Abstract
Midbrain dopaminergic activity seems to be important in forming the prediction of future events such as rewards. The nucleus accumbens (NAc) plays an important role in the integration of reward with motor function, and it receives dense dopamine innervation and extensive limbic and cortical afferents. Here, we examined the specific role of the dopamine D2 receptor (D2R) in mediating associative learning, locomotor activity, and regulating NAc neural responses by using D2R-knockout (KO) mice and their wild-type littermates. D2R-KO mice displayed reduced locomotor activity and slower acquisition of a place-learning task. D2R-KO eliminated the prereward inhibitory response of neurons in the NAc. In contrast, an increased number of neurons in D2R-KO mice displayed place-related activity. These results provide evidence that D2R in the NAc participates in coding for a specific type of neural response to incentive contingencies and partly in spatial learning.
The ability to predict future events such as rewards permits an organism to interact with its environment in a manner that is essential for its survival (1). Research on motivation, locomotion, addiction, and reward processes has emphasized the essential contribution of the mesolimbic dopamine system (2, 3). Dopaminergic activity at the level of the nucleus accumbens (NAc) seems to be important in forming a prediction of reward (4, 5) that is an essential determinant for learning-approach behaviors to reward sources (2, 6). In addition to a dense dopamine innervation from the ventral tegmental area (VTA; refs. 5 and 7), the extensive cortical and limbic inputs (8–10) also are integrated in the NAc, a brain structure closely involved in motivation and goal-directed behavior (6). However, the involvement of dopamine receptor subtypes in assessing reward information still remains to be specified. Knockout (KO) strategies offer a prospectively unique approach for revealing the function of specific proteins and neurotransmitter systems in behavior. For example, the effect of dopamine D2 receptor (D2R) in opioid analgesia was demonstrated in the D2R-KO mouse (11). Therefore, we trained KO mice lacking D2R (D2R-KO) and their wild-type (WT) littermates in spatial tasks using intracranial self-stimulation (ICSS) as rewards. To investigate the involvement of D2R functions in reward and spatial associative processes, we recorded neural activity from the NAc of D2R-KO mice and their WT littermates, which were well trained to perform two spatial tasks: random reward place-search task (RRPST) and place-learning task (PLT). In the RRPST, mice learned to move randomly in the open field to obtain ICSS rewards. The PLT, a type of associative learning task, required mice to learn and memorize two reward places set at fixed locations in the open field.
Methods
Subjects.
Six male D2R-KO (27–33 g) and seven WT (28–37 g) mice were used in the present experiments. We obtained the mice from a collaborative laboratory (Laboratory of DNA Biology and Embryo Engineering, University of Tokyo, Tokyo). The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrode Implantation and ICSS Training.
Mice were implanted bilaterally with monopolar stimulating electrodes for ICSS in the posterior lateral hypothalamic area (anteroposterior = −2.3 mm, mediolateral = ±0.70 mm, and dorsoventral = −5.3 mm from the bregma) (12). The recording electrodes consisting of a bundle of eight nichrome wires inserted into a stainless steel cannula were mounted on a moveable microdrive, enabling later adjustment of electrode position. This electrode assembly was implanted into the dorsal part of the NAc (AP = +1.42 mm, ML = ±0.75 mm from the bregma) at the same surgery. After the mice recovered from surgery, the efficacy of electrical stimulation was verified in a nose-poking chamber with a small round hole in the center. Each time a mouse poked its nose into the hole, a 500-ms train of 0.3-ms biphasic square waves pulsed at 80 Hz was generated by a stimulator. The mice were trained daily to self-stimulate in a 30–60-min session for 5–7 days until stable nose-poking was obtained.
Spatial Task Training.
The mice were trained to perform spatial tasks (13) in an 80-cm-diameter open field. The cumulative distance traveled in extended trials of 10-min duration on the first day of training served as a measure of spontaneous locomotor activity. In the second task, mice performed a distance movement (DM) task, in which the mice could obtain rewards if their moving distance met a predetermined distance. DM criteria began at 30 cm and was increased incrementally to 100 cm when the mice acquired 50 ICSS rewards within 10 min. The mice were tested subsequently in the RRPST and PLT. (i) For RRPST, a reward place was delineated with its center chosen at random within the open field. The mouse was rewarded with ICSS when it entered the reward place. (ii) For PLT, two reward places were located diametrically opposite to one another in the open field. The mouse was rewarded in both places when it returned to one reward place after visiting to the other. A trial was terminated when the mouse had received 50 ICSS or 10 min had passed, whichever occurred first.
