Abstract
The heterogeneity of γ-aminobutyric acid type A (GABAA) receptors contributes to the diversity of neuronal inhibition in the regulation of information processing. Although most GABAA receptors are located synaptically, the small population of α5GABAA receptors is largely expressed extrasynaptically. To clarify the role of the α5GABAA receptors in the control of behavior, a histidine-to-arginine point mutation was introduced in position 105 of the murine α5 subunit gene, which rendered the α5GABAA receptors diazepam-insensitive. Apart from an incomplete muscle relaxing effect, neither the sedative, anticonvulsant, nor anxiolytic-like activity of diazepam was impaired in α5(H105R) mice. However, in hippocampal pyramidal cells, the point mutation resulted in a selective reduction of α5GABAA receptors, which altered the drug-independent behavior. In line with the role of the hippocampus in certain forms of associative learning, trace fear conditioning, but not delay conditioning or contextual conditioning, was facilitated in the mutant mice. Trace fear conditioning differs from delay conditioning in that the conditioned and unconditioned stimulus are separated by a time interval. Thus, the largely extrasynaptic α5GABAA receptors in hippocampal pyramidal cells are implicated as control elements of the temporal association of threat cues in trace fear conditioning.
The diversity in inhibition through interneurons is an important aspect in the regulation of neuronal information processing. The synchronous firing of inhibitory interneurons is thought to contribute to synaptic plasticity (1–4). Concomitantly, the structurally diverse γ-aminobutyric acid type A (GABAA) receptor subtypes would also be expected to play a role in adaptive brain functions, such as learning and memory (5, 6). The structurally diverse GABAA receptors in hippocampal pyramidal cells are a case in point with their striking domain-specific distribution for the fine tuning of their neuronal activity (7). Although α1GABAA receptors occur mainly in axodendritic and axosomatic synapses, α2GABAA receptors are particularly prominent in axoaxonic and specific axosomatic synapses (8–10). Among brain GABAA receptors, those containing the α1-, α2-, and α3-subunit are largely synaptic. However, the small receptor population containing the α5-subunit is not concentrated in synapses, as demonstrated in olfactory bulb and in hippocampal pyramidal cells (11, 12). Various types of extrasynaptic GABAA receptors have been found to mediate tonic inhibition as demonstrated in patch clamp recordings (13, 14). In the present work, an attempt was made to identify the functional significance of a specific, extrasynaptic GABAA receptor in behavioral terms. To clarify the in vivo relevance of the α5GABAA receptor, a mouse line was generated containing a point mutation in position 105 of the α5-subunit by gene targeting.
Methods
Mouse Breeding.
The histidine residue in position 105 was replaced by arginine (H105R) in the mouse α5-subunit gene essentially as described (15, 16). RW-4 embryonic stem cells were purchased from Genome Systems (St. Louis). These cells were derived from the 129/SvJ substrain. Chimeras were bred with EIIa-cre mice (17) on the 129/SvJ background (RCC, Füllinsdorf, Switzerland), and the neomycin-resistance cassette was permanently removed. Offspring carrying the cre transgene and the mutation were then bred with wild type 129/SvJ mice and animals carrying the point mutation but not the EIIa–cre transgene were selected. These mice were further bred against 129/SvJ wild-type mice. Subsequently, heterozygotes were intercrossed. Between 20 and 40 breeding pairs of both homozygous mutant and wild-type mice produced the experimental animals, which were used at 8–12 weeks of age. Each mouse was injected with diazepam only once. All animal experiments were approved by the Cantonal Veterinary Office in Zurich.
Immunohistochemistry.
Immunoperoxidase staining for the α1-, α2-, α3-, and α5-subunit was performed in perfusion-fixed adult mouse brain parasagital sections using antigen retrieval to enhance the staining (9). The densitometric analysis was carried out with the MCID M5 imaging system (Imaging Research, St. Catherines, ON, Canada) in digital images from sections of wild type and α5(H105R) mutant processed simultaneously under identical conditions. The relative staining intensity in regions of interest was measured in the fornix and subtracted. Values are given as mean ± SD using an arbitrary scale. Statistical comparison was done with one-way ANOVA, using the Tukey–Kramer test for posthoc multiple comparisons. Double-immunofluorescence staining was done in cryosections from fresh-frozen brain, using guinea pig antibodies against the α2- or α5-subunit (1:4,000) (18) together with the monoclonal antibody mAb7a against gephyrin (1:300; Connex, Martinsried, Germany) with a protocol optimizing detection of postsynaptic GABAA receptor clusters (9). Images from both markers were acquired sequentially by multitracking with a Zeiss Pascal confocal laser scanning microscope, digitally merged, and processed for background subtraction by using identical parameters for wild type and mutant. Autoradiography was performed as described (19).
