Abstract
Background
Transcatheter tricuspid valve edge-to-edge repair (T-TEER) can be effective for patients with tricuspid regurgitation (TR), but TR may recur, requiring transcatheter tricuspid valve replacement (TTVR). Although TTVR has been described in patients with single leaflet device attachment of edge-to-edge devices, implantation in presence of well-attached devices has not been described.
Case Summary
We present a patient with recurrent TR after T-TEER. Transesophageal echocardiography revealed that 3 previously placed MitraClip XTW devices were well attached to the tricuspid leaflets, with recurrent severe TR. After laceration of 1 leaflet attached to the central XTW clip with transcatheter electrosurgery, we implanted an EVOQUE TTVR with trivial paravalvular leakage.
Discussion
Transcatheter electrosurgery can free a tricuspid clip from leaflets, allowing implantation of an EVOQUE TTVR with excellent TR resolution.
Take-Home Message
Transcatheter electrosurgical liberation of T-TEER devices might be a viable technique in patients who require a TTVR for recurrent tricuspid regurgitation.
Key words: transcatheter tricuspid valve replacement, tricuspid edge-to-edge repair, tricuspid valve electrosurgery, tricuspid valve regurgitation
Graphical Abstract

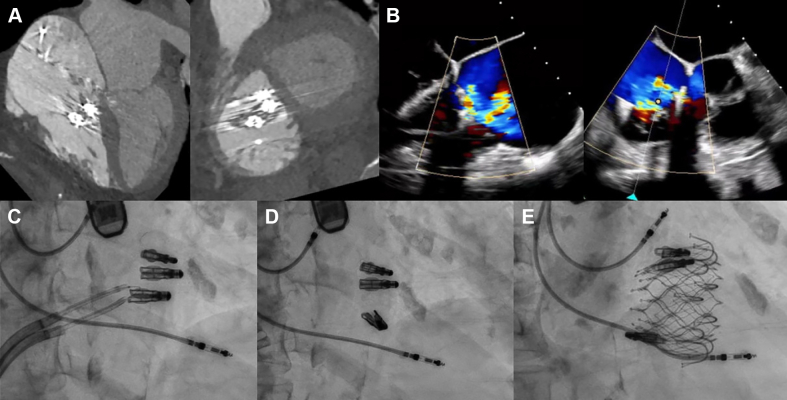
Visual Summary.
Illustration of Tricuspid Clip Liberation and Transcatheter Tricuspid Valve Replacement Implantation
(A) Computed tomography of tricuspid clips. (B) Echocardiography of tricuspid regurgitation after tricuspid clips. (C) Flying V in position to lacerate anterior leaflet. (D) Laceration of anterior leaflet. (E) Implantation of EVOQUE transcatheter tricuspid valve replacement.
History of Presentation
The patient was a 98-year-old woman with recurrent tricuspid regurgitation (TR) after history of transcatheter tricuspid valve edge-to-edge repair (T-TEER) 3 years ago. Despite high-dose diuretics, she had NYHA functional class III symptoms. Physical examination revealed jugular venous distension, lower extremity edema, and II/VI holosystolic murmur at left lower sternal border.
Past Medical History
The patient had a history of chronic atrial fibrillation and permanent pacemaker. In 2021, she presented with severe dyspnea and lower extremity edema from severe TR. Echocardiography revealed a moderately dilated right atrium and mild right ventricular (RV) dilation with normal function. Tricuspid valve (TV) coaptation gap was 8 mm with a TR effective orifice area of 0.50 cm2, consistent with severe (3+) atrial secondary TR.1 Because her anatomy was consistent with ≥90% probability of achieving moderate TR or less,2 we proceeded with T-TEER. She received 2 MitraClip XTW devices (Abbott Vascular) near the anteroseptal commissure and 1 MitraClip XTW device on anterior-septal leaflets near the midorifice, reducing TR from severe to moderate (Figure 1, Video 1).