Behavioral Analysis.
A microcomputer was programmed to monitor (i) the number of nose pokes that occurred in the operant chamber, (ii) spontaneous locomotion in the open field, and (iii) the number of rewards acquired, distance traveled, and duration of each trial in the three tasks. Comparisons of the number of nose pokes, spontaneous locomotor activity, and trial duration in the DM tasks were made by a two-tailed Student's t test. The number of rewards and distance traveled in the RRPST and PLT were analyzed using two-way ANOVA (type, between subjects; day, within subjects). Individual comparisons between subjects used the two-tailed Student's t test, and within subjects by Fisher's probable least-squares difference test.
Electrophysiological Recording.
The recording electrode assembly was advanced in the NAc at 40–60 μm per day. Single neural activities were recorded when the mice perform the RRPST and PLT by using a conventional recording procedure (13).
Analysis of Neural Correlates with Reward and Location.
Unit data in 4-s bins before and after the starting points of reward delivery were chosen and used to generate histograms and rastergrams. The firing rate seen before receiving rewards in the RRPST trial served as the baseline rate, which was compared with the firing rate seen in the reward period by using a t test. Based on responses of neurons to ICSS in the RRPST, recorded neurons were classified into three categories: the inhibitory (I), excitatory (E), and no response (N) types if neurons displayed significant increase, decrease, or no change in firing rate, respectively. Prereward and postreward responses of I, E, and N type neurons were determined in the PLT (Table 1) by comparing neural activity for 1.5 s before and after ICSS with the baseline rate. The neurons were tabulated as having prereward inhibitory (Ipre), excitatory (Epre), and no response (Npre) and postreward inhibitory (Ipost), excitatory (Epost), and no response (Npost) if neurons showed relevant response in these phases. Place fields were delineated in RRPST by processes described previously (13). A place field is a cluster of pixels with a firing rate exceeding twice the identified mean firing rate, and each place field contains at least nine contiguous pixels.
Table 1.
Comparisons of accumbens neural responses between WT and D2R-KO mice
| Mice | Prereward
|
Postreward
|
Place-related
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ipre | Epre | Npre | Ipost | Epost | Npost | Number | Size, cm2 | ||
| WT | |||||||||
| ICSS | I 30 | 11 | 4 | 15 | 9 | 6 | 15 | 4 | 435 ± 88 |
| E 13 | 0 | 8 | 5 | 1 | 6 | 6 | 3 | ||
| N 40 | 4 | 4 | 32 | 3 | 1 | 36 | 2 | ||
| Total | n = 83 | 15 | 16 | 52 | 13 | 13 | 57 | 9 | |
| D2R-KO | |||||||||
| ICSS | I 29 | 0 | 1 | 28 | 9 | 5 | 15 | 9 | 738 ± 89* |
| E 7 | 0 | 2 | 5 | 0 | 4 | 3 | 4 | ||
| N 18 | 0 | 3 | 15 | 2 | 1 | 15 | 2 | ||
| Total | n = 54 | 0** | 6 | 48 | 11 | 10 | 33 | 15* | |
A total of 83 and 54 neurons recorded, respectively, from the WT and D2R-KO mice were classified as inhibitory (I), excitatory (E), and no response (N) according to their responses to ICSS in the RRPST. The responses of classified neurons prior to and after reward were examined in the PLT. Place field sizes were measured in the RRPST.
, P < 0.05; and
, P < 0.001 (χ2 test).