Electrophysiology.
Parasagital brain slices were obtained from both wild-type and α5(H105R) mice (3–6 weeks of age) and superfused (20) during the recording at 1–2 ml/min with equilibrated (95% O2/5% CO2) artificial cerebrospinal fluid containing 125 mM NaCl, 26 mM NaHCO3, 25 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 2.5 mM CaCl, and 1 mM MgCl. Bipolar stimulus electrodes were placed in the middle third of the stratum radiatum of the hippocampal CA1 region for the application of current pulses of 30 μs duration and 10–100 μA. Field excitatory postsynaptic potentials were measured, amplified and digitized by using a Digidata 1200 A/D interface and pclamp software (Axon Instruments, Foster City, CA).
Behavior.
The behavioral tests were performed as described (21, 22).
Motor and locomotor activity.
Recordings were made in automated circular arenas for 1 h starting 30 min after drug administration.
Pentylenetetrazole test.
Mice were injected i.p. with 120 mg/kg of pentylenetetrazole 30 min after oral diazepam or vehicle administration. The latency to tonic convulsion was recorded during a 10-min period of observation.
Light–dark choice test.
Mice were tested in a novel two-chamber apparatus with the dark and the lit (500 Lux) areas interconnected by a small tunnel 30 min after drug administration. The time spent in the two areas was recorded for 5 min after the first entry into the dark tunnel.
Elevated plus-maze test.
Mice were video recorded in an elevated crossbar with two walled and two open arms for 5 min starting 30 min after drug administration.
Horizontal wire test.
The number of mice unable to grasp the wire with the two forepaws and at least one hindpaw within three trials was noted 30 min after drug administration.
Delay fear conditioning.
After 3 min of exposure to the chamber, mice received three successive tone-shock pairings (3 min apart; tone, 80 dB, 1 kHz, 10 s; footshock, 0.5 mA, 50 Hz, 0.5 s), with the shock delivered during the last 500 ms of the tone. Freezing was recorded 48 h later in a modified context (new olfactive, tactile, and visual cues) for 3 min and subsequently in the presence of the tone for 8 min.
Trace fear conditioning.
The procedure was similar to that for delay conditioning, except that an empty trace interval of 1 s was interposed between the tone and the footshock in three learning trials.
Contextual fear conditioning.
Mice placed individually into a chamber for 3 min were exposed to three consecutive footshocks (0.5 mA, 50 Hz, 1-s duration, 1 min apart). Freezing to the same context was measured during 6 min, 24 h after conditioning.
Results
Molecular and Cellular Characterization.
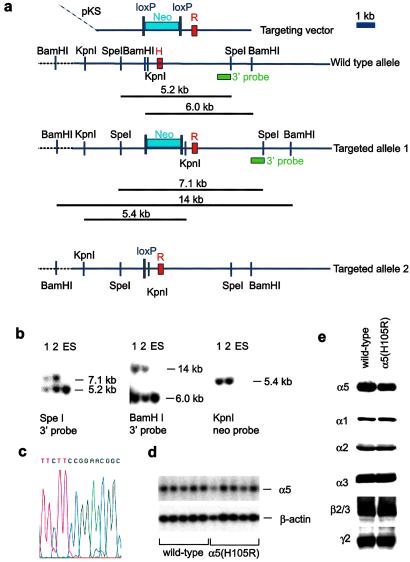
In recombinant α5β3γ2 receptors, the GABA response was largely unaltered when the histidine residue in position 105 of the α5-subunit was replaced by arginine, but diazepam showed practically no affinity (23, 24). This point mutation was therefore introduced into the germ line of mice by gene targeting (Fig. 1). A replacement vector RK-A5, which contained the desired point mutation in exon 4 and a loxP-flanked neomycin-resistance marker in intron 4, was electroporated into RW-4 embryonic stem (ES) cells. Correctly targeted ES cells containing the point mutation and the neo marker (Targeted allele 1, Fig. 1a) were injected into blastocysts. Mice carrying this mutant allele were bred to Ella-cre mice (17). The Ella-cre transgene efficiently eliminated the loxP-flanked neomycin resistance cassette from the germ line, thus generating targeted allele 2 (Fig. 1a). The Ella-cre transgene was bred out. The mutant alleles were analyzed by Southern blotting (Fig. 1b), sequence analysis (Fig. 1c), and Northern blotting (Fig. 1d).
