Abstract
Background
The diagnosis of cardiac amyloidosis can be achieved noninvasively for transthyretin amyloid cardiomyopathy through nuclear scintigraphy with bone tracers. For other subtypes of cardiac amyloidosis (eg, light-chain amyloid cardiomyopathy), or in cases of suspected transthyretin amyloid cardiomyopathy with nondiagnostic scintigraphy results, histologic confirmation via tissue biopsy and amyloid subtype identification is required.
Case Summary
We present a case of a 66-year-old male with heart failure and left ventricular hypertrophy with evidence of systemic amyloidosis. Despite multiple biopsies of different organs and extensive testing, including mass spectrometry, the amyloid subtype could not be clearly identified. The patient's condition rapidly deteriorated without the initiation of targeted therapy, leading to multiple hospitalizations and an unfavorable prognosis.
Take-Home Message
This case emphasizes the diagnostic challenges in cardiac amyloidosis and highlights the importance of continuous advancements in diagnostic techniques to improve patient outcomes with this complex disease.
Key words: cardiac amyloidosis, heart failure, mass spectrometry, monoclonal gammopathy of undetermined significance, subtyping
Graphical Abstract
Visual Summary.
Patient History, Diagnostic Approach, and Follow-Up
| Time | Event |
|---|---|
| Presentation | HF clinic: exertional dyspnea and peripheral edema ECG: pseudoinfarction pattern in V1 to V4. TTE: LV hypertrophy, EF = 42% |
| Further investigation | Lab tests: serum protein electrophoresis with hypogammaglobulinemia; elevated serum free lambda chain. Proteinuria of 8.7 g/dL. Urine immunofixation: positive for IgD lambda light chain. Serum immunofixation: positive for IgD lambda light chain CMR: biventricular hypertrophy, subendocardial late gadolinium enhancement, elevated T1 and T2 mapping values, and increased extracellular volume |
| 2 months after presentation | Hospital admission due to acute HF |
| During hospitalization | 99mTc-HMDP bone scintigraphy: score Perugini 1 Bone marrow biopsy: positive for amyloid, inconclusive immunohistochemistry; 8% plasma cells. Abdominal fat biopsy: negative for amyloid |
| Further investigation | Renal biopsy: positive for amyloid, inconclusive immunohistochemistry; MS: gelsolin |
| 6 months after presentation | Hospital admission due to acute HF |
| Further investigation | Endomyocardial biopsy: positive for amyloid; inconclusive immunohistochemistry; MS: SAA1-3 |
| 7 months after presentation | Hospital admission due to acute HF |
| Further investigation | Bone marrow biopsy: positive for amyloid, inconclusive immunohistochemistry; MS: inconclusive |
| 8 months after presentation | Hospital admission due to acute HF |
99mTc-HMDP bone scintigraphy = 99mTc–hydroxyl-methylene-diphosphonate bone scintigraphy; CMR = cardiac magnetic resonance; ECG = electrocardiogram; EF = ejection fraction; HF = heart failure; Ig = immunoglobulin; Lab = laboratory; LV = left ventricular; MS = mass spectrometry; SAA 1-3 = serum amyloid A subtypes 1-3; TTE = transthoracic echocardiogram.
Cardiac amyloidosis, an infiltrative restrictive cardiomyopathy, results from amyloid fibril deposition in the heart, often presenting as left ventricular hypertrophy.1 Of 36 identified amyloidogenic proteins,2 transthyretin (TTR) and immunoglobulin light chain account for 95% of cases.3
The disease manifests with both cardiac and extracardiac symptoms, and various noninvasive diagnostic techniques, including electrocardiography, echocardiography, cardiac magnetic resonance (CMR), and laboratory tests, can raise suspicion for cardiac amyloidosis through the identification of various red flags.4,5 Transthyretin amyloid cardiomyopathy (ATTR-CM) can often be diagnosed noninvasively using bone scintigraphy, whereas light-chain amyloid cardiomyopathy (AL-CM) requires histologic confirmation through tissue biopsy following initial screening for monoclonal proteins.4, 5, 6
Amyloid is identified by the apple-green birefringence seen with Congo red staining when viewed under cross-polarized light.4,7 Determining the amyloid subtype has traditionally relied on immunohistochemistry using antibodies directed toward different amyloid proteins.1,7 However, the sensitivity and specificity of this technique are often suboptimal.7 Mass spectrometry (MS), now the gold standard, is limited to a few reference laboratories.7,8
Early diagnosis and timely treatment are crucial for improving prognosis in cardiac amyloidosis. The clinical management of the disease depends on the specific type of amyloid involved. Thus, accurate subtyping is essential for selecting the appropriate treatment and improving clinical outcomes.4,5,7
We describe a case of a 66-year-old male with progressive heart failure (HF) and systemic amyloid deposition. However, no clear diagnosis was achieved after multiple diagnostic tests, including MS, the current diagnostic gold standard.