Results
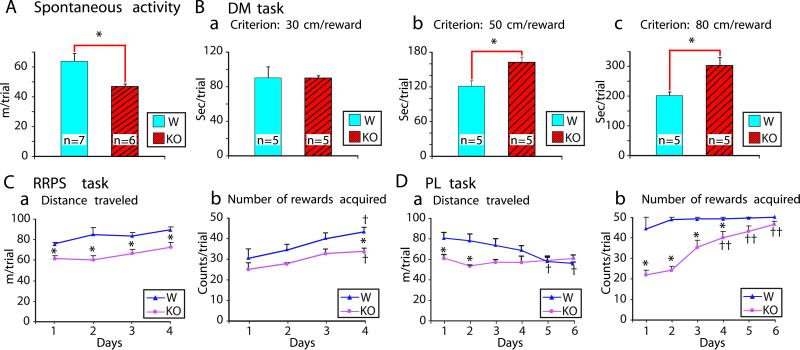
First, we tested the effect of D2R-KO at the behavior level. There were no significant differences between the two types of mice in the mean current intensity or mean number of nose pokes for ICSS in the operant chamber, which indicates that D2R-KO disrupted neither self-stimulation behavior nor simple motor performance. However, in tests of forward locomotion over extended periods, the D2R-KO mice showed a marked reduction in activity compared with their WT littermates (Fig. 1A). We found no difference in the elapsed time per trial between the two groups when a shorter DM criterion was used (Fig. 1Ba) but did find differences when the DM criterion was set at longer intervals (Fig. 1 Bb or Bc), which shows that the D2R-KO mice tended to move more slowly than the WT mice (14, 15). The reduced locomotion seen in the D2R-KO mice continued in the RRPST training through 4 consecutive days (Fig. 1Ca). For the number of rewards acquired in the RRPST, there was no significant difference between the two groups except on day 4 (Fig. 1Cb).
Figure 1.
Comparisons of spontaneous locomotor activity and performance on spatial tasks between WT and D2R-KO mice. (A) Spontaneous locomotor activity. (B) Performance on the DM task. (a–c) Mean elapsed time per trial in DM tasks with predetermined criteria were 30, 50, and 80 cm per reward. (C and D) Performance on the RRPST and PLT, respectively. (a) Mean distance traveled per trial. (b) Mean number of rewards acquired per trial. D2R-KO mice showed slower acquisition of the PLT. All data are expressed as mean ± SEM. *, P < 0.05 versus WT group (Student t test); †, P < 0.05; ††, P < 0.01 versus same group on day 1 (Fisher's probable least-squares difference test). W, WT mice; KO, knockout mice.
In contrast to the RRPST performance, the D2R-KO mice clearly were delayed in the acquisition of the PLT (Fig. 1D). The D2R-KO mice traveled less than the WT in the PLT only during the first 2 days (Fig. 1Da) but acquired fewer rewards than the WT mice until the 4th day (Fig. 1Db). At the end of the training sessions, the D2R-KO mice acquired approximately the same number of rewards as the WT mice. However, examination of the trails traveled in the PLT showed that whereas the WT mice visited two fixed rewarding places in an efficient shuttle manner (Fig. 2 Ba and Da), the D2R-KO mice displayed an inefficient pattern of movement between these significant places in the open field (Fig. 3Ba and Da).
Figure 2.