Figure 1.
Targeting of the GABAA receptor α5 subunit (GABRA5) gene and molecular analysis. (a) Scheme of targeting strategy. The 3′ probe used for Southern blot analysis is depicted as green box. The “H” and the “R” on the red boxes depicting exon 4 specify whether the exon contains a codon for the naturally occurring histidine or the mutant arginine, respectively, at the amino acid position 105. The neomycin-resistance marker (“Neo”) is flanked by two parallel loxP sites. (b) Southern blot analysis of wild-type embryonic stem cells and mutant clones 1 and 2. (c) Verification of the α5(H105R) point mutation in a homozygous mutant α5(H105R) mouse by DNA sequencing. The codons for amino acids 104–106 are TTC CGG AAC. Exon 4 sequences were amplified by PCR and sequenced on an ABI Prism 310 Genetic Analyzer. (d) Northern blot analysis of the α5 transcript. Total RNA was prepared from whole brain without cerebellum, subjected to agarose gel electrophoresis and blotted onto Nylon membranes (Amersham Pharmacia Hyond N+). The membrane was first hybridized with an α5-exon 4 probe. After etching of the signals, the same blot was hybridized to a β-actin probe to assess the amount of material loaded per lane. (e) Western blot of GABAA receptor subunits. Crude membranes prepared from 3 distinct pools of 10 brains each of wild-type and α5(H105H) mice after removal of the cerebellum were used for Western blotting. To avoid saturation of the signals on the films, Western blotting was performed at protein concentrations ranging between 5 and 40 μg and at least 3 different times of exposure to the x-ray films (ranging from 30 s to 3 min). Densitometric analysis of α5 subunit signals was performed on films derived from blots containing 5, 10, 15, and 20 μg of protein and 3 different exposure times to control for linearity of the signals.
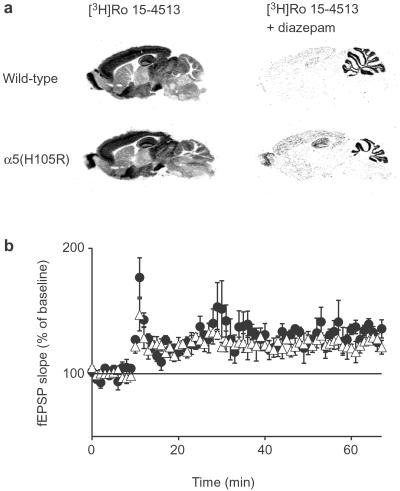
α5(H105R) mice showed no overt distinctive phenotype and bred normally. The major GABAA receptor subunits (α1, α2, α3, β2/3,and γ2) were expressed at normal levels in the α5(H105R) mice, as shown by Western blotting apart from a slight reduction of the α5 subunit protein (20% ± 5%) (Fig. 1e). In keeping with the introduction of the point mutation, the number of diazepam-insensitive sites was increased in α5(H105R) mice with no change in Kd as shown by radioligand binding in whole brain without cerebellum by using [3H]Ro 15–4513 in the presence of 10 μM diazepam (Bmax = 0.046 ± 0.008 pmol/mg protein in wild type, 0.111 ± 0.009 pmol/mg protein in mutant; Kd = 3.1 ± 0.8 nM in wild type, 3.2 ± 0.7 nM in mutant, n = 4 each). Autoradiographically, the newly generated diazepam-insensitive binding sites were present exclusively in areas expressing the α5 subunit in wild type with the highest density in olfactory bulb and hippocampus and low levels in cerebral cortex and some brainstem nuclei (Fig. 2a).
Figure 2.
Distribution of diazepam-insensitive sites and induction of LTP. (a) Autoradiographic distribution of benzodiazepine binding sites in parasagital brain sections of wild-type and α5(H105R) mice. (Left) Labeling of both diazepam-sensitive and -insensitive GABAA receptors by incubation with 20 nM [3H]Ro 15–4513. (Right) Incubation with the radioligand in the presence of 10 μM diazepam (labeling of diazepam-insensitive receptors). Nonspecific binding was assessed in the presence of 10 μM flumazenil. (b) Schaffer collateral LTP in wild-type (●) and α5(H105R) mice (▵). After 10 min of baseline recording in hippocampal slices, 20 pulses were given at 100 Hz repeated four times with a 20-s interval for a total of 80 stimuli. The slope (20–80%) of the field excitatory postsynaptic potential was measured and normalized to baseline. The results are given as mean ± SE as percentage of baseline. There was no significant difference in the potentiation throughout the time course of the experiment.