History of Presentation
A 66-year-old White man was referred to the HF clinic due to exertional dyspnea and peripheral edema over the past 3 months. No relevant familial history was present.
On examination, he was afebrile and hemodynamically stable (blood pressure: 123/65 mm Hg; regular pulse rate: 89 beats/min), with decreased pulmonary sounds at the right base. Bilateral pretibial edema was observed.
Past Medical History
The patient's medical history was notable for hypertension and type 2 diabetes.
Investigations
The electrocardiogram showed a pattern of pseudoinfarction in the anterior leads (Figure 1A). Transthoracic echocardiogram revealed severe concentric left ventricular hypertrophy (interventricular septum [IVS] thickness: 19 mm; posterior wall thickness: 17 mm) and reduced contractility (left ventricular ejection fraction: 42%), with an echogenic myocardium described as "granular sparkling" and a reduction in global longitudinal strain (−7.3%) without an "apical sparing" pattern. Grade 3 diastolic dysfunction was noted, along with hypertrophy of the free wall of the right ventricle (13 mm), moderate mitral regurgitation, and mild to moderate circumferential pericardial effusion (Figure 1B). CMR (Figure 1C) demonstrated subendocardial late gadolinium enhancement, along with elevated T1 (Figure 1D) and T2 mapping (Figure 1E).
Figure 1.
Electrocardiogram, Echocardiogram, and Cardiac Magnetic Resonance of the Patient During the Initial Diagnostic Work-Up
(A) Electrocardiogram showing sinus rhythm and a pseudoinfarction pattern in the anterior leads. (B) Transthoracic echocardiogram demonstrating concentric left ventricular hypertrophy (evident in the short-axis view), biatrial dilation, and hypertrophy of the right ventricular free wall (evident in the apical view). The bull's-eye view shows reduced global longitudinal strain (−7.3%) without an "apical sparing" pattern. (C) Cardiac magnetic resonance showing evident hypertrophy of all 4 cardiac chambers (4-chamber view). (D) Native T1 mapping diffusely increased (1215 ± 77 milliseconds). (E) Native T2 mapping diffusely increased (60 ± 9 milliseconds).
Laboratory testing was abnormal, with creatinine of 1.43 mg/dL and significant elevations of N-terminal pro–B-type natriuretic peptide and troponin T levels at 29,115 pg/mL and 178 ng/L, respectively. Hemoglobin and calcium levels were normal.
The presentation of HF with moderately reduced ejection fraction was presumed to be secondary to cardiac amyloidosis; therefore, we began to rule out light-chain amyloidosis (AL). Serum protein electrophoresis showed hypogammaglobulinemia (2.6 g/dL), and an elevated serum-free lambda chain (kappa to lambda free light chain ratio: 0.053; difference between involved and uninvolved free light chains [dFLC]: 425.4 mg/L) was observed. Serum immunofixation revealed a monoclonal immunoglobulin D (IgD) lambda component. Urine immunofixation was positive for immunoglobulin light chain (Bence-Jones protein), with proteinuria of 8.7 g/dL. These findings confirmed the presence of a monoclonal gammopathy. Additionally, beta-2 microglobulin levels were elevated (9.43 mg/L).
Examination of the bone marrow biopsy specimen was consistent with amyloidosis (Figure 2A), showing deposits of hyaline substance with apple-green birefringence under polarized light in Congo red staining (Figure 2B). Immunohistochemistry was negative for kappa and lambda light chains, TTR, and amyloid A (AA), precluding the characterization of the amyloid subtype. Additionally, the myelogram revealed 8% plasma cells, indicating monoclonal gammopathy of undetermined significance (MGUS).
Figure 2.