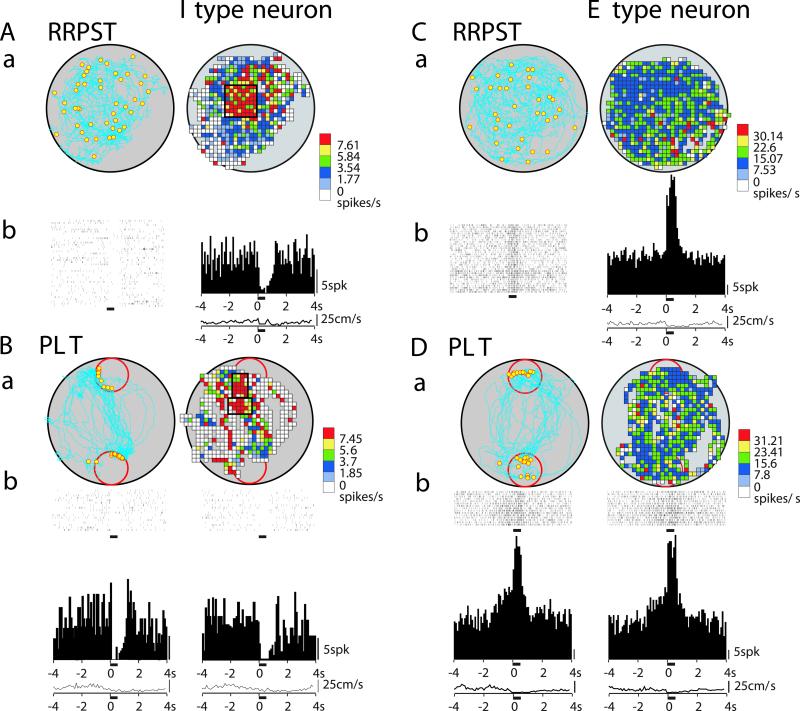
Examples of reward and place correlates of accumbens neurons during the RRPST and PLT in WT mice. (A and C) Performance of mice and neural responses on the RRPST. (a) Trail of mouse (Left) and firing-rate map (Right). Yellow dots in the trail map indicate the locations of reward delivery. (b) Single sweep of responses to ICSS (Left) and its expanded display (Right). The bars above the sweeps indicate the ICSS period. (Cb) Activation of a neuron by ICSS (Left) and superimposed responses (Right) show activation with a fixed latency of 2.0 ms (●) in an all-or-none manner, and other responses with varied latencies of 4–9 ms. ▾, stimulation delivered at 10-ms intervals. (c) Rastergrams (Left), histograms of firing (Right), and curve of averaged locomotion speed (Bottom Right). (B and D) Performance of mice and neural responses on the PLT. (a) Trail of mouse reward locations (Left) and firing-rate map (Right). (b) Rastergram (Upper Left), histogram of firing (Middle Left), and curve of averaged locomotion speed (Bottom Left) corresponding to data recorded at the upper red-circled place. (Right) Rastergram, histogram, and curve of averaged speed corresponding to data recorded at lower red-circled place. Most neurons responded similarly at both reward sides, but note that this I-type neuron had higher prereward inhibition at upper reward side and was insensitive to movement speed. The horizontal bars below the rastergrams, histograms, and speed curves indicate the ICSS period. The color-scale tables to the right of the firing maps show calibration for firing rate. The thick black open rectangles in color-coded firing rate maps demarcate place fields.
Figure 3.
Examples of reward and place correlates of accumbens neurons during RRPST and PLT in D2R-KO mice. (A and C) Performance of mice and neural responses on the RRPST. (a) Trail of mouse, reward locations (Left), and firing-rate map (Right). (b) Rastergrams (Left), histograms of firing (Right), and curves of averaged locomotion speed (Bottom Right). (B and D) Performance of mice and neural responses on the PLT. Other notations are similar to those described for Fig. 2. Note that prereward inhibition is absent in I-type neurons (Bb), whereas E-type neurons still displayed prereward excitation (Db).
We next tested the plasticity of the NAc neurons to address the issue of how the D2R influences neural responses in the NAc to reward information. All neurons presented here were recorded from the medial core part of the NAc. Fig. 2 A and C shows typical examples of inhibitory (I-type) and excitatory (E-type) responses in the RRPST, respectively, during the ICSS period recorded in the WT mice. During the ICSS period, suppression in firing of the I-type neurons ranged from 40 to 94% (Fig. 2 Ab and Ac) and facilitation of the E-type ranged from 70 to 480% (Fig. 2 Cb and Cc). These I- and E-type neurons in the RRPST also showed prereward inhibitory (Fig. 2Bb) or excitatory responses (Fig. 2Db) in the PLT, respectively. In the WT mice, the majority of the I-type neurons showed inhibitory responses of 1–1.5 s preceding the reward delivery, with decreases in firing from 36% to a nadir of 80%, and most of the E-type neurons showed prereward excitatory responses with increases in firing from 40 to 216%. Postreward responses lasted for 0.5–1.5 s, with decreases in firing from 38 to 80% or increases in firing from 40 to 450%.
