Abstract
Background
There is a theoretical drug interaction between enzalutamide, a potent cytochrome P 3A4 (CYP 3A4) inducer, and diltiazem, a CYP3A4 substrate. Resources recommend avoiding the combination.
Case Summary
The patient was taking diltiazem for rate control of permanent atrial fibrillation. After initiating enzalutamide for prostate cancer, he became persistently tachycardic, developed heart failure, and was admitted. On admission, enzalutamide was held, and diltiazem was stopped. His left ventricular ejection fraction was reduced from a baseline of 52% to <15%. The patient regained rate control with metoprolol and was asymptomatic at discharge.
Discussion
This case describes the interaction between enzalutamide and diltiazem that resulted in a loss of rate control and subsequent heart failure. It provides a cautionary example of the complications that may arise with gaps in drug interaction management and timely monitoring and follow-up.
Take-Home Message
Streamlined, multidisciplinary approaches to monitoring and managing drug interactions of uncertain clinical significance, particularly in the field of cardio-oncology, are needed.
Key Words: acute heart failure, atrial fibrillation, cancer, cardiomyopathy, complication, ejection fraction
Graphical Abstract
History of Presenting Illness
An 80-year-old man with permanent atrial fibrillