Abstract
Background
Thyrotoxicosis can lead to high-output cardiac failure, and although beta-blockers have a relative contraindication in decompensated congestive heart failure, they are commonly used in thyrotoxicosis to control hypersympathetic activity.
Case summary
This is the case of a 38-year-old female who arrived with a thyroid storm, atrial fibrillation, and volume overload in physical examination. Echo revealed severely reduced left ventricular systolic function with normal diastology. She started on esmolol and successfully transitioned to propranolol.
Discussion
This case highlights the need to tailor beta-blocker therapy based on the underlying cause of left ventricular dysfunction, particularly when sustained tachycardia and decreased systemic vascular resistance are the main drivers of thyrotoxic cardiac failure.
Take-home message
Suspect thyrotoxic cardiac failure, especially in the setting of normal diastology, and consider starting BB.
Key Words: acute heart failure, atrial fibrillation, beta blocker, tachycardia-mediated cardiomyopathy, thyroid storm, thyrotoxicosis
Graphical abstract
History of presentation
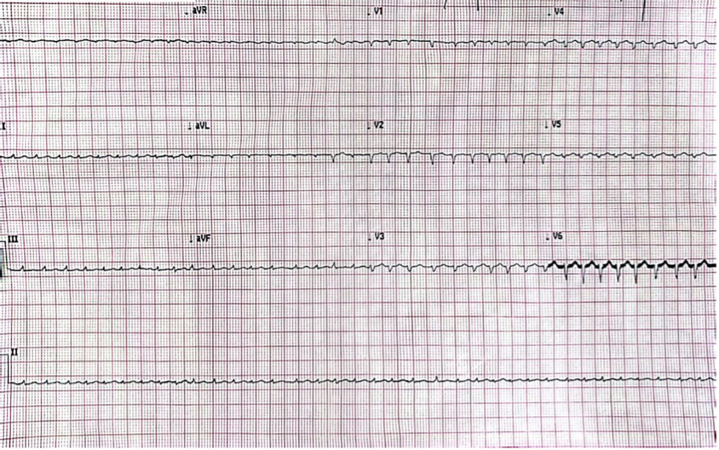
A 38-year-old female (G3P2A1) with a history of recently diagnosed hyperthyroidism, presented to the emergency department with shortness of breath that had progressively intensified overnight. She also reported increasing lower extremity edema and intermittent episodes of palpitations over the past few weeks. She denied drug use, smoking, or alcohol consumption and had no recent hospitalizations or surgeries. On arrival, her vital signs were as follows: blood pressure 108/60 mm Hg, heart rate 150-160 beats/min, respiratory rate 18 breaths/min, and oxygen saturation 100% on 3 L of oxygen via nasal cannula. The electrocardiogram revealed atrial fibrillation (AF) with a rapid ventricular response (Figure 1).
Take-Home Messages
-
•
Suspect thyrotoxic cardiac failure in patients with reduced ejection fraction, volume overload, and normal diastology, with tachycardia-mediated cardiomyopathy likely being the etiology.
-
•
In decompensated heart failure with reduced ejection fraction due to thyrotoxicosis, consider esmolol to manage tachycardia.
Figure 1.
Electrocardiogram
Electrocardiogram showing atrial fibrillation with fast ventricular response.
The physical examination showed a tremulous, chronically ill female with jugular venous distention, exophthalmos, and crackles up to mid lung bilaterally. The cardiac exam showed a regular rhythm with no gallops or murmurs. Her extremities were warm with symmetrical +2 lower extremity edema. Laboratory values were remarkable for undetectable thyroid stimulating hormone (<0.015 ng/dL) and supranormal triiodothyronine (T3) (12.3 μg/dL) and thyroxine (6.94 μg/dL).
Past medical history
She had a recent hospitalization due to palpitations a few months prior. At that time, she was diagnosed with hyperthyroidism but was unable to start medical therapy due to social limitations. Her only medication was an albuterol inhaler as needed.