Two neurons shown in Fig. 3 Ab and Cb are typical I and E types, respectively, recorded from the D2R-KO mice. The prereward inhibitory response in the predictable reward (i.e., PLT) is absent in the I-type neuron (Fig. 3Bb), which still displays an inhibitory response in the reward and postreward phases (Fig. 3Ab). Conversely, the prereward excitatory response appeared normally (Fig. 3Db) in the E-type neuron (Fig. 3Cb).
Population comparisons of neurons with reward and place correlates between the WT and D2R-KO mice revealed further interesting differences (Table 1). The number of neurons with prereward excitatory responses in WT mice (n = 16) was nearly equal to those with inhibitory responses (n = 15), which is consistent with our previous study using rats (16). In the D2R-KO mice, in contrast, there were no neurons with prereward inhibitory responses, whereas the number of prereward excitatory neurons was comparable to the WT mice. The number of neurons with prereward excitatory responses of E-type neurons in the WT and D2R-KO mice had no difference. Although the total number of responding neurons in the reward and postreward phases did not differ between the WT and D2R-KO mice, during the rewarding ICSS the inhibitory responses were observed more frequently in the KO mice.
Paradoxically, in contrast to the low number of neurons that responded during the prereward phase, place-related cells (i.e., neurons that fire in a specific location in the open field) were observed much more frequently in the D2R-KO mice (nearly 3-fold relative to the WT mice). Fig. 2A also shows an example of a place-related neuron in the WT mice, with a small place field located near the center of the open field. This neuron showed both prereward and place correlates. Thus, integration of spatial and reward information is possible at the level of individual neurons in the NAc. An I-type neuron of the D2R-KO mice with a larger place field having no prereward response is illustrated in Fig. 3 Aa and Ba. The averaged place-field size of the place-related cells in the D2R-KO mice was about twice that of the WT mice (Table 1).
Discussion
Nigrostriatal dopamine neurons participate in motor acts, whereas the mesolimbocortical dopamine pathway, arising from the VTA and innervating the ventral striatum including the NAc, plays a role in incentive motivational processes (3, 17, 18). Therefore, locomotion impairment observed in the D2R-KO mice may reflect changes to the D2R system located in the nigrostriatal area (19). Furthermore, changes in neural responses to incentive stimuli in these mice may result from disruption of D2R in the mesolimbocortical dopaminergic pathway. In the D2R-KO mice, the prereward inhibitory response was eliminated, whereas the inhibitory responses recorded during and after reward were unchanged. These observations lead to a firm point that the D2R plays a crucial role in determining the specific type of response displayed by NAc neurons in a prereward period. In addition to this role for the D2R system, the present results do not preclude the involvement of other transmitter systems such as the noradrenergic inputs (20, 21) from the locus coeruleus (22) in the reward or consummatory phase (2, 23). In the D2R-KO mice, although no D2R was found in the NAc, the expression of other substances such as enkephalin increased (14, 15). These phenomena also might influence the behavior of D2R-KO mice. The fact that the D2R-KO mice engaged in self-stimulation behavior seems not to support a conjecture for a role of the D2R in brain stimulation (24).
The PLT requires the use of previously acquired information about the location of reward to guide the animal's prospective search behavior. Reward-predicting information is recruited also in establishing approach behavior (4, 5). The slower acquisition of the PLT observed in the D2R-KO mice could reflect changes in processes used to acquire associative environmental information and prereward coding essential for efficient approach response to the reward. Drug-infusion studies have confirmed the involvement of D2R in spatial tasks with an emphasis on the memory-consolidation process (25). We assume that the repetition of PLT trials on consecutive days, in conjunction with a number of exclusively excitatory prereward responses, both contributed to the ability of the D2R-KO mice to acquire reward-predicting responses and thereby to improve their performance. The strong bias to place-related neural activity in NAc neurons in the D2R-KO mice (Table 1) suggests that neural substrates for spatial mapping of the environment were compromised in mice lacking the D2R.