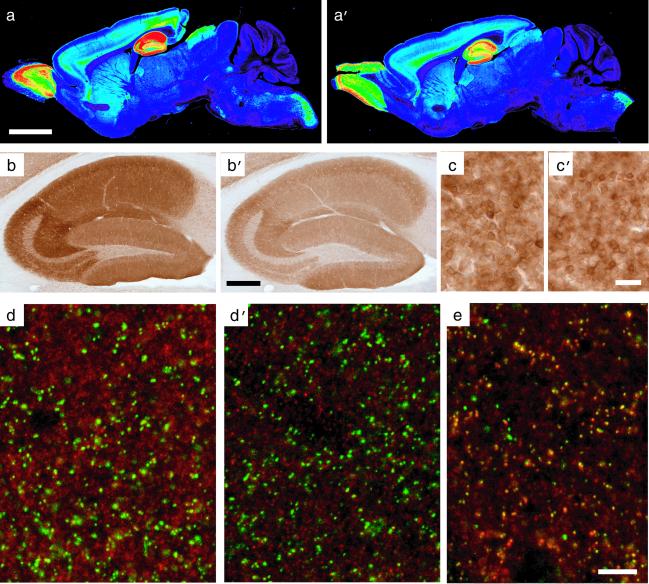
Immunohistochemically, the α5 subunit staining was prominent in the olfactory bulb, caudal spinal trigeminal nucleus, and dorsal horn of the spinal cord, and was moderate in deep cortical layers and superior colliculus to a similar extent in both wild-type and mutant mice (Fig. 3 a, a′, c, and c′). This finding was established by densitometric analysis (Table 1). Based on the lack of clustering and colocalization with gephyrin (Fig. 3d), it was confirmed by confocal laser scanning microscopy in wild-type mice that α5GABAA receptors in CA1 pyramidal cells were extrasynaptic (11, 12). In the α5(H105R) mutants, however, the dendritic α5-subunit staining was reduced exclusively in hippocampal pyramidal cells compared with wild type. The differential laminar staining pattern seen in CA1 and CA3 was nevertheless retained (Fig. 3 b and b′; Table 1). Despite the striking reduction of α5GABAA receptors in hippocampal pyramidal cells (Fig. 3d′), the expression of the postsynaptic α2GABAA receptors in the same cells was unaltered in the mutant mice (Fig. 3e). The expression of the α1- and α3-subunits in hippocampus of the mutants was likewise unchanged. This result was obtained by the densitometric analysis of the level of expression and laminar distribution of the α1-, α2-, and α3-subunit (Table 2). Thus, in hippocampal pyramidal cells, the point mutation selectively interfered with the assembly, transport, or turnover of the α5GABAA receptor. The α5(H105R) mice were therefore expected to display not only a pharmacological phenotype through the lack of the diazepam response at α5GABAA receptors, but also a drug-independent physiological phenotype because of the exclusive and cell-specific deficit of α5GABAA receptors in hippocampal pyramidal cells.
Figure 3.
Regional and cellular expression of the α5-subunit protein. Selective loss of extrasynaptic α5GABAA receptors in α5(H105R) mice [a, b, c, and d: wild type; a′, b′, c′, d′, and e: α5(H105R) mutant]. (a and a′) False-color images depicting the regional distribution of the α5-subunit in parasagital sections of adult mice, as detected by immunoperoxidase staining. Staining in wild type corresponded to that described (11, 31). Note the reduction of staining selectively in the hippocampal formation of mutant mice. (b and b′) Enlargement of the hippocampal formation showing the global reduction of α5-subunit immunoreactivity in CA1 and CA3 in the mutant compared with control. (c and c′) By comparison, no change in α5-subunit staining intensity was observed in olfactory bulb granule cells (see Table 1 for quantification). (d and d′) Images from confocal laser scanning microscopy, depicting a double-immunofluorescence staining for the α5-subunit (red) and gephyrin (green) in the stratum radiatum of CA1. Gephyrin is a postsynaptic marker of GABAergic synapses. The lack of colocalization with the α5-subunit staining reflects the extrasynaptic distribution of α5GABAA receptors. The staining intensity of these receptors is markedly reduced in mutant mice, whereas gephyrin immunoreactivity is unchanged. (e) For comparison, double staining for the α2 subunit (red) and gephyrin (green) reveals the extensive postsynaptic localization of α2GABAA receptors (yellow dots) in α5 mutant mice. (Scale bars: a, a′, 2.5 mm; b, b′, 0.5 mm; c, c′, 25 μm; d, d′, and e, 10 μm.)