Differential diagnosis
In this patient with thyroid storm, volume overload may arise from the hypermetabolic state, where reduced systemic vascular resistance (SVR) contributes to elevated venous return and increased cardiac output. Initially, this hyperdynamic circulation helps maintain stroke volume but can eventually lead to pulmonary congestion and peripheral edema as the body struggles to compensate for excessive fluid accumulation. In contrast, if the volume overload were a manifestation of low-output heart failure, where impaired systolic function and reduced ejection fraction (EF) lead to inadequate tissue perfusion, then tachycardia is a compensatory response to the low-output state. In this scenario, we were concerned that starting beta-blockers (BBs) could further worsen the clinical scenario. Distinguishing between these 2 causes of volume overload was critical for guiding treatment, particularly in the decision to use BBs. Therefore, a transthoracic echocardiogram (TTE) was promptly ordered to help guide further management.
Investigation
The TTE revealed left ventricular (LV) dysfunction with severely reduced EF of 25% to 30% with normal diastology. The pulsed-wave tissue Doppler imaging (PW-TDI) revealed an e’ septal velocity of 12 cm/s (Figure 2A) and e’ lateral velocity of 14 cm/s (Figure 2B). Tricuspid regurgitation maximal velocity was 2.45 m/s (Figure 3A) and left atrial volume index was 29.5 mL/m2 (Figure 3B). The calculated E/e’ septal ratio was 9.5 and E/e’ lateral ratio was 8.2, indicating normal LV filling pressures.
Figure 2.
Echocardiographic Diastology Evaluation
Transthoracic echocardiogram apical 4-chamber view with pulsed-wave tissue Doppler imaging showing normal diastology. (A) E’ septal velocity = 12 cm/s. (B) E’ lateral velocity = 14 cm/s.
Figure 3.
Left Ventricular Filling Pressures Echocardiographic Evaluation
(A) Transthoracic echocardiogram parasternal short-axis view with continuous-wave Doppler imaging at the tricuspid valve. Tricuspid regurgitation max velocity is 2.45 m/s. (B) End-systolic left atrial volume equal to 29.5 mL/m2. (C) Mitral inflow E velocity is 1.15 m/s.
Management
Because of hemodynamic instability, the patient underwent synchronized cardioversion, which successfully restored sinus tachycardia (Figure 4). She started intravenous diuresis and therapeutic anticoagulation for stroke prevention. The endocrinology team started propylthiouracil and steroids. Esmolol to control her heart rate was also started. After 24 hours, she was transitioned to oral propranolol and successfully began guideline-directed medical therapy for congestive heart failure (CHF).
Figure 4.
Electrocardiogram Post-Cardioversion
Electrocardiogram post cardioversion showing sinus tachycardia.
Discussion
Although symptoms of CHF are common in patients with hyperthyroidism, the development of dilated cardiomyopathy with impaired LV systolic function is less frequent.1 In hyperthyroidism, cardiac contractility is usually enhanced due to the decrease in SVR and the increase in blood volume, leading to higher venous return and, consequently, greater preload.1 Over time, as a compensatory mechanism to chronic volume overload and elevated cardiac output, the LV mass increases.2
Systolic heart failure in hyperthyroidism occurs due to impaired diastolic relaxation mediated by tachycardic-mediated cardiomyopathy, as sustained high heart rates weaken the heart's contractile ability.2 Subclinical hyperthyroidism, particularly when prolonged, can result in cardiotoxic effects due to overexpression of beta-adrenergic receptors and heightened adrenergic stimulation.1
Excess thyroid hormone, particularly elevated levels of T3, plays a key role in the pathophysiology of thyroid-induced cardiac dysfunction. T3 reduces systemic vascular resistance by promoting vasodilation through nitric oxide production in vascular smooth muscle.3 Moreover, catecholaminergic-induced cardiomyopathy due to thyroid hormone toxicity has been proposed as a cause of LV dysfunction in thyrotoxicosis.4
In the management of patients with thyrotoxic heart failure, BBs are used to control heart rate despite the evidence of volume overload, relatively contraindicated in other forms of heart failure. When initiating BBs in patients with significant heart failure, their use should be closely monitored due to their negative chronotropic effects.2 Esmolol, a short-acting BB, may be a reasonable alternative in such cases. In the context of hemodynamically stable decompensated CHF with AF, amiodarone, a class III antiarrhythmic, is classically used for rate control due to its mild negative inotropic effect.5 However, amiodarone should be avoided during acute episodes of thyrotoxicosis, as it can exacerbate thyroid hormone synthesis due to its high iodine content.6 Except in specific etiologies of heart failure, such as tachycardia-mediated cardiomyopathy, BBs may be withheld in cases of marked volume overload or marginally low cardiac output, as an increased heart rate compensates for low stroke volume to maintain cardiac output.5,7 However, in thyrotoxicosis, BBs are used to attenuate hypersympathetic activity. Propranolol, a nonselective BB, is commonly used in thyroid storm to reduce T3 levels and inhibit the conversion of thyroxine to T3.8
In this case, we suggest using esmolol, a beta-1 selective blocker with a much shorter half-life of 9 minutes, compared to propranolol's longer half-life (2.4 hours), for early rate control in patients with LV dysfunction.6 In young patients presenting with thyroid storm and newly diagnosed heart failure with low EF and relatively normal diastology, as seen in our case, intravenous BB therapy is advisable.
