Abstract
Acid-sensing ion channel 3 (ASIC3), a proton-gated ion channel of the degenerins/epithelial sodium channel (DEG/ENaC) receptor family is expressed predominantly in sensory neurons including nociceptive neurons responding to protons. To study the role of ASIC3 in pain signaling, we generated ASIC3 knockout mice. Mutant animals were healthy and responded normally to most sensory stimuli. However, in behavioral assays for pain responses, ASIC3 null mutant mice displayed a reduced latency to the onset of pain responses, or more pain-related behaviors, when stimuli of moderate to high intensity were used. This unexpected effect seemed independent of the modality of the stimulus and was observed in the acetic acid-induced writhing test (0.6 vs. 0.1–0.5%), in the hot-plate test (52.5 and 55 vs. 50°C), and in tests for mechanically induced pain (tail-pinch vs. von Frey filaments). We postulate that ASIC3 is involved in modulating moderate- to high-intensity pain sensation.
Cation channels of the degenerin/epithelial sodium channel family (DEG/ENaC) have been proposed as transducers of somatosensory stimuli in several species (1). The structural hallmarks of these proteins are two hydrophobic transmembrane domains, with short N and C termini and a large extracellular loop. In vertebrates, the DEG/ENaC family includes several related subunits of Na+-selective (PNA/PK, 8–40) acid-sensing ion channels (ASIC1a, ASIC1b, ASIC2a, ASC2b, ASIC3, and ASIC4; previously named ASIC-α/BNC2, ASIC-β, MDEG1/BNC1, MDEG2, DRASIC, and SPASIC, respectively) (2–4). ASIC proteins associate as homo- or heteromultimers to form functional receptors. Because these receptors are gated by protons, it has been suggested that they might be involved in the perception of pain during tissue acidosis (5). However, there is evidence also that they are involved in mechanosensation; many DEG/ENaC proteins are localized to mechanosensitive cells in Caenorhabditis elegans, Drosophila melanogaster, rat, and mouse (1, 6, 7). In C. elegans, mutations in DEG/ENaC proteins such as MEC-4 and MEC-10 lead to impaired touch responses (8, 9), and the targeted deletion of ASIC2 in mice resulted in a reduced sensitivity of low-threshold mechanoreceptors (7).
One member of the ASIC family, ASIC3, seems to be a particularly good candidate for the transduction of proton and mechanical stimuli, because ASIC3 is expressed predominantly in dorsal root ganglia neurons (10) including large-diameter mechanoreceptors and unmyelinated small-diameter peptidergic nociceptors (3, 6, 11). In addition, ASIC3 protein was found to be present in sensory nerve terminals in Meissner corpuscles lanceolate fibers, which correspond to rapidly adapting low-threshold mechanoreceptors, as well as in free nerve endings, which may correspond to nociceptors (6).
Previous studies have shown that ASIC3 can be activated by protons and generate biphasic inward currents when it was expressed in heterologous cells (12). A transient inward current can be induced when extracellular pH falls to 7.0, which is well within the physiological range. In these same cells, a further pH drop to 5.0 also can induce a sustained current (13–15).
A recent physiological analysis of ASIC3-deficient mouse mutants has revealed alterations in mechano- and acid-sensitive neurons such as a reduced sensitivity of some mechanoreceptors to noxious pinch and an enhanced sensitivity to light touch (6). However, the contribution of ASIC3 to pain signaling remains unclear. In this study we show that ASIC3−/− animals display enhanced behavioral responses to high-intensity nociceptive stimuli regardless of their modality. These results reveal a role for ASIC3 in the tonic inhibition of high-intensity pain signals.
Materials and Methods
Generation and Breeding of ASIC3−/− Mice.
Genomic clones containing the mouse ASIC3 gene were screened from a 129/Sv mouse genomic library by using a 710-bp (nucleotides 1–710) fragment from the rat ASIC3 cDNA (10). λ phage clones containing the entire coding region were isolated, and the inserts were subcloned into pBluescript II KS(+) (Stratagene). The targeting vector was constructed by replacing a 2.5-kb BsrGI fragment (containing exons 2–11) with a lacZ-neo cassette. This targeting construct was introduced into embryonic stem cells by homologous recombination using standard protocols (16). Mutant embryonic stem cell lines were injected into C57BL/6J blastocysts, and chimeras were backcrossed to CD-1 mice. In most behavioral assays, F2 offspring (8–12 weeks) were tested. F4 mice with a CD-1 background were used in the von Frey fiber, tail-pressure, and carrageenan tests.