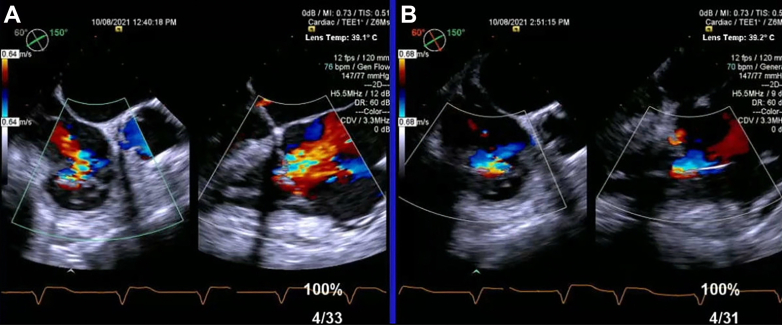
Figure 1.
TEE of Tricuspid Valve Edge-to-Edge Repair
(A) Transesophageal echocardiography (TEE) of tricuspid valve before edge-to-edge repair. (B) TEE of tricuspid valve after edge-to-edge repair with 3 MitraClip XTW devices.
Differential Diagnosis
The patient had no findings concerning for infection; therefore, endocarditis was unlikely. Echocardiography revealed preserved biventricular function and normal pulmonary artery pressures, consistent with recurrent TR being the primary cause of her symptoms.
Investigations
Transesophageal echocardiography (TEE) revealed that all 3 tricuspid clips were well attached to the leaflets with severe TR (Figure 2, Video 2). Computed tomography revealed 2 clips near the anteroseptal commissure and 1 near the midorifice (Figure 3, Video 3). Annulus perimeter was 136 mm (mean diameter: 43.3 mm), suggesting that 44-mm EVOQUE (Edwards Lifesciences) would be feasible, if the central clip was liberated so that is could be pushed aside during transcatheter tricuspid valve edge-to-edge repair (TTVR) implantation.
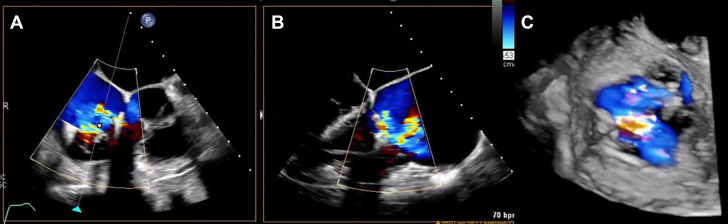
Figure 2.
TEE of Severe Recurrent TR after Tricuspid Valve Clips
(A) Transesophageal echocardiography (TEE) (intercommissural view) of recurrent tricuspid regurgitation (TR) after transcatheter tricuspid valve edge-to-edge repair (T-TEER). (B) TEE (long-axis view) of recurrent TR after T-TEER. (C) Three-dimensional TEE of recurrent TR after T-TEER.
Figure 3.
CT of Tricuspid Clips
(A) Computed tomography (CT) (long-axis view) of 3 tricuspid clips. (B) CT (short-axis view) of 3 tricuspid clips and pacemaker lead.
Management
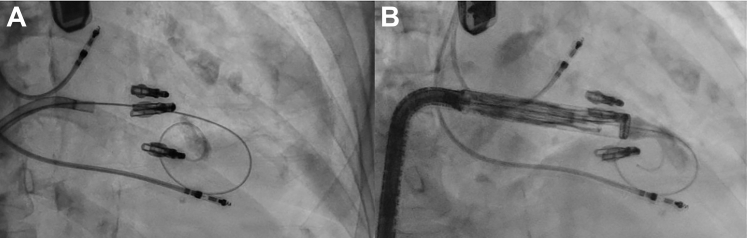
Using a modified Seldinger technique with ultrasound, the right femoral vein was accessed and 2 Perclose Proglides (Abbott Vascular) placed, with insertion of a 26-F Gore DrySeal sheath (Gore Medical). After heparinization, 2 Agilis NxT catheters (Abbott Vascular) were advanced into the right atrium (Figure 4A). Via a large curve Agilis NxT, a multipurpose A guide was advanced between clips 2 and 3 into the right ventricle; via the small curve Agilis NxT, a JR4 guide was advanced beneath clip 3 into the right ventricle and a 30-mm EnSnare (Merit Medical) followed (Figure 4B). Via multipurpose A guide, an Astato XS 20 wire (Asahi Intecc Medical) inside a PiggyBack catheter (Teleflex Medical) was advanced and snared by the 30-mm EnSnare via the JR4 guide (Figure 4C). The Astato wire was externalized from the JR4 guide until a flying V was across the anterior leaflet (Figures 4D and 4E). Electrocautery (50 W) was applied to the back end of the Astato wire, and the flying V was retracted, lacerating the anterior leaflet and leaving the tricuspid clip attached to the septal leaflet (Figure 4F, Video 4), resulting in torrential TR (Video 5).