Follow-up
The patient had an uneventful hospitalization and was discharged with appropriate medical therapy. A follow-up TTE performed in the subsequent months showed recovery of EF 50% to 55% (Video 1).
Conclusions
LV systolic dysfunction with CHF is a rare presentation of thyrotoxicosis, and there are limited data on how to treat these conditions when presenting together. Sustained tachycardia, fluid retention, and decreased SVR are the main factors driving cardiac failure in this female; therefore, rate control becomes critical. Longer periods of tachyarrhythmia seem to contribute to the development of LV dysfunction.1 Several observational studies suggest that LV dysfunction in younger patients is often reversible.5 Targeting rate control should improve volume status, as addressing the underlying cause of tachycardia-mediated cardiomyopathy seems appropriate. In contrast, older patients with LV dysfunction and reduced EF, where ischemia may be a contributing factor, may demonstrate tachycardia as a compensatory response to a low-output state. For these patients, there should be a low threshold for considering direct current cardioversion, particularly in those with AF. We recommend that in patients with low EF but normal PW-TDI in the setting of thyroid storm, BBs should be initiated, as the underlying mechanism of LV dysfunction is more likely tachycardia-mediated cardiomyopathy secondary to metabolic compromise. In contrast, in patients with low EF and underlying diastolic dysfunction, characterized by low PW-TDI (e’ septal and e’ lateral velocities), careful administration of rate control agents is warranted, as tachycardia may be a compensatory response to a low-output state.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The patient provided informed consent for the use of his data and images.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Echocardiographic Evaluation
The left images show an ejection fraction (EF) of 25% to 30% before cardioversion. (A) Parasternal long-axis view. (B) Apical 4-chamber view. The images on the right show an EF of 50% to 55% after several months. (C) Parasternal long-axis view. (D) Apical 4-chamber view. Follow-up imaging was technically difficult to perform, limiting visualization of certain regions of the endocardium.
References
- 1.Siu C.W., Yeung C.Y., Lau C.P., Kung A.W., Tse H.F. Incidence, clinical characteristics, and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483–487. doi: 10.1136/hrt.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danzi S., Klein I. Thyroid hormone and the cardiovascular system. Minerva Endocrinol. 2004;29(3):139–150. [PubMed] [Google Scholar]
- 3.Kannan L., Shaw P.A., Morley M.P., et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail. 2018;11(12) doi: 10.1161/CIRCHEARTFAILURE.118.005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira N., Parisi A., Dec W., Choo J., Hajjar R., Gordon P. Myocardial stunning in hyperthyroidism. Clin Cardiol. 2009;23(4):298–300. doi: 10.1002/clc.4960230417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joglar J.A., Chung M.K., Armbruster A.L., et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2024;83(1):109–279. doi: 10.1016/j.jacc.2023.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riaz K., Forker A.D., Isley W.L., Hamburg M.S., McCullough P.A. Hyperthyroidism: a "curable" cause of congestive heart failure — three case reports and a review of the literature. Congest Heart Fail. 2003;9(1):40–46. doi: 10.1111/j.1527-5299.2003.01124.x. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Obi M.F., Namireddy V., Garg Y., Sharma M. Benefit and preference of propranolol over metoprolol in thyrotoxicosis-induced atrial fibrillation: a case report and review of literature. Cureus. 2023;15(1) doi: 10.7759/cureus.34474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic Evaluation
The left images show an ejection fraction (EF) of 25% to 30% before cardioversion. (A) Parasternal long-axis view. (B) Apical 4-chamber view. The images on the right show an EF of 50% to 55% after several months. (C) Parasternal long-axis view. (D) Apical 4-chamber view. Follow-up imaging was technically difficult to perform, limiting visualization of certain regions of the endocardium.