Mouse Genotyping.
The ASIC3 mutation was confirmed by Southern blotting with a 1.8-kb external SpeI/SacI DNA fragment from the genomic clone. Subsequent genotypings were performed by PCR. A genomic 5′ primer, TGTGGTCCCAGGACTTGGTA, and a 3′ primer, ATACTTGCTGTTGCTGGCAG, were used to identify the wild-type allele (0.7 kb). The DRASIC knockout allele was identified with the same 5′ primer and a 3′ lacZ primer, ATTCAGGCTGCGCAACTGTT (0.5 kb).
Northern Analysis.
RNA was isolated from tissues by using TRIzol (Invitrogen). Total RNA (5–10 mg) was loaded in each lane in a formaldehyde agarose gel. Gel electrophoresis and Northern blotting were performed by using a standard method. 32P-labeled cDNA probes were hybridized with blots in 5 ml of ExpressHyb solution (CLONTECH) at 68°C for 1 h. Blots were washed in 0.2 × SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7.0)/0.5% SDS for 40 min. For ASIC3 expression, a 650-bp EcoRI/BsrGI DNA fragment (nucleotides 1–650) corresponding to the first exon was used as probe. For other members of the ASIC family, the similar region of exon 1 was chosen. For vanilloid receptor (VR)1, the blots were probed with a 400-bp PCR fragment amplified by using the primers 5′-AGACAGACAGCCTGAAGCAGTTT-3′ and 5′-CTTGTCACGAACTTGGTGTTGTC-3′. For VRL1, the blots were probed with a 430-bp PCR fragment corresponding to amino acids 78–220. For cyclophilin, the probe was a 300-bp PCR DNA fragment that was amplified by using the primers 5′-ACCCCACCGTGTTCTTCGGAC-3′ and 5′-CATTTGCCATGGACAAGATG-3′.
Immunohistochemistry.
Immunostaining was performed as described (10). Primary antibodies against peripherin, N52, substance P (dilutions of 1:500, 1:500, and 1:100 respectively, Chemicon) were diluted in blocking solution comprising 1× PBS, 1% BSA, and 0.5% Triton X-100. Secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes) were used at a 1:500 dilution in blocking solution. isolectin B4-fluorescein isothiocyanate (4 μg/ml) was diluted in PBS containing 0.1 mM CaCl2, MgCl2, MnCl2, and 0.2% Triton X-100.
Behavioral experiments.
Mice were housed in a temperature- and humidity-controlled vivarium kept on a 12-h dark/light cycle (light on at 0700 Eastern standard time). Animals had free access to food and water. Male animals, 8–12-weeks old, were used for behavioral studies. All experiments were approved by local ethical committees.
For the acetic acid-induced writhing test, mice (n = 8–13 per group) were injected i.p. with 10 ml/kg 0.1–0.6% acetic acid (pH 2.8) and separated into individual cages. A few minutes after the injection, animals showed abdominal constrictions and lengthwise stretches of the torso with a concomitant concave arching of the back. The number of abdominal constrictions was counted for 20 min after the injection. The onset time of the first constriction was recorded also.
The tail-flick assay was performed by using an automated tail-flick apparatus (Columbus Instruments, Columbus, OH) by standard procedures (17, 18). The cut-off time was 12 sec. The number of animals was 10 per group.
The hot-plate test was performed with an electronically controlled hotplate analgesia meter (Columbus Instruments) at 5, 52.5, and 55°C. The latency until mice showed first signs of discomfort (hind-paw lifting, licking, or shaking, and jumping) was recorded. The number of animals was 10 per group.
To determine mechanical pain sensation and hyperalgesia, a von Frey filament test was used. Briefly, mice were placed on a wire mesh platform in a transparent Plexiglas chamber (7 × 11 × 12 cm). Mice (n = 10 per group) were allowed to habituate for 30 min before the test. A series of von Frey fibers (0.23–3.63 g) were applied through the wire mesh onto the plantar surface of both hind paws in ascending order beginning with the finest fiber. A withdrawal response was considered valid only if the hind paw was removed completely from the platform. If the paw withdrawal response was ambiguous, the application was repeated. For each paw, a von Frey hair was applied five times at 5-sec intervals. The threshold was determined when paw withdrawal was observed in more than three of five applications.