Bone Marrow and Endomyocardial Biopsy Images From the Patient
Bone marrow (A and B) and endomyocardial biopsy (C and D) images from the patient, prior to (A) Pathologic examination results demonstrating deposition of an amorphous, hyaline, and eosinophilic substance in the capillary walls, hematoxylin and eosin stain (original magnification, 10×). (B and C) Capillary wall deposits showing apple-green birefringence under polarized light, compatible with amyloid deposits, Congo red staining, (original magnification, 5×). (D) Immunohistochemical staining showing amyloid immunolabeling for P component (original magnification, 5×). Arrows indicate amyloid deposits.
At this point, systemic amyloidosis with cardiac and renal involvement overlapping with MGUS was the most likely diagnosis. Therefore, we proceeded with a tissue biopsy to establish the definitive diagnosis.
Due to the procedure's safety and accessibility, an abdominal fat pad excisional biopsy was initially performed, which yielded a negative result. A subsequent renal biopsy revealed the presence of amyloid substance in the walls of glomerular capillaries and arterioles, as demonstrated by Congo red staining. However, although immunostaining for the amyloid P component was positive, subtype characterization by immunohistochemistry could not be achieved. To further characterize the amyloid subtype, a shotgun proteomics technique using liquid chromatography–tandem MS was performed at a national reference laboratory, and protein gelsolin was identified (intensity-based absolute quantification 12.57 ± 3; counts: 65), with no evidence of immunoglobulin light chains or TTR in the renal sample. Although the patient exhibited no signs or symptoms suggestive of gelsolin amyloidosis (eg, progressive cranial and peripheral neuropathies, corneal lattice dystrophy, cutis laxa), evaluation by ophthalmology, neurology, and dermatology specialists was performed, definitively excluding these manifestations. Furthermore, pathogenic or likely pathogenic mutations in the GNS gene were investigated but were not identified.
This time, a new attempt at a tissue biopsy was made, with an endomyocardial biopsy of the left ventricle (left ventricle free wall and IVS). The sample showed hypertrophy of cardiomyocytes, interstitial fibrosis, and amyloid substance (Figure 2C); however, subtype characterization was not possible with immunohistochemistry (Figure 2D), and MS identified serum amyloid A subtypes 1-3 (SAA1-3), which are characteristic of secondary AA amyloidosis, with no evidence of AL (immunoglobulin kappa to lambda free light chain ratio: 0.71; reference range: 0.35–1.40). Our patient did not exhibit symptoms typically associated with systemic diseases that can lead to AA amyloidosis (eg, fever, joint pain, swollen lymph nodes, hemoptysis, night sweats, abdominal pain, diarrhea). Moreover, the patient's inflammatory markers were low or nonspecific, with a C-reactive protein of 1.82 mg/dL and an erythrocyte sedimentation rate (ESR) of 89 mm; tests for hepatitis B and C, and HIV were negative. Therefore, we continued our diagnostic work-up to further characterize the amyloid subtype.
Finally, a second bone marrow biopsy was conducted. It showed amyloid deposition without diagnostic features of multiple myeloma or identification of AL subtype by MS (serum amyloid P-component − log2 intensity-based absolute quantification = 10.06 ± 0.66; counts: 31).
During the diagnostic work-up, a 99mTc–hydroxyl-methylene-diphosphonate bone scintigraphy was performed to exclude the coexistence of ATTR-CM, which revealed a Perugini score of 1, and a TTR genetic test was negative. Although a Perugini score of 3 would have been fairly specific for ATTR-CM, a score of 1 is a nonspecific finding which can also be seen in AL and some other TTR subtypes. The absence of TTR deposits in multiple organ biopsies evaluated by both immunohistochemistry and MS and the overall clinical presentation did not support this amyloid subtype.
Management
After a multidisciplinary team discussion that included cardiology, hematology, and pathology, it was decided not to initiate chemotherapy due to the absence of an established diagnosis.
Outcome and Follow-Up
During the exhaustive clinical diagnostic process, the patient experienced multiple episodes of acute HF, leading to 4 hospitalizations with inadequate response to diuretic therapy. Although there is limited evidence from clinical trials, adjuvant therapy with doxycycline 100 mg once daily was initiated. However, no improvement in functional capacity or target organ response was observed, with severe left ventricular hypertrophy (IVS: 23 mm; posterior wall: 22 mm), significant biventricular dysfunction, pericardial and pleural effusion noted on transthoracic echocardiogram (Figures 3A to 3D), and a significant rise in cardiac biomarkers (N-terminal pro–B-type natriuretic peptide: 265,782 pg/mL and troponin T: 343 ng/L).