Table 1.
Selective loss of α5-subunit immunoreactivity in dendritic layers of the hippocampus in α5(H105R) mice
| Region | α5-subunit immunoreactivity
|
|||
|---|---|---|---|---|
| OD wild type | OD mutant | % | P | |
| CA1, s. or | 219 ± 26 | 141 ± 34 | −36 | <0.001 |
| CA1, s. pyr | 259 ± 25 | 215 ± 40 | −17 | NS |
| CA1, s. rad | 244 ± 19 | 158 ± 34 | −35 | <0.001 |
| CA1, s. lm | 208 ± 18 | 133 ± 25 | −36 | <0.001 |
| CA3, s. or | 237 ± 22 | 170 ± 35 | −28 | <0.001 |
| CA3, s. pyr | 227 ± 26 | 198 ± 36 | −13 | NS |
| CA3, s. lucidum | 141 ± 27 | 99 ± 18 | −30 | <0.001 |
| CA3, s. rad | 289 ± 32 | 182 ± 31 | −37 | <0.001 |
| DG, granule cell layer | 185 ± 36 | 173 ± 28 | −7 | NS |
| DG, s. mol | 184 ± 27 | 142 ± 31 | −23 | NS |
| DG, hilus | 89 ± 28 | 74 ± 19 | −18 | NS |
| Olfactory bulb, gcl | 164 ± 42 | 138 ± 29 | −16 | NS |
| Striatum | 40 ± 10 | 35 ± 14 | −12 | NS |
| Frontal cortex, I–III | 17 ± 11 | 18 ± 13 | +3 | NS |
| Frontal cortex, VI | 108 ± 31 | 125 ± 27 | +15 | NS |
| Superior colliculus | 73 ± 23 | 57 ± 14 | −22 | NS |
| Vs, pars caudalis | 97 ± 14 | 108 ± 22 | +12 | NS |
Densitometry was performed in sections processed for immunoperoxidase staining (adult mice, n = 8 per genotype). For each region, two sections were analyzed per animal; background was measured in the fornix and subtracted. The overall lack of a decrease in α5-subunit immunoreactivity in the stratum pyramidale (s. pyr.) might represent an increase in intracellular receptors, because membrane-associated α5GABAA receptors are reduced in mutant mice (see Fig. 3 d and d′). %, percent deviation from values in wild type. DG, dentate gyrus; OD, optical density values (arbitrary scale); s. lm, stratum laconosum-moleculare; s. lucidum, stratum lucidum; s. mol., stratum moleculare; s. or, stratum oriens; s. rad., stratum radiatum; Vs, spinal trigeminal nucleus. Results are expressed as mean ± SD; NS, not significant (P > 0.05).
Table 2.
Lack of change of α1-, α2-, and α3-subunit immunoreactivity in α5 (H105R) mice
| Region | Subunit-IR, % of wild type
|
|||||
|---|---|---|---|---|---|---|
| α1 | α2 | α3 | ||||
| CA1, s. or | +1.9 | NS | +3.8 | NS | +15.0 | NS |
| CA1, s. pyr | +1.6 | NS | +10.1 | NS | +8.8 | NS |
| CA1, s. rad | +4.0 | NS | +1.2 | NS | +1.4 | NS |
| CA1, s. lm | −0.8 | NS | −0.2 | NS | +8.9 | NS |
| DG, s. mol | +5.4 | NS | −9.7 | NS | −1.9 | NS |
Changes in subunit immunoreactivity (IR) were measured by densitometry. None of the differences between wild type and mutant mice were statistically significant (NS). For abbreviations, see Table 1.
Pharmacology.