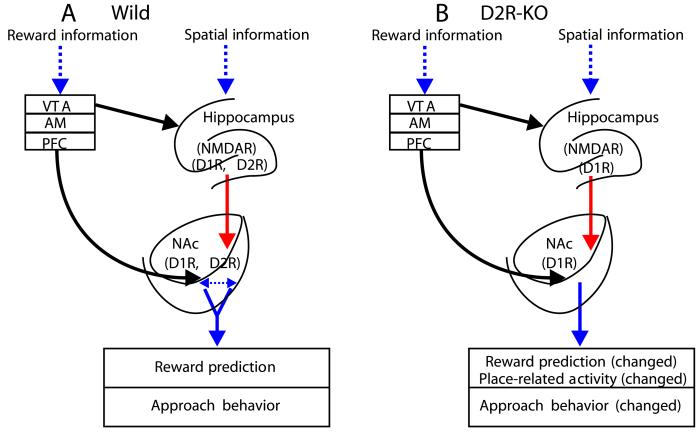
Finally, we would like to discuss the anatomical and functional connections of the NAc and propose putative neural circuits to rationalize the present finding at neural and behavioral levels (Fig. 4). The HF, AM, and PFC send fibers to the NAc (8). Spatial and contextual information are associated in the HF (13, 26–28). Specific rewards are coded in the activity of the AM (29) and PFC (30–32). In addition, massive dopaminergic inputs arising from the VTA to the NAc are widely recognized to mediate rewards and drug abuse (3, 33). In the WT mice (Fig. 4A), relevant reward information would be processed in the AM, VTA, and PFC, and environmental information would be processed in the HF, both of which then converge onto NAc neurons (9, 16). The action of dopamine through the D1 and D2 receptors in the NAc and its afferent sources may interact with the glutamatergic action through N-methyl-D-aspartate receptors (34, 35). This mutual interaction might be critical for the function of neural ensembles in the NAc (10) and its sources, which then affect spatial learning. On one level, the absence of inhibitory prediction in the D2R-KO mice may reflect the crucial involvement of D2R in the NAc in the incentive phase of motivated behavior (Fig. 4B). However, it also might reflect the changes in the D2R system of structures providing reward information to the NAc such as the PFC and AM. Unchanged excitatory responses related to reward prediction may have been sufficient to permit the D2R-KO mice to perform spatial tasks based on memory of a place associated with reward (e.g., the PLT). Last, the effect of D2R-KO might bias the activation of the HF cells, thereby increasing the flow of spatial information to efferent structures, which could trigger more potent NAc neurons representing place information. Nevertheless, at present it is still difficult to explain the significant change in the number of place-related cells and place-field size in the NAc of the D2R-KO mice. Many HF place cells are reported to show a prereward response in a reward place if it is set within the place field; furthermore, this responsiveness to a reward place is thought to be involved in place recognition (13). Given that NAc neurons recorded in the present study displayed prereward responses at both reward places, which were independent of the place field, it is highly unlikely that such prereward responses reflect the prereward responses of HF place cells. We conclude therefore that NAc I- and E-type neurons are involved in prediction of reward but not in place recognition.
Figure 4.
Putative neural circuits for reward and place-related responses in the WT and D2R-KO mice. (A) In the WT mice, reward information passed through the VTA, amygdala (AM), and prefrontal cortex (PFC) converge onto NAc neurons. Spatial, contextual information signaling rewards are processed in the hippocampal formation (HF) with action of N-methyl-D-aspartate receptors (NMDAR) in interaction with dopamine receptors (D1R and D2R). Association of two processed flows is integrated in the NAc, also with the involvement of dopamine receptors. (B) In the D2R-KO mice, the absence of D2R leads to imbalance of dopamine receptors, which results in changes of approach behavior and NAc neural responses to incentive stimuli and place. Note that information inputs from the AM, PFC, and VTA to the NAc are separated but were simplified by sharing one arrow line as shown in the figure. See Discussion for detail.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (C), Advanced Brain Science Project, from the Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Abbreviations
- NAc
nucleus accumbens
- VTA
ventral tegmental area
- D2R
dopamine D2 receptor
- KO
knockout
- WT
wild type
- ICSS
intracranial self-stimulation
- RRPST
random reward place-search task
- PLT
place-learning task
- DM
distance movement
- AM
amygdala
- PFC
prefrontal cortex
- HF
hippocampal formation
References