Figure 4.
Fluoroscopic Views of Procedural Steps
(A) Agilis catheters in right atrium above clips. (B) Multipurpose A guide in between second and third clip; JR4 guide is below third clip with 30-mm EnSnare positioned in the right ventricle. (C) Astato wire snared by EnSnare. (D) Right anterior oblique view of flying V. (E) Left anterior oblique view of flying V. (F) Anterior leaflet lacerated with tricuspid clip on septal leaflet.
After laceration, a small curve Safari wire (Boston Scientific Corp) was advanced via one of the Agilix NxT catheters into RV apex (Figure 5A); the 26-F Gore DrySeal sheath was changed to the 28-F EVOQUE delivery system and advanced across the annulus (Figure 5B). After confirming depth, a capsule gap was created (Figure 6A), and the prosthesis was stepwise deployed (Figures 6B to 6I). TEE revealed excellent TTVR function with mild paravalvular leak (PVL) near the anteroseptal commissure (Video 6).
Figure 5.
Fluoroscopic Views Safari Wire and EVOQUE Delivery System in Right Ventricle
(A) Small curve Safari wire in the right ventricle. (B) Evoque delivery system positioned across the tricuspid annulus.
Figure 6.
Fluoroscopic Views of EVOQUE Deployment
(A) Evoque delivery system with capsule gap. (B) Evoque anchors at 45°. (C) Evoque anchors at 90°. (D) Evoque anchors partially flipped. (E) Evoque anchors fully flipped. (F) Evoque fully expanded. (G) Evoque deployed. (H) Evoque deployed with delivery system retracted. (I) Left anterior oblique fluoroscopic view of Evoque deployed.
Outcome and Follow-Up
The patient had no vascular complications, and warfarin was restarted on day 0. Postoperative echocardiography revealed a well-positioned EVOQUE TTVR with mild TR (mean gradient: 4 mm Hg). Pacemaker interrogation showed normal function, and she was discharged home on postoperative day 4. At 1 month, the patient reported reduced lower extremity edema and improved functional status (NYHA functional class II). Echocardiography revealed preserved RV function and stable TTVR function with trivial TR and mean gradient of 4.5 mm Hg.
Discussion
Transcatheter tricuspid repair and replacement has been shown to improve outcomes compared with medical therapy.3,4 In patients with complex anatomy, such as wide coaptation gaps or lead-induced TR, TTVR is often required for sufficient TR reduction.5 In patients with less complex anatomy, T-TEER can be effective, while minimizing risk of pacemakers and obviating need for anticoagulation.
It is challenging however to manage patients with history of T-TEER who develop recurrent severe TR. In some patients, TR is driven by volume overload and can be medically managed. In others, TR recurs because of single leaflet device attachment (SDLA), which occurred in 7.0% of patients in TRILUMINATE.3 In limited experience, SLDA does not appear to impede TTVR implantation with the EVOQUE system,6,7 likely because clips with SLDA are pushed out of the way by the TTVR frame. Also, EVOQUE has multiple anchors that engage the chordae and leaflets around the annulus, increasing the probability of anchoring. Finally, tricuspid clips with SLDA are stabilized by anchor engagement and the flexible outer frame conforms to the annulus, thereby mitigating PVL.