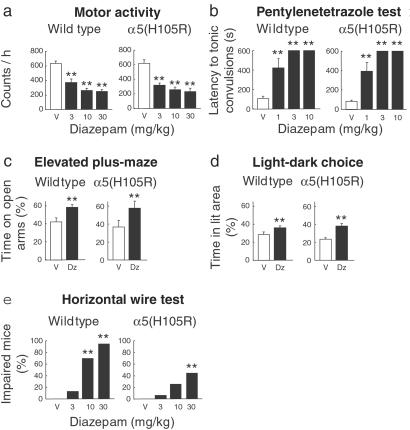
The behavioral responses of the α5(H105R) point mutation were first assessed pharmacologically. In both wild-type and mutant mice, diazepam was similarly effective in reducing the motor activity (Fig. 4a) and the locomotor activity (not shown), and was similarly protective against pentylenetetrazole-induced tonic convulsions (Fig. 4b). Furthermore, the anxiolytic-like action of diazepam remained unaltered in the α5(H105R) mice, as demonstrated in the elevated plus–maze test and in the light–dark choice test (Fig. 4 c and d). It was only in the horizontal wire test that α5(H105R) mice differed from wild-type mice by their reduced responsiveness to the diazepam-induced impairment of the grasping reflex (Fig. 4e; P < 0.01 versus wild type, Newman–Keuls). This effect is attributed to the presence of α5GABAA receptors on motoneurons and in the dorsal horn of spinal cord (25). It is noteworthy that in all of the above test paradigms, the diazepam-independent behavior of the α5(H105R) mice did not differ from that of wild-type mice as revealed by the vehicle controls. Thus, the hippocampal deficit of α5GABAA receptors was not critical for the drug-independent behavior in these tests, which included the anxiety-related behavior.
Figure 4.
Behavioral responses to diazepam. (a) Motor activity. Diazepam (3–30 mg/kg per os) dose-dependently reduced the activity counts to the same extent in wild-type and α5(H105R) mice [F3, 95 = 39.26; **, P < 0.001; n = 9–24]. The two vehicle-treated groups were likewise comparable. (b) Pentylenetetrazole test. Increasing doses of diazepam (1–10 mg/kg per os) protected both wild-type and α5(H105R) mice against pentylenetetrazole-induced convulsions as shown by the similarly increasing delay of occurrence of the tonic convulsion [F3,74 = 90.81; **, P < 0.001; n = 6–15]. (c) Elevated plus-maze. Wild-type and α5(H105R) mice displayed a similar increase in the percentage of time spent on the open arms in response to diazepam (1 mg/kg per os) [F1,32 = 9.24; **, P < 0.01; n = 9 per group]. The time spent on the enclosed arms did not differ between the two groups. (d) Light–dark choice test. Diazepam (1 mg/kg per os) increased the percentage of time spent in the illuminated area in the same way in wild-type and α5(H105R) mice [F1,37 = 17.67; **, P < 0.001; n = 9–12]. The time spent in the dark area did not differ between the two groups. (e) Horizontal wire test. In α5(H105R) mice, diazepam (10 mg/kg per os) failed to produce an impairment of the grasping reflex compared with wild-type mice [F3,132 = 5.43; **, P < 0.01; n = 16–24] with a clear genotypic distinction being retained at a higher dose (30 mg/kg). (P < 0.01 versus wild type, Newman–Keuls). Results are expressed as means ± SE. V, vehicle; Dz, diazepam.
Long-Term Potentiation (LTP) and Fear Conditioning.
When the neuronal plasticity of hippocampal pyramidal cells was assessed by the induction of LTP, there was no difference in the response properties between wild-type and α5(H105R) mice (Fig. 2b). Submaximal LTP, induced in hippocampal slices by four trains of 20 pulses at 100 Hz with a 20-s intertrain interval, resulted in a similar potentiation (29% ± 6% wild type, 23% ± 3% mutant mice, n = 6 each). This finding suggests that the ability to undergo activity-dependent increases in excitatory synaptic strength was unaltered in α5(H105R) mice.