For the tail-pressure test, an analgesia meter (Ugo Basile, Comerio, Italy) was used. The tail was placed on a small plinth under a cone-shaped pusher. The force applied to the tail by the plinth increases at a constant rate. We determined the force at the moment of tail flick. The number of animals was 20 per group.
Hargreave's test was performed basically as described (19). Briefly, each mouse was placed on a 3/16th-inch-thick glass floor and restrained in a glass jar (6.5 × 7.5 × 9.5 cm). Mice (n = 10 per group) were allowed to habituate for at least 2 h before testing. The plantar surface of mouse hind paws was stimulated by a high-intensity beam (45 W) from a projector lamp bulb located 6 cm below the glass floor. The paw-withdrawal latency was measured.
For carrageenan hypersensitivity, λ carrageenan (20 mg/ml, Sigma) was suspended in an isotonic saline solution and injected into the plantar surface of the right hind paw in a volume of 20 μl by using a 27-gauge needle. After 4 h, mice (n = 10 per group) were tested either for thermal sensitivity of both hind paws by the Hargreave's test or mechanical sensitivity by the von Frey fiber test. For capsaicin hypersensitivity, capsaicin (0.1 μg/μl in an isotonic saline containing 10% ethanol and 0.5% Tween 80, Sigma) was injected into the plantar surface of the right hind paw in a volume of 20 μl. After 4 h, thermal and mechanical sensitivity was tested as described above.
Data were analyzed by one- or two-way ANOVA followed by post hoc tests when appropriate with the STATVIEW 5.0 program (SAS Institute, Cary, NC).
Results
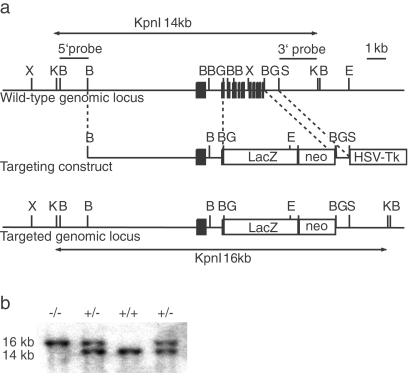
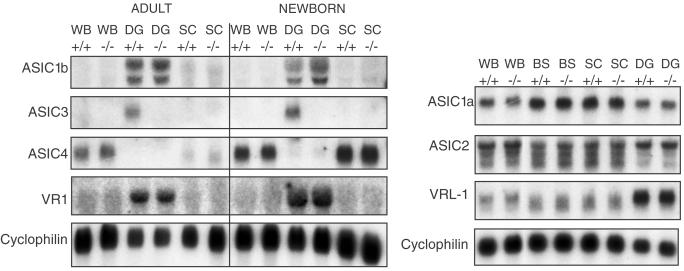
To inactivate the ASIC3 gene, we replaced exons 2–11 with a lacZ-neo cassette by homologous recombination in embryonic stem cells (Fig. 1). This deletion removes the second transmembrane domain and most of the extracellular domain. Homozygous ASIC3−/− mice were obtained with almost the expected Mendelian frequency (ASIC3+/+, 336 animals, 27%; ASIC 3+/−, 591 animals, 49%; ASIC3−/−, 286 animals, 24%). They were healthy and fertile and cared for their offspring. The absence of ASIC3 expression in homozygous mutant mice was confirmed by Northern blot analysis using an exon 1-specific probe. ASIC3 is expressed as a single transcript in dorsal root ganglion cells of wild-type animals, whereas no expression can be detected in homozygous ASIC3−/− mice. We also probed Northern blots with ASIC1a-, ASIC1b-, ASIC2-, ASIC4-, VR1-, and VRL1-specific probes to determine whether the ASIC3 mutation interferes with the expression of other members of the ASIC gene family. As shown in Fig. 2, the expression levels of these genes were not altered in dorsal root ganglia, spinal cord, brainstem, and brain. These results indicate that no compensatory up-regulation of other ASIC genes occurred in ASIC3−/− knockout mice. To examine whether the absence of ASIC3 affects the development of sensory neurons, serial sections of dorsal root ganglia from ASIC3+/+ and ASIC3−/− mice were stained with cell-specific markers and counted. As shown in Table 1, the numbers of large- (N52-positive) and small- (peripherin-positive) diameter neurons were similar in both genotypes. Among small-diameter (nociceptive) neurons, the ratios of peptidergic (substance P-positive) and nonpeptidergic (isolectin B4-positive) cells also were similar. Thus, the ASIC3 mutation did not result in any gross developmental alterations of the dorsal root ganglia.
