biochemical processing of α-Syn. Finally, the pathology in these mice is clearly associated with abnormal accumulation of ubiquitin in neurons and proteolytic processing/aggregation of α-Syn polypeptide, because truncations of α-Syn have been associated with increased ability to aggregate (35, 36), cause toxicity to cells in culture (37), and accumulate in a variety of Hu α-synucleinopathies (38–41), the biology underlying the accumulation of truncated α-Syn will be important for understanding the pathogenesis of PD and other α-synucleinopathies.
Supplementary Material
Acknowledgments
We are greatly indebted to Ms. Debbi Swing for her technical assistance in creating the transgenic mice described in this study. We thank Drs. David Borchelt and Philip C. Wong for helpful discussions. Thanks to Ms. Hilda Slunt for the technical help. Special thanks to Dr. Anne M. Andrews for the neurochemical analysis of dopamine and dopaminemetabolites. This work was supported by National Institutes of Health Grants AG14248, NS38065, and NS38377.
Abbreviations
- PD
Parkinson's disease
- SNpc
substantia nigra pars compacta
- α-Syn
α-synuclein
- LB
Lewy body
- LN
Lewy neurite
- WT
wild type
- Hu
human
- Mo
mouse
- PrP
prion protein promoter
- NF
neurofilament
- GFAP
glial fibrillary acidic protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fahn S, Przedborski S. In: Merritt's Neurology. Rowland L P, editor. Williams & Wilkins, New York: Lippincott; 2000. pp. 679–695. [Google Scholar]
- 2.Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 3.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J T, Schols L, Riess O. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 4.George J M, Clayton D F. Neurosci News. 1998;1:12–17. [Google Scholar]
- 5.Maroteaux L, Campanelli J T, Scheller R H. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero D A, Kondo J, Ihara Y, Saitoh T. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedert M. Nature (London) 1997;388:232–233. doi: 10.1038/40767. [DOI] [PubMed] [Google Scholar]
- 8.Irizarry M C, Kim T W, McNamara M, Tanzi R E, George J M, Clayton D F, Hyman B T. J Neuropathol Exp Neurol. 1996;55:889–895. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Clayton D F, George J M. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 10.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho W H, Castillo P E, Shinsky N, Verdugo J M, Armanini M, Ryan A, et al. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee F J S, Liu F, Pristupa Z B, Niznik H B. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 12.Spillantini M G, Schmidt M L, Lee V M Y, Trojanowski J Q, Jakes R, Goedert M. Nature (London) 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 13.Spillantini M G, Crowther R A, Jakes R, Hasegawa M, Goedert M. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson D W. Curr Opin Neurol. 2001;14:423–432. doi: 10.1097/00019052-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Galvin J E, Uryu K, Lee V M, Trojanowski J Q. Proc Natl Acad Sci USA. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvin J E, Lee V M Y, Trojanowski J Q. Arch Neurol (Chicago) 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 17.Goedert M, Spillantini M G. Mol Psychiatry. 1998;3:462–465. doi: 10.1038/sj.mp.4000458. [DOI] [PubMed] [Google Scholar]
- 18.Feany M B, Bender W W. Nature (London) 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 19.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 20.Van der Putten H, Wiederhold K H, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren W P J M, Ruegg M A, et al. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen T E, Muzyczka N, Mandel R J, Bjorklund A. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M K, Slunt H H, Martin L J, Thinakaran G, Kim G, Gandy S E, Seeger M, Koo E, Price D L, Sisodia S S. J Neurosci. 1996;16:7513–7525. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borchelt D R, Davis J, Fischer M, Lee M K, Slunt H H, Ratovitsky T, Regard J, Copeland N G, Jenkins N A, Sisodia S S, et al. Genet Anal Biomed Eng. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee M K, Borchelt D R, Kim G, Thinakaran G, Slunt H H, Ratovitski T, Martin L J, Kittur A, Gandy S, Levey A I, et al. Nat Med. 1997;3:756–760. doi: 10.1038/nm0797-756. [DOI] [PubMed] [Google Scholar]
- 25.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovitski T, Davenport F, Nordstedt C, Seeger M, et al. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 26.Borchelt D R, Ratovitski T, Van Lare J, Lee M K, Gonzales V B, Jenkins N A, Copeland N G, Price D L, Sisodia S S. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 27.Galvin J E, Lee V M Y, Baba M, Mann D M A, Dickson D W, Yamaguchi H, Schmidt M L, Iwatsubo T, Trojanowski J Q. Ann Neurol. 1997;42:595–603. doi: 10.1002/ana.410420410. [DOI] [PubMed] [Google Scholar]
- 28.Hill W D, Lee V M Y, Hurtig H I, Murrary J M, Trojanowski J Q. J Comp Neurol. 1991;309:150–160. doi: 10.1002/cne.903090111. [DOI] [PubMed] [Google Scholar]
- 29.Manetto V, Perry G, Tabaton M, Mulvihill P, Fried V A, Smith H T, Gambetti P, Autilio-Gambetti L. Proc Natl Acad Sci USA. 1988;85:4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempermann G, Kuhn H G, Gage F H. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conway K A, Lee S J, Rochet J C, Ding T T, Williamson R E, Lansbury P T., Jr Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway K A, Lee S J, Rochet J C, Ding T T, Harper J D, Williamson R E, Lansbury P T., Jr Ann NY Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston W J, McGowan E, et al. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- 34.Gerlach M, Riederer P. J Neural Transm. 1996;103:987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- 35.Crowther R A, Jakes R, Spillantini M G, Goedert M. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 36.Park S M, Jung H Y, Chung K C, Rhim H, Park J H, Kim J. Biochemistry. 2002;41:4137–4146. doi: 10.1021/bi015961k. [DOI] [PubMed] [Google Scholar]
- 37.Kanda S, Bishop J F, Eglitis M A, Yang Y, Mouradian M M. Neuroscience. 2000;97:279–284. doi: 10.1016/s0306-4522(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 38.Baba M, Nakajo S, Tu P H, Tomita T, Nakaya K, Lee V M, Trojanowski J Q, Iwatsubo T. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell B C, McLean C A, Culvenor J G, Gai W P, Blumbergs P C, Jakala P, Beyreuther K, Masters C L, Li Q X. J Neurochem. 2001;76:87–96. doi: 10.1046/j.1471-4159.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 40.Goedert M. Philos Trans R Soc London B. 1999;354:1101–1118. doi: 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duda J E, Giasson B I, Gur T L, Montine T J, Robertson D, Biaggioni I, Hurtig H I, Stern M B, Grossman M, Lee V M, et al. J Neuropathol Exp Neurol. 2000;59:830–841. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.