Figure 3.
Echocardiographic Image Documenting Disease Progression, With Marked Left Ventricular Hypertrophy
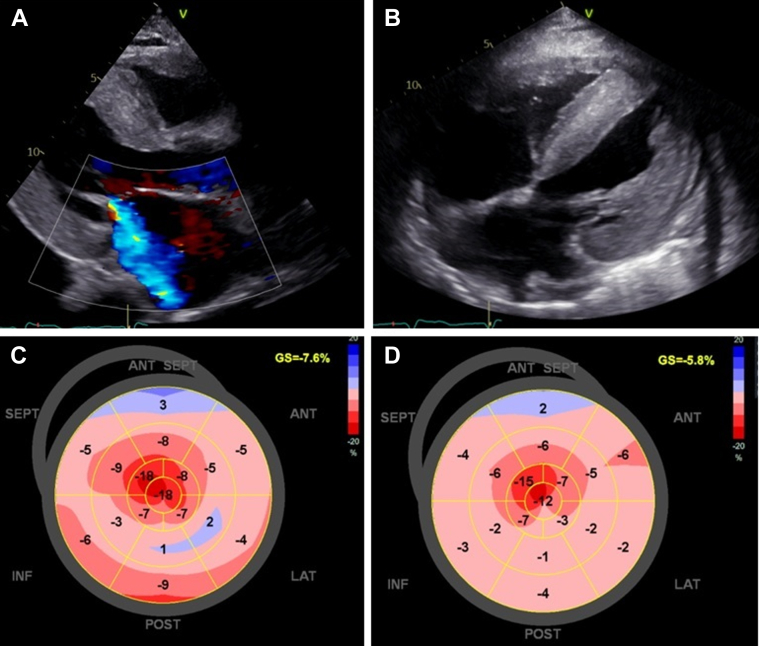
(A) Echocardiogram at 6 months after clinical presentation, showing severe concentric left ventricular hypertrophy and mitral regurgitation (long-axis view). (B) Echocardiogram at 8 months, demonstrating biventricular hypertrophy, interatrial septum hypertrophy, atrial enlargement, and findings concerning cardiac amyloidosis (4-chamber subcostal view). (C and D) Evolution of the bull's-eye plot pattern with further reduction in global longitudinal strain and the typical apical sparing pattern at 6 and 8 months after clinical presentation, respectively.
During the last hospital admission, the patient progressed to cardiogenic shock with acute kidney injury (creatinine: 6.89 mg/dL), requiring continuous veno-venous hemodiafiltration. A temporary transvenous pacemaker was also implanted due to complete atrioventricular block. Despite comprehensive organ support, the patient had an unfavorable outcome and died 8 months after the initial manifestations of the disease.
Discussion
We report a case of systemic amyloidosis where subtype characterization could not be achieved despite using immunohistochemistry and MS. Given the rapid progression and clinical deterioration of the patient, with multiple hospital admissions for acute HF, progression to nephrotic syndrome, and the presence of IgD MGUS, we think this is a case of AL-CM; however, we were unable to confirm it. Serum IgD monoclonal protein is extremely rare, found in <1% of patients with monoclonal gammopathies. IgD-related disorders (eg, myeloma, amyloidosis) are usually associated with poor prognosis due to their aggressive nature. However, recent studies show that survival outcomes in IgD amyloidosis are similar to those in IgG, IgA, or AL amyloidosis.9 Additionally, the lower secretion and shorter half-life of IgD make its detection and diagnosis more challenging.9
Diagnosing AL amyloidosis requires confirming amyloid deposits in tissue or on bone marrow biopsy in patients with evidence of plasma cell dyscrasia on monoclonal protein screening.5 Prompt diagnosis is critical, especially in suspected cardiac amyloidosis, because early therapy initiation improves outcomes.4 Identifying the amyloid fibril protein after biopsy is essential.10
During the diagnostic work-up, we first performed an abdominal fat pad biopsy, after an inconclusive bone marrow biopsy, due to its accessibility, minimally invasive nature, and safety. However, no amyloid deposits were found. The sensitivity of this procedure is variable, with reported rates of 79% to 84% in AL amyloidosis, depending on the amyloid subtype, overall amyloid burden, and small sample size.11
In cases with significant renal involvement (eg, proteinuria, nephrotic syndrome), renal biopsy can be helpful in diagnosis. MS identified gelsolin protein, raising the possibility of hereditary gelsolin amyloidosis, a rare autosomal dominant condition. This subtype usually presents with ophthalmologic (corneal lattice dystrophy), neurologic (facial paralysis), and dermatologic (cutis laxa) symptoms,12 none of which were seen in our patient. Renal and cardiac involvement are less common in hereditary gelsolin amyloidosis, and cardiomyopathy and HF hospitalizations are rare.12 The patient had no family history of the condition and no Nordic European ancestry, where it is more common.12 Genetic testing showed no mutations in the GNS gene. According to the National Finnish Gelsolin Amyloidosis Registry (FIN-GAR), this subtype does not significantly affect lifespan before 75 years of age.12 Therefore, our case, with its clinical presentation and rapid poor prognosis, was not suggestive of hereditary gelsolin amyloidosis.