Well-attached T-TEER devices, however, prevent anchor engagement and stent frame expansion by limiting leaflet excursion and decreasing valve orifice area. Knowing how to manage these patients requires an understanding of the index T-TEER procedure and the resultant anatomic constraints as defined by multimodal imaging. For example, in a patient with a single T-TEER device firmly attached to the anteroseptal commissure, the residual orifice and leaflet mobility might be adequate for direct TTVR implantation without an adjunctive procedure. In our patient, however, in addition to 2 anteroseptal commissural clips, the clip near the mid-TV orifice clearly limited expansion of any prosthesis. Given these limitations, we decided to intentionally create an SLDA of the center clip using the technique CLEFT (Clip Liberation to Facilitate Transcatheter Tricuspid Valve Replacement) ELASTA-Clip procedure for the liberation of mitral edge-to-edge devices to allow transcatheter mitral valve implantation.8,9 We decided not to lacerate the 2 anteroseptal commissural clips because we had to balance the risk of commissural PVL vs the risk of creating a large flail. If there is adequate leaflet capture with good stent frame expansion, the outer sealing skirt of EVOQUE likely conforms to the anteroseptal commissure even with clip(s) in place, resulting in no more than mild PVL, as in our case. Conversely, a large flail might limit adequate leaflet capture and anchoring by EVOQUE, particularly on the septal side, causing even more instability and PVL. Although we are not sure our approach predictably prevents large flails, mechanistically, we speculate that lacerating just anterior to the central most clip replicates a simple SLDA-type anatomy, which typically does not result in a large flail, probably because chordal attachments remain intact. If a large flail does result, leaflet capture might still be possible, but would require careful understanding of residual anatomy using TEE with multiplanar reconstruction and likely intracardiac echocardiography before proceeding with EVOQUE implantation.
After laceration, TTVR implantation with EVOQUE entails several critical steps. Foremost, echocardiographic multiplanar reconstruction with spin is necessary to evaluate leaflet capture at each of the 9 anchors, especially because TEER devices are hyperechoic and may be confused with anchors. It is critical to understand the location of each of these clips in relation to commissures before the EVOQUE anchors are flipped.
Several uncertainties about this approach remain, including whether to lacerate anterior vs septal or posterior vs septal TV leaflets. Laceration of the shorter septal leaflet might leave a very short leaflet length that could jeopardize TTVR anchoring; anterior leaflet laceration might reduce this risk. Regardless of whichever TV leaflet is chosen for laceration, it might be preferable to lacerate as close to the clip as possible. Further uncertainty exists on which and how many clips to liberate. Although early experience suggests that clips close to commissures may be left alone, >1 securely attached clip may not allow TTVR valve frame expansion, resulting in early commissural perivalvular leak. Finally, it is unknown if full clip removal as described by Elison et al10 would be an alternative, but we fear that EVOQUE leaflet capture and anchoring might be inadequate after full removal given the adjacent leaflet tissue removed with this technique.
Whether full removal or our technique is suitable for other TTVR devices is uncertain. Other prostheses with anchors for leaflet capture, such as Cardiovalve (Venus MedTech) and TriSol (Trisol Medical), likely behave similarly to EVOQUE. Conversely, prostheses that do not require leaflet capture should anchor and might be better suited after full clip removal or in presence of large flail; for example, septal anchoring with the LuxValve (Jenscare Scientific) should be unimpeded and the Topaz valve (TriCares GmbH) should anchor secondary to its outer sealing skirt conforming to the annulus. Ultimately, more experience will clarify if this approach works with a broad range of TTVRs in a broad range of patients.
Conclusions
We present a successful case of transcatheter electrosurgical liberation of a tricuspid edge-to-edge repair device to allow successful TTVR with the EVOQUE system. We hope this encourages similar efforts so we can understand if this technique is feasible, safe, and effective for a broader range of patients.
Funding Support and Author Disclosures
Dr Thourani receives grant/research support from Abbott Vascular, Boston Scientific, Atricure, Croívalve, Edwards Lifesciences, Highlife, Jenavalve, LaPlace, Medtronic, Pi-Cardia, Tricares, and Trisol; and has equity in Dasi Simulations, Trisol, and Pi-Cardia. Dr Yadav is a speaker and consultant for Edwards Lifesciences, Abbott, and Medtronic; is on the advisory board for Dasi Simulations, PiCardia, and Trisol; and has investment in Dasi Simulations and Excision Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Take-Home Message
-
•
Transcatheter electrosurgical liberation of well-attached T-TEER devices might be a viable technique in patients who require a TTVR for recurrent tricuspid regurgitation.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transesophageal Echocardiography of Tricuspid Valve Edge-to-Edge Repair
Significant reduction in tricuspid regurgitation after transcatheter tricuspid valve edge-to-edge repair (right image) from baseline (left image).
Transesophageal Echocardiography of Severe Recurrent Tricuspid Regurgitation After Tricuspid Valve Clips
Severe tricuspid regurgitation after 3 MitraClip XTW devices.