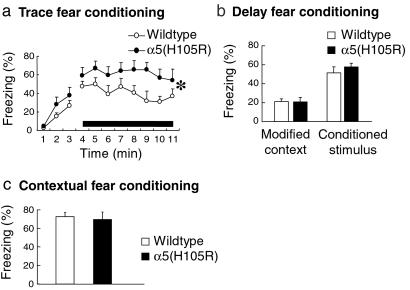
The hippocampus plays an essential role in certain types of associative learning and memory (5, 6, 26). Trace conditioning is a hippocampus-dependent form of associative learning in which the conditioned stimulus (CS) and the unconditioned stimulus (US) are separated by a certain time interval. However, when the CS coterminates or overlaps with the US, as in delay conditioning, the associative learning does not involve the hippocampus (5, 6, 26, 27). Wild-type and α5(H105R) mice were therefore compared with regard to their ability to acquire a trace fear conditioning task in which the tone and the shock were separated by an empty interval of 1 s. Wild-type and α5(H105R) mice displayed a similar increase in the amount of freezing during the learning session. However, when reexposed 48 h later to the tone (8 min), the α5(H105R) mice showed an enhanced percentage of freezing in comparison to wild type (Fig. 5a). As the facilitation in trace fear conditioning might reflect an altered processing of the CS–US association, the mice were tested in delay fear conditioning. This learning task differed from trace fear conditioning only by the lack of time interval between the auditory cue and the shock. Under these conditions, no difference in the fear response was seen between wild-type and α5(H105R) mice during the learning session or when the animals were reexposed to the tone (8 min) 48 h later (Fig. 5b). Similarly, the two groups of mice showed comparable freezing responses when reexposed to the same context (Fig. 5c). In addition, the pain sensitivity was unaltered in mutant mice compared with wild type as tested in the hot plate test. These control experiments demonstrate that the hippocampus-independent association of the CS and US in delay and contextual conditioning was not impaired in the α5(H105R) mice. These results underline the selectivity of the behavioral response to the mutation-induced receptor deficit. It was only in trace conditioning that the α5(H105R) mice displayed a behavioral alteration. Thus, the extrasynaptic α5GABAA receptors in hippocampal pyramidal cells are critically involved in the processing of the temporal discontiguity of the CS–US association.
Figure 5.
Fear conditioning. (a) Trace fear conditioning. In three learning trials the conditioned stimulus (tone) was followed 1 s later by a foot shock. When tested 48 h later, the α5(H105R) mice showed a higher amount of freezing than wild-type mice over the period of exposure to the tone (solid bar) [F1,84 = 4.44, *, P < 0.05; n = 7 per group]. No difference in the freezing response was observed during the first 3 min of exposure to a modified context. (b) Delay fear conditioning. In three learning trials, the tone coterminated with the foot shock. When tested 48 h later, the mean percentage of time spent freezing to the tone (8 min) was similar in wild-type and α5(H105R) mice (n = 8 per group). No group difference was seen in the freezing response to a modified context (first 3 min). (c) Contextual fear conditioning. After three trials of exposure to a foot shock, wild-type and α5(H105R) mice displayed a similar mean percentage of time freezing when re-exposed to the same context 24 h later (n = 8 per group). Results are expressed as means ± SE.
Discussion
The α1GABAA receptor has been shown to mediate the sedative, amnestic, and anticonvulsant activity of diazepam (15), whereas the anxiolytic action was mediated via α2GABAA receptors (16). The pharmacological analysis of α5(H105R) mice confirmed this view in that the drug responses attributed to α1 and α2 receptors remained unaffected. Only the muscle relaxant action of diazepam was impaired in the α5(H105R) mutant mice, most likely because of α5GABAA receptors located on motoneurons and in the dorsal horn of spinal cord (25). Thus, the muscle relaxant action of diazepam is mediated by neural circuits expressing more than one type of GABAA receptor, with the α5GABAA receptor being added to the previously identified α2 and α3 GABAA receptors (21). It should be noted in this context that GABAA receptor subtypes are identified only by the presence of one distinctive α-subunit.
In the α5(H105R) mice, the distribution and level of expression of the mutated α5GABAA receptor was unaltered in all brain areas except in hippocampal pyramidal cells, which displayed a striking dendritic receptor deficit (Fig. 3, Table 1). Apparently, the point mutation interfered with a cell-specific process required for the assembly, transport, or turnover of the α5GABAA receptors. Although the underlying mechanism remains to be identified, the mutation-induced receptor deficit was specific for the extrasynaptic α5GABAA receptors because the expression of the synaptic α2GABAA receptors was unaltered in the same cells. The distinction of synaptic and extrasynaptic location is based on immunohistochemical evidence. GABAA receptors, which are clustered in membrane domains apposed to the anchoring protein gephyrin, are termed synaptic. The presence of gephyrin, which interacts indirectly with the γ2-subunit of GABAA receptors, is a prerequisite for GABAA receptor clustering (28). Receptors that are not clustered and do not colocalize with gephyrin are considered extrasynaptic. By this definition, the α5 receptors were largely extrasynaptic in both wild-type and point-mutated mice, indicating that the point mutation did not interfere with the membrane targeting of the mutated receptor. This finding held for all brain regions expressing α5GABAA receptors and included the hippocampus, where the remaining α5GABAA receptors were largely extrasynaptic. Furthermore, because the hippocampal expression of the α1-, α2-, and α3-subunits was unaltered in the mutants (Table 2), a substitution of the α5 subunit by another α-subunit in the pentameric structure of the mutated α5GABAA receptors is unlikely.