Figure 1.
Generation of ASIC3-deficient mice. (a) Restriction map of the murine wild-type ASIC3 locus, the targeting vector, and the mutant ASIC3 locus. The targeting vector was generated by replacing a 2.5-kb BsrGI fragment, containing exons 2–11, with lacZ-neo. Correctly targeted clones were identified by Southern blotting using 5′ and 3′ outside probes as indicated. (b) Southern blot analysis of tail biopsies after digestion with KpnI. The wild-type and mutant ASIC3 alleles can be identified as 14- or 16-kb fragments, respectively. X, XmaI; K, KpnI; B, BamHI; BG, BsrGI; S, SpeI; E, EcoRI.
Figure 2.
Northern blot analysis of neonatal (day 4) and adult mouse tissues with probes for ASIC1–ASIC4 and VR1 and VRL1. Cyclophilin served as a control. WB, whole brain; DG, dorsal root ganglia; SC, spinal cord; BS, brainstem.
Table 1.
Neuronal cell composition of dorsal root ganglia from ASIC3+/+ and ASIC3−/− mice
| Marker | ASIC3 genotype | Total cells | Positive cells | Percentage |
|---|---|---|---|---|
| N52 | +/+ | 2689 | 1206 | 44.9 |
| Peripherin | +/+ | 2689 | 1623 | 60.4 |
| N52/peripherin | +/+ | 2689 | 142 | 5.3 |
| N52 | −/− | 3364 | 1416 | 42.1 |
| Peripherin | −/− | 3364 | 2117 | 63.0 |
| N52/peripherin | −/− | 3364 | 169 | 5.0 |
| IB4 | +/+ | 879 | 309 | 35.2 |
| IB4 | −/− | 607 | 221 | 36.4 |
| Substance P | +/+ | 1258 | 129 | 10.3 |
| Substance P | −/− | 1553 | 199 | 12.8 |
Sections (10 μM) from dorsal root ganglia of ASIC3+/+ and ASIC3−/− mice were stained immunofluorescently for the indicated cell markers. IB4, isolectin B4; N52, 200-kDa neurofilmentprotein.
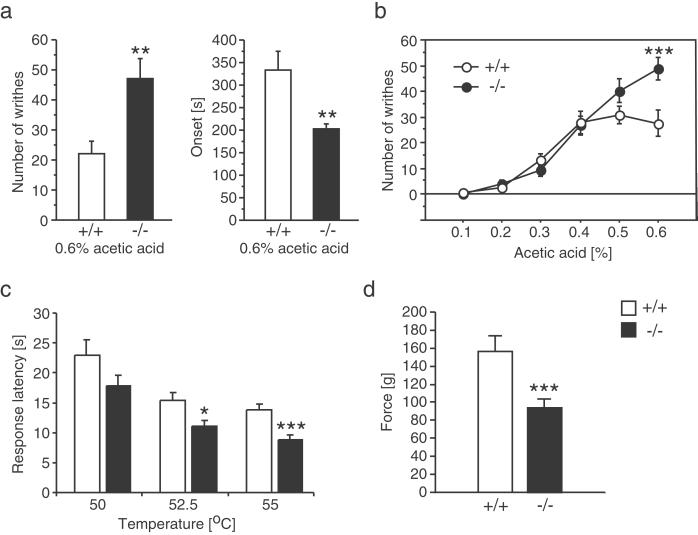
To examine acid-evoked pain responses, we used the acetic acid-induced writhing test. Unexpectedly, ASIC3−/− mice showed more writhings and had an earlier response onset than ASIC3+/+ animals (Fig. 3a). Thus, ASIC3−/− animals appeared to be more pain-sensitive in this test. Because this result was surprising, we wished to establish a dose-response curve for the acetic acid effect by using concentrations ranging from 0.1 to 0.6%. We found that the writhing response was similar in wild-type and knockout mice below a concentration of 0.4%. At higher concentrations there was no further increase in the writhing response of wild-type mice, but there was in knockout animals (Fig. 3b). Interestingly, we found that all ASIC3−/− mice showed an immediate pain response (vocalization) during the acetic acid injection. This response was not observed in any of the wild-type mice tested, further suggesting that ASIC3−/− mice are more sensitive to acid-evoked pain.