We performed an endomyocardial biopsy, which, on MS analysis, identified SAA1-3 proteins, which would normally signify a diagnosis of secondary amyloidosis—a potential consequence of chronic inflammatory or infectious disease. Our patient had no significant past medical history or symptoms suggestive of rheumatologic diseases, autoinflammatory syndromes, tuberculosis, chronic inflammatory conditions (eg, Crohn disease), malignancies, or conditions predisposing to recurrent infections, including HIV.2 During the diagnostic work-up, C-reactive protein was mildly elevated, and ESR was also increased. However, although ESR reflects inflammation, it is not disease-specific. Notably, amyloid deposition itself can trigger an inflammatory response.2 Although nephrotic syndrome and renal involvement are common in AA amyloidosis, and cardiac involvement is rare but has been reported,2 the patient showed no clinical signs of an underlying condition predisposing to AA amyloidosis. Thus, we think AA amyloidosis alone does not fully explain this presentation. However, cases of coexisting AA and AL amyloidosis have been documented in the literature.13
Immunohistochemistry is a widely used, simple, and cost-effective technique; however, its sensitivity varies depending on antibody affinity, pathologist expertise, and laboratory protocols.10,14 Standard antibody panels primarily detect common amyloid types (AA, transthyretin amyloidosis [ATTR], AL), limiting their utility in identifying rare forms.15 These antibodies target native proteins, but amyloid fibrils often undergo conformational and posttranslational modifications, potentially altering binding sites and reducing antibody efficacy—an issue particularly relevant for AL fibrils.10 To address this limitation, Linke16 and several amyloidosis research laboratories have developed custom antibodies tailored to different amyloid types to improve protein identification. In our case, all samples were analyzed at a central reference laboratory by pathologists with extensive experience in amyloidosis. The renal biopsy, in particular, was reviewed by a dedicated renal pathologist specializing in kidney biopsy analysis.
MS is the preferred method for amyloid typing, with 88% sensitivity and 96% specificity.5 Dasari et al15 analyzed 16,175 specimens over 11 years, identifying 21 amyloid types, including rare forms (10%), undetectable by immunohistochemistry. MS provides unambiguous results from small tissue samples in a single assay. However, it is complex, requires specialized expertise and equipment, and is unavailable in many amyloidosis centers. Despite analyzing multiple tissue samples from different organs (kidney, heart, and bone marrow) at a national reference laboratory integrated into the Portuguese Mass Spectrometry Network, we were unable to determine the amyloid subtype in our patient.