The α5GABAA receptor deficit in hippocampal pyramidal cells of α5(H105R) mice permitted the analysis of its contribution to hippocampus-dependent learning and memory. In associative learning, the temporal processing is thought to involve the hippocampus (5, 6, 26, 27). Indeed, in trace conditioning where the CS and the US are temporally discontiguous, the α5(H105R) mice showed a facilitation of the freezing response. Thus, the α5GABAA receptors appear to be a molecular substrate for the hippocampal processing of the temporally discontiguous stimuli. In contrast, when the CS and US coterminate or overlap, the hippocampus is not required for CS–US association (5, 6, 26, 27). Indeed, in delay or in contextual fear conditioning, the response of the mutants did not differ from wild type, which underlines the selectivity of the α5GABAA receptors for the behavioral response in trace fear conditioning.
The findings point to a critical role of hippocampal extrasynaptic α5GABAA receptors in associative learning. Rhythmic synchronous neuronal activities provide the associative firing needed to trigger changes in synaptic strength (1–6). The simultaneous firing of interneurons is known to occur (29, 30), and GABA spills over to adjacent extrasynaptic GABAA receptors (13, 14). In this way, extrasynaptic α5GABAA receptors can be considered to contribute to the regulation of dendritic excitability of hippocampal pyramidal cells and the efficacy of excitatory inputs, and thereby contribute to the synaptic plasticity regulating the temporal association of threat cues.
Acknowledgments
We are grateful to C. Sidler, C. Michel, S. Lambert, and E. Calame for expert technical assistance. The work was supported by the Swiss National Science Foundation and the National Centre of Competence in Research on Neural Plasticity and Repair.
Abbreviations
- GABAA
γ-aminobutyric acid type A
- CS
conditioned stimulus
- US
unconditioned stimulus
- LTP
long-term potentiation
References
- 1.Cobb S R, Buhl E H, Halasy K, Paulsen O, Somogyi P. Nature (London) 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 2.Whittington M A, Traub R D, Jeffreys J G. Nature (London) 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Wang Y, Markram H. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 4.Tamas G, Buhl E H, Lorincz A, Somogyi P. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 5.Wallenstein G V, Eichenbaum H, Hasselmo E. Trends Neuosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen O, Moser E. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- 7.Maccaferri G, Roberts J D B, Szucs P, Cottingham C A, Somogyi P. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusser Z, Sieghart W, Benke D, Fritschy J M, Somogyi P. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritschy J M, Weinmann O, Wenzel A, Benke D. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 10.Nyiri G, Freund T F, Somogyi P. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- 11.Fritschy J M, Johnson D K, Möhler H, Rudolph U. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- 12.Brünig I, Scotti E, Sidler C, Fritschy J M. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- 13.Brickley S G, Cull-Candy S G, Farrant M. J Physiol (London) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mody I. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph U, Crestani F, Benke D, Brünig I, Benson J A, Fritschy J M, Martin J R, Bluethmann H, Möhler H. Nature (London) 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 16.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson J A, Fritschy J M, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 17.Lakso M, Pichel J G, Gorman J R, Sauer B, Okamoto Y, Lee E, Alt F W, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritschy J M, Möhler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 19.Benke D, Michel C, Möhler H. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- 20.Vogt K, Regehr W G. J Neurosci. 2001;21:75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 22.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent J P, Belzung C, Fritschy J M, Lüscher B, Möhler H. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 23.Benson J A, Löw K, Keist R, Möhler H, Rudolph U. FEBS Lett. 1998;432:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 24.Kelly M D, Smith A, Banks G, Wingrove P, Whiting P W, Atack J, Seabrook G R, Maubach K A. Br J Pharmacol. 2002;135:248–256. doi: 10.1038/sj.bjp.0704459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohlhalter S, Weinmann O, Möhler H, Fritschy J M. J Neurosci. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassermann E A, Miller R R. Annu Rev Psychol. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- 27.Medina J F, Repa J C, Mauk M D, LeDoux J E. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- 28.Essrich C, Lorez M, Benson J, Fritschy J M, Lüscher B. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 29.Galarretta M, Hestrin S A. Nature (London) 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 30.Gibson J R, Beierlein M, Connors B W. Nature (London) 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 31.Fritschy J M, Benke D, Johnson D K, Mohler H, Rudolph U. Neuroscience. 1997;81:1043–1053. doi: 10.1016/s0306-4522(97)00244-3. [DOI] [PubMed] [Google Scholar]