Figure 3.
Analysis of nociceptive responses in ASIC3+/+ and ASIC3−/− mice. (a) The number of writhing bouts was increased significantly and their onset was reduced significantly in ASIC3-deficient mice after i.p. injection of 0.6% acetic acid (10 ml/kg). (b) Injection of different acetic acid concentrations showed that the hyperalgesic phenotype of ASIC3−/− mice is observed only at high but not at low acid concentrations. (c) ASIC3-deficient mice have reduced response latencies in the hot-plate test at high- but not low-temperature settings. (d) The pain thresholds in the tail-pressure assay also were reduced significantly in ASIC3−/− mice. *, P ≤ 0.05; **, P ≤ 0. 01; ***, P ≤ 0.005.
To determine whether other acute pain responses were affected also by the ASIC3 mutation, we assessed thermal pain sensitivity by using the tail-flick and hot-plate tests. Mutant mice displayed normal responses in the tail-flick test (data not shown), but had significantly lower response latencies in the hot-plate test (Fig. 3c). Mechano-nociception was evaluated by the tail-pressure and von Frey fiber tests. We found that knockout mice were more sensitive to tail pressure than wild-type animals (Fig. 3d), whereas there was no significant difference between two genotypes in the von Frey fiber test (Fig. 4 b and c). It should be noted that the pain stimuli in the von Frey fiber test are much milder than that applied in the tail-pressure test. The dose-response analyses of the acetic acid-induced writhing and hot-plate tests suggested that genotype differences in nociception correlated with the intensity of the pain stimuli. To confirm this inference, we performed two-way ANOVA. In the writhing test, we found significant genotype (F1,106 = 5.425, P = 0.0217) and concentration (F5,106 = 47.5, P < 0.0001) effects and a significant interaction (F5,106 = 4.288, P = 0.0014). Post hoc analysis showed that the genotype effect was significant (P < 0.005) only at the highest concentration tested. For the hot-plate test, we found significant genotype (F1,66 12.747, P = 0.0007) and temperature (F2,66 = 16.36, P = 0.0001) effects but no interaction. Paired comparisons (t test), however, showed a significant difference in the response latency between knockout and wild-type mice only at 52.5 and 55 but not at 50°C. Together, these results indicate that the ASIC3 mutation affected nociceptive responses only at high stimulus intensities.
Figure 4.
Normal inflammation-induced hyperalgesia and allodynia in ASIC3+/+ and ASIC3−/− mice. Thermal hyperalgesia as determined with the Hargreave's test (a) and mechanical allodynia as measured with von Frey filaments (b) were similar in mutant and wild-type animals after s.c. injection of carrageenan. (c) Capsaicin injection also produced similar levels of mechanical allodynia in ASIC3+/+ and ASIC3−/− mice.
Finally, we tested the effects of the ASIC3 deletion on animal models of inflammation-induced hyperalgesia and allodynia. Thermal hyperalgesia and mechanical allodynia were assessed with the Hargreave's and von Frey fiber tests, respectively. Four hours after carrageenan injection, both ASIC3+/+ and ASIC3−/− mice showed mechanical allodynia and thermal hyperalgesia, but there was no difference between the two genotypes (Fig. 4 a and b). A similar result was found in mice injected with capsaicin (Fig. 4c). We still found a significant mechanical allodynia 24 (F1,17 = 7.28, P = 0.015) and 72 h (F1,17 = 5.50, P = 0.032) after carrageenan injection but no genotype effect (24 h: F1,17 = 0.46, P = 0.509; 72 h: F1,17 = 3.04, P = 0.100), suggesting that ASIC3 does not contribute to inflammation-induced changes in these nociceptive responses.