Computed Tomography of Tricuspid Clips
Gated computed tomography of tricuspid valve with 2 MitraClip XTW devices attached to anteroseptal commissure and 1 MitraClip XTW device near middle of the valve.
Transcatheter Electrosurgical Liberation of 1 Tricuspid Clip
Transesophageal Echocardiography of Tricuspid Valve After Leaflet Laceration
Transesophageal echocardiography showing mobile central clip with torrential tricuspid regurgitation.
Transesophageal Echocardiography of EVOQUE Valve
Transesophageal echocardiography showing well-seated EVOQUE valve with trace paravalvular leak.
References
- 1.Hahn R.T., Lawlor M.K., Davidson C.J., et al. Tricuspid valve academic research consortium definitions for tricuspid regurgitation and trial endpoints. J Am Coll Cardiol. 2023;82(17):1711–1735. doi: 10.1016/j.jacc.2023.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Lurz P., Besler C., Schmitz T., et al. Short-term outcomes of tricuspid edge-to-edge repair in clinical practice. J Am Coll Cardiol. 2023;82(4):281–291. doi: 10.1016/j.jacc.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Sorajja P., Whisenant B., Hamid N., et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388(20):1833–1842. doi: 10.1056/NEJMoa2300525. [DOI] [PubMed] [Google Scholar]
- 4.Hahn R.T., Makkar R., Thourani V.H., et al. Transcatheter valve replacement in severe tricuspid regurgitation. N Engl J Med. 2025;392(2):115–126. doi: 10.1056/NEJMoa2401918. [DOI] [PubMed] [Google Scholar]
- 5.Hausleiter J., Stolz L., Lurz P., et al. Transcatheter tricuspid valve replacement. J Am Coll Cardiol. 2025;85(3):265–291. doi: 10.1016/j.jacc.2024.10.071. [DOI] [PubMed] [Google Scholar]
- 6.Wild M.G., Zahr F., Nabauer M., Massberg S., Hausleiter J. Transfemoral transcatheter tricuspid valve replacement after failed leaflet repair. Eurointervention. 2021;17(12):e1022–e1023. doi: 10.4244/EIJ-D-21-00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traynor B.P., Alnasser S., Bisleri G., Ong G., Fam N.P. EVOQUE transcatheter tricuspid valve replacement for double TriClip single-leaflet device attachment. JACC Cardiovasc Interv. 2024;17(22):2701–2702. doi: 10.1016/j.jcin.2024.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Samim D., Sorajja P., Lanz J., et al. Transapical transcatheter mitral valve replacement after failed transcatheter edge-to-edge repair: a multicenter experience. JACC Cardiovasc Interv. 2025;18(3):311–321. doi: 10.1016/j.jcin.2024.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Lisko J.C., Greenbaum A.B., Guyton R.A., et al. Electrosurgical detachment of MitraClips from the anterior mitral leaflet prior to transcatheter mitral valve implantation. JACC Cardiovasc Interv. 2020;13(20):2361–2370. doi: 10.1016/j.jcin.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elison D., Aldea G., Jelacic S., Chung C.J., Mackensen G.B., McCabe J.M. First-in-Human percutaneous excision of a failed MitraClip followed by transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2024;17(4):571–573. doi: 10.1016/j.jcin.2023.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal Echocardiography of Tricuspid Valve Edge-to-Edge Repair
Significant reduction in tricuspid regurgitation after transcatheter tricuspid valve edge-to-edge repair (right image) from baseline (left image).
Transesophageal Echocardiography of Severe Recurrent Tricuspid Regurgitation After Tricuspid Valve Clips
Severe tricuspid regurgitation after 3 MitraClip XTW devices.
Computed Tomography of Tricuspid Clips
Gated computed tomography of tricuspid valve with 2 MitraClip XTW devices attached to anteroseptal commissure and 1 MitraClip XTW device near middle of the valve.
Transcatheter Electrosurgical Liberation of 1 Tricuspid Clip
Transesophageal Echocardiography of Tricuspid Valve After Leaflet Laceration
Transesophageal echocardiography showing mobile central clip with torrential tricuspid regurgitation.
Transesophageal Echocardiography of EVOQUE Valve
Transesophageal echocardiography showing well-seated EVOQUE valve with trace paravalvular leak.