- 1.Schultz W, Dayan P, Montague P. Science. 1997;245:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 2.Ikemoto S, Panksepp J. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 3.Kupfermann I, Kandel E R, Iversen S. In: Principles of Neural Science. 4th Ed. Kandel E R, Schwartz J H, Jessell T M, editors. New York: The McGraw-Hill Companies; 2000. pp. 998–1013. [Google Scholar]
- 4.Blackburn J R, Phillips A G, Jakubovic A, Fibiger H C. Behav Neurosci. 1989;103:15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Schultz W. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Robbins T W, Everitt B J. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 7.Mogenson G J, Jones D L, Yim C Y. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 8.Brog J S, Salyapongse A, Deutch A Y, Zahm D S. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 9.Floresco S B, Seamans J K, Phillips A G. Behav Brain Res. 1996;80:161–168. doi: 10.1016/0166-4328(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell P, Greene J, Pabello N, Lewis B L, Grace A A. Ann NY Acad Sci. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- 11.King M A, Bradshaw S, Chang A H, Pintar J E, Pasternak G W. J Neurosci. 2001;21:7788–7792. doi: 10.1523/JNEUROSCI.21-19-07788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 13.Kobayashi T, Nishijo H, Fukuda M, Bures J, Ono T. J Neurophysiol. 1997;78:597–613. doi: 10.1152/jn.1997.78.2.597. [DOI] [PubMed] [Google Scholar]
- 14.Baik J H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Nature (London) 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi H, Aiba A, Nakamura K, Nakao K, Sakagami H, Goto K, Kondo H, Katsuki M. Genes Cells. 1996;1:253–268. doi: 10.1046/j.1365-2443.1996.d01-238.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin P D, Ono T. Behav Brain Res. 2000;116:23–38. doi: 10.1016/s0166-4328(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 17.Horvitz J C. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 18.Hajnal A, Norgren R. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung M P, Sankoorikal E B. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barone F C, Wayner M J, Tsai W H, Zarco de Coronado I. Brain Res Bull. 1981;7:181–193. doi: 10.1016/0361-9230(81)90083-6. [DOI] [PubMed] [Google Scholar]
- 21.Ono T, Nakamura K, Fukuda M, Kobayashi T. J Neurophysiol. 1992;67:265–279. doi: 10.1152/jn.1992.67.2.265. [DOI] [PubMed] [Google Scholar]
- 22.Delfs J M, Zhu Y, Druhan J P, Aston-Jones G S. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 23.Rolls E T. The Brain and Emotion. New York: Oxford Univ. Press; 1999. [Google Scholar]
- 24.Nakajima S, Patterson R L. Brain Res. 1997;760:74–79. doi: 10.1016/s0006-8993(97)00304-1. [DOI] [PubMed] [Google Scholar]
- 25.Setlow B, McGaugh J L. Behav Neurosci. 1998;112:603–610. doi: 10.1037//0735-7044.112.3.603. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura N, Nishijo H, Tamura R, Eifuku S, Endo S, Ono T. J Neurosci. 1999;15:2381–2393. doi: 10.1523/JNEUROSCI.19-06-02381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huerta P T, Sun L D, Wilson M A, Tonegawa S. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- 28.Wiener S I, Korshunov V A, Garcia R, Berthoz A. Eur J Neurosci. 1995;7:2206–2219. doi: 10.1111/j.1460-9568.1995.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 29.Ono T, Nishijo H, Uwano T. Prog Neurobiol. 1995;46:401–422. doi: 10.1016/0301-0082(95)00008-j. [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Nishino H, Fukuda M, Sasaki K, Nishijo H. Brain Res. 1984;8:323–332. doi: 10.1016/0006-8993(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.Schoenbaum G, Chiba A A, Gallagher M. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M. Nature (London) 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 33.Spanagel R, Weiss F. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 34.Kalivas P W, Duffy P. Brain Res. 1997;761:173–177. doi: 10.1016/s0006-8993(97)00464-2. [DOI] [PubMed] [Google Scholar]
- 35.Wilkerson A, Levin E D. Neuroscience. 1999;89:743–749. doi: 10.1016/s0306-4522(98)00346-7. [DOI] [PubMed] [Google Scholar]