Discussion
We have generated mice with targeted disruptions of the ASIC3 gene and studied their responses to acid injections and other painful stimuli. ASIC3−/− mice have no obvious developmental effects. Their dorsal root ganglia appear to have normal numbers of neurons and neuronal subtypes. Northern blot analysis did not reveal any compensatory changes in RNA levels of other ASIC gene family members or VR1 and VRL1.
ASIC3 is highly sensitive to extracellular protons (pH0.5 activation = 6.7). It has a steep activation curve with a Hill coefficient of 4.3 and shows a rapid recovery from desensitization after prolonged exposure to pH ≤ 6.0 (14). These properties convinced earlier workers that ASIC3 channels may be used by sensory neurons to detect tissue acidosis. In this way, they could participate in mediating acid-evoked behavioral responses. However, recent physiological studies by Price et al. (6) showed that the loss of ASIC3 in mice has only subtle effects on proton-induced currents in C fibers. These subtle changes did not result in a loss of acid-induced behavioral responses, because recuperative behaviors were not changed in these animals after subdermal injections of acetic acid. It seems likely that the majority of proton-gated currents in these neurons are contributed by VR1 (20, 21).
To determine how these cellular alterations would affect the animals' responses to painful stimuli, we analyzed ASIC3−/− mice in a variety of nociceptive tests. We were surprised to find that in the acetic acid-induced writhing test, ASIC3−/− animals screamed while they were injected, had more writhing bouts, and an earlier onset of pain responses. Clearly, these behavioral responses suggest that the acid injection was more painful in the absence of ASIC3. However, the enhanced responses of mutant mice are not an indication for an increased sensitivity to protons. On the contrary, we only observed these effects when a high acid concentration (0.6%) was used but not at low acid concentrations. ASIC3−/− animals thus displayed exaggerated responses at high stimulus intensities. Most strikingly, a similar intensity-response relationship was observed for a thermal pain stimulus in the hot-plate test. Furthermore, with mechanical stimuli, we also did not find any differences between ASIC3+/+ and ASIC3−/− for low-intensity stimuli (von Frey test), but we found significantly enhanced pain responses in mutant mice when a high-intensity stimulus (tail pinch) was used. Together these results provide strong evidence that ASIC3 modulates moderate- to high-intensity pain stimuli regardless of their modality.
The modulatory role of ASIC3 on high-intensity pain signaling could be attributed to direct effects on the signaling properties of polymodal nociceptive C fibers. This idea is supported by the notion that ASIC3 can form heteromultimers with other ASIC proteins (5, 22–24). However, electrophysiological studies have demonstrated that the loss of ASIC3 reduces responses of C fibers to noxious stimuli (pressure, heat, and protons; ref. 6). Furthermore, alterations in thermal responses were seen only with low-intensity stimuli, when the stimulating temperatures were lower than 52°C. It thus seems that the loss of ASIC3 would increase rather than decrease the threshold of nociceptive C-fibers.
Alternatively, the loss of ASIC3 may alter behavioral nociceptive responses by changing the balance of dorsal horn inputs from various sensory afferents. The importance of this balance on the spinal processing of nociceptive signals was conceptualized first in Melzak and Wall's 1965 gate-control theory (25). Although many aspects of the original theory could not be sustained, its central concept, the inhibitory effect of A fiber input on C fiber evoked activity, has been confirmed in many studies (26). This effect is thought to be a key element in the segmental inhibition of pain signaling through transcutaneous electrical nerve stimulation (TENS) and vibration (27, 28). In good agreement with this hypothesis is the recent demonstration that the deletion of ASIC3 leads to changes in large-diameter mechanoreceptor responses including an enhanced sensitivity of rapidly-adopting mechanoreceptors and a decreased sensitivity of A fiber mechanonociceptors (6). Furthermore, several properties of proton-gated currents were altered in large-diameter mechanoreceptors in the absence of ASIC3. The significance of the latter finding is unclear at present, however, because peripheral fibers from these neurons do not respond to acid stimulation (29).
Because ASIC3 mRNA levels increase in inflamed tissues (11, 30), it has been suggested that ASIC3 may contribute to inflammation-induced mechanical allodynia and thermal hyperalgesia. However, we found similar levels of hyperalgesia and allodynia after carrageenan or capsaicin injection into ASIC3+/+ or ASIC3−/− mice. Again, these results are consistent with the idea that ASIC3 modulates the perception of high- but not low-intensity stimuli, which are important for the production of pain after tissue inflammation.