According to Phull et al,17 who retrospectively assessed 226 patients with cardiac amyloidosis (both wild-type ATTR and hereditary ATTR with the V122I mutation), a high prevalence of MGUS was found (39% and 49%, respectively), and MS studies reveal that >10% of patients with monoclonal gammopathy can have ATTR deposits in the bone marrow.18 During the diagnostic process, we also decided to exclude the coexistence of TTR amyloidosis, knowing that in ATTR cases, circulating kappa immunoglobulin light chains can contaminate TTR fibrils and limit their diagnosis using proteomic methods.10 However, we only identified a low-grade radiotracer uptake, a finding that has previously been described in patients with AL amyloidosis.19
Treatment options for systemic amyloidosis vary significantly based on the subtype; therefore, a clear understanding and accurate diagnosis are essential for effective patient management. Our case highlights the challenges in diagnosing cardiac amyloidosis and the need to improve current diagnostic methods to minimize their pitfalls. Recent research has focused on improving the noninvasive diagnosis of amyloid subtypes through targeted amyloid-binding positron emission tomography (PET) tracer imaging. Genovesi et al20 propose [18F]-florbetaben PET/computed tomography as a valuable tool for diagnosing AL amyloidosis. Their study, which included 40 patients, showed that AL amyloidosis was associated with higher and more persistent cardiac uptake compared with ATTR, with tracer retention declining rapidly after the early scan in non-AL cases. These findings suggest that [18F]-florbetaben PET could serve as an initial rule-in tool for suspected AL amyloidosis. Ongoing studies (NCT06048601 and NCT05184088) are further evaluating its diagnostic value.20 Additionally, [18F]-florbetapir and [11C]PiB PET have demonstrated high affinity for AL cardiac amyloidosis and can detect the disease at an early stage, even before significant left ventricular wall thickening occurs.21
With the introduction of artificial intelligence (AI) to optimize the diagnosis of cardiac amyloidosis by recognizing patterns in electrocardiograms, echocardiograms, CMR, and bone scintigraphy, there is potential for significant improvements in identifying high-risk patients. AI could also be a valuable additional pathology tool.22 Because tissue acquisition, processing, and interpretation demand considerable expertise, less experienced centers may face challenges in data interpretation. Therefore, using AI models could help to reduce observer variability and enhance accuracy in pathologic evaluations. Palstrøm et al23 used a Boruta method on a random forest classifier with proteomics data to identify novel amyloid signature proteins. This achieved near-perfect accuracy in distinguishing amyloid biopsies from controls and accurately categorizing the subtypes. Similarly, Kamel et al22 applied Raman spectroscopy and AI to renal tissue, successfully detecting and classifying amyloidosis with accuracy ranging from 95.6% to 98.4%, suggesting potential for broader application in other organs.
The group led by Riefolo et al10 argues that a sequential approach combining immunohistochemistry with specific antibodies and liquid chromatography–MS is likely the gold standard for amyloid typing. Additionally, establishing specialized amyloidosis centers with dedicated teams of cardiologists, pathologists, and biochemists/chemical engineers is essential. Promoting these centers within cardiology clinics to facilitate the early referral of patients suspected of having this disease is crucial for achieving optimal outcomes through a team-based approach.
Conclusions
In this report, we presented a case of systemic amyloidosis with cardiac and renal involvement, where the fibril subtype was not clearly identified after immunohistochemistry and MS analysis. Characterizing the amyloid subtype is essential for initiating targeted therapy; without this identification, the prognosis remains unfavorable.
Despite advanced techniques like MS, this case underscores challenges in definitive subtype identification. This highlights the need for continuous advancements in diagnostic methods, integrating AI and establishing specialized amyloidosis centers to enhance accuracy and patient management.
Funding Support and Author Disclosure
This work was supported by Fundação para a Ciência e a Tecnologia (grant number UIDB/00306/2020). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Take-Home Messages
-
•
This case highlights the importance of identifying signs and symptoms suggestive of cardiac amyloidosis to initiate early diagnostic work-up.
-
•
Understanding the diagnostic methods for cardiac amyloidosis, including the identification of the amyloid protein subtype, is crucial for accurate diagnosis.
-
•
Improving the precision of current diagnostic methods is essential for timely and accurate diagnosis, which plays a key role in enhancing patient outcomes.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Brito D., Albrecht F.C., de Arenaza D.P., et al. World heart federation consensus on transthyretin amyloidosis cardiomyopathy (ATTR-CM) Glob Heart. 2023;18(1):59. doi: 10.5334/gh.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirioglu S., Uludag O., Hurdogan O., et al. AA amyloidosis: a contemporary view. Curr Rheumatol Rep. 2024;26(7):248–259. doi: 10.1007/s11926-024-01147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Embry-Dierson M., Farrell M.B., Schockling E., Warren J., Jerome S. Cardiac amyloidosis imaging, part 1: amyloidosis etiology and image acquisition. J Nucl Med Technol. 2023;51(2):83–89. doi: 10.2967/jnmt.123.265415. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail. 2021;23(4):512–526. doi: 10.1002/ejhf.2140. [DOI] [PubMed] [Google Scholar]
- 5.Kittleson M.M., Ruberg F.L., Ambardekar A.V., et al. 2023 ACC Expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(11):1076–1126. doi: 10.1016/j.jacc.2022.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Bashir Z., Younus A., Dhillon S., Kasi A., Bukhari S. Epidemiology, diagnosis, and management of cardiac amyloidosis. J Investig Med. 2024;72(7):620–632. doi: 10.1177/10815589241261279. [DOI] [PubMed] [Google Scholar]
- 7.Noborn F., Thomsen C., Vorontsov E., et al. Subtyping of cardiac amyloidosis by mass spectrometry-based proteomics of endomyocardial biopsies. Amyloid. 2023;30(1):96–108. doi: 10.1080/13506129.2022.2127088. [DOI] [PubMed] [Google Scholar]
- 8.Schönland S.O., Hegenbart U., Bochtler T., et al. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119(2):488–493. doi: 10.1182/blood-2011-06-358507. [DOI] [PubMed] [Google Scholar]
- 9.Gertz M.A., Buadi F.K., Hayman S.R., et al. Immunoglobulin D amyloidosis: a distinct entity. Blood. 2012;119(1):44–48. doi: 10.1182/blood-2011-07-366206. [DOI] [PubMed] [Google Scholar]
- 10.Riefolo M., Conti M., Longhi S., Fabbrizio B., Leone O. Amyloidosis: what does pathology offer? The evolving field of tissue biopsy. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1081098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitaoka H., Izumi C., Izumiya Y., et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ J. 2020;84(9):1610–1671. doi: 10.1253/circj.CJ-20-0110. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt E.K., Mustonen T., Kiuru-Enari S., Kivelä T.T., Atula S. Finnish gelsolin amyloidosis causes significant disease burden but does not affect survival: FIN-GAR phase II study. Orphanet J Rare Dis. 2020;15(1):19. doi: 10.1186/s13023-020-1300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini F., Buda G., De Tata V., et al. Different types of amyloid concomitantly present in the same patients. Hematol Rep. 2019;11(4):7996. doi: 10.4081/hr.2019.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal F., Rosenzweig M. Amyloidosis with cardiac involvement: identification, characterization, and management. Curr Hematol Malig Rep. 2021;16(4):357–366. doi: 10.1007/s11899-021-00626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasari S., Theis J.D., Vrana J.A., et al. Amyloid typing by mass spectrometry in clinical practice: a comprehensive review of 16,175 samples. Mayo Clin Proc. 2020;95(9):1852–1864. doi: 10.1016/j.mayocp.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Linke R.P. On typing amyloidosis using immunohistochemistry. Detailled illustrations, review and a note on mass spectrometry. Prog Histochem Cytochem. 2012;47(2):61–132. doi: 10.1016/j.proghi.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Phull P., Sanchorawala V., Connors L.H., et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR) Amyloid. 2018;25(1):62–67. doi: 10.1080/13506129.2018.1436048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gertz M.A. Immunoglobulin light chain amyloidosis: 2024 update on diagnosis, prognosis, and treatment. Am J Hematol. 2024;99(2):309–324. doi: 10.1002/ajh.27177. [DOI] [PubMed] [Google Scholar]
- 19.Hutt D.F., Quigley A.M., Page J., et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15(11):1289–1298. doi: 10.1093/ehjci/jeu107. [DOI] [PubMed] [Google Scholar]
- 20.Genovesi D., Vergaro G., Giorgetti A., et al. [18F]-Florbetaben PET/CT for differential diagnosis among cardiac immunoglobulin light chain, transthyretin amyloidosis, and mimicking conditions. JACC Cardiovasc Imaging. 2021;14(1):246–255. doi: 10.1016/j.jcmg.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Fontana M., Ioannou A., Cuddy S., et al. The last decade in cardiac amyloidosis: advances in understanding pathophysiology, diagnosis and quantification, prognosis, treatment strategies, and monitoring response. JACC Cardiovasc Imaging. 2025;18(4):478–499. doi: 10.1016/j.jcmg.2024.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamel M.A., Abbas M.T., Kanaan C.N., et al. How artificial intelligence can enhance the diagnosis of cardiac amyloidosis: a review of recent advances and challenges. J Cardiovasc Dev Dis. 2024;11(4):118. doi: 10.3390/jcdd11040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palstrøm N.B., Rojek A.M., Møller H.E.H., et al. Classification of amyloidosis by model-assisted mass spectrometry-based proteomics. Int J Mol Sci. 2021;23(1):319. doi: 10.3390/ijms23010319. [DOI] [PMC free article] [PubMed] [Google Scholar]