In summary, we suggest that ASIC3 is not essential for acid-induced pain sensation, although it seems to be required for full-blown responses to acids, but it rather plays an important role in modulating the perception of moderate- to high-intensity pain stimuli. It remains to be determined whether reduction in pain perception is because of effects of ASIC3 on nociceptive C fibers, mechanoreceptive A fibers, or both. Additional studies with cell-specific ASIC3 deletions will be required to distinguish between these possibilities.
Acknowledgments
This work was supported in part by grants from the Land Nordrhein-Westphalen (Innovationsprogramm Forschung) and Deutsche Forschungsgemeinschaft Grants SFB400 and FOR425.
Abbreviations
- DEG/ENaC
degenerins/epithelial sodium channel
- ASIC
acid-sensing ion channel
- VR
vanilloid receptor
References
- 1.Mano I, Driscoll M. BioEssays. 1999;21:568–578. doi: 10.1002/(SICI)1521-1878(199907)21:7<568::AID-BIES5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann R, Lazdunski M. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 3.Akopian A N, Chen C C, Ding Y, Cesare P, Wood J N. NeuroReport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman B T, Corey D P. Proc Natl Acad Sci USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann R, Champigny G, Lingueglia E, De Weille J R, Heurteaux C, Lazdunski M. Ann NY Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- 6.Price M P, McIlwrath S L, Xie J, Cheng C, Qiao J, Tarr D E, Sluka K A, Brennan T J, Lewin G R, Welsh M J. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 7.Price M P, Lewin G R, McIlwrath S L, Cheng C, Xie J, Heppenstall P A, Stucky C L, Mannsfeldt A G, Brennan T J, Drummond H A, et al. Nature (London) 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 8.Hong K, Driscoll M. Nature (London) 1994;367:470–473. doi: 10.1038/367470a0. [DOI] [PubMed] [Google Scholar]
- 9.Huang M, Chalfie M. Nature (London) 1994;367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen C C, England S, Akopian A N, Wood J N. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voilley N, de Weille J, Mamet J, Lazdunski M. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 13.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. Nature (London) 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland S P, Benson C J, Adelman J P, McCleskey E W. Proc Natl Acad Sci USA. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Weille J R, Bassilana F, Lazdunski M, Waldmann R. FEBS Lett. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 16.Zimmer A. Annu Rev Neurosci. 1992;15:115–137. doi: 10.1146/annurev.ne.15.030192.000555. [DOI] [PubMed] [Google Scholar]
- 17.Konig M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer A, Zimmer A M, Baffi J, Usdin T, Reynolds K, Konig M, Palkovits M, Mezey E. Proc Natl Acad Sci USA. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogil J S, Wilson S G, Bon K, Lee S E, Chung K, Raber P, Pieper J O, Hain H S, Belknap J K, Hubert L, et al. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 20.Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Petersen-Zeitz K R, Koltzenburg M, Basbaum A I, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 21.Caterina M J, Julius D. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 22.Lingueglia E, de Weille J R, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 23.Bassilana F, Champigny G, Waldmann R, de Weille J R, Heurteaux C, Lazdunski M. J Biol Chem. 1997;272:28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Canessa C M. J Gen Physiol. 2001;117:563–572. doi: 10.1085/jgp.117.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melzack R, Wall P D. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 26.Melzack R. Can J Exp Psychol. 1993;47:615–629. doi: 10.1037/h0078871. [DOI] [PubMed] [Google Scholar]
- 27.Garrison D W, Foreman R D. Pain. 1994;58:309–315. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 28.Woolf C J, Thompson J W. In: Textbook of Pain. Wall P D, Melzack R, editors. Edinburgh: Churchill Livingstone; 1994. pp. 1191–1208. [Google Scholar]
- 29.Steen K H, Reeh P W, Anton F, Handwerker H O. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yiangou Y, Facer P, Smith J A, Sangameswaran L, Eglen R, Birch R, Knowles C, Williams N, Anand P. Eur J Gastroenterol Hepatol. 2001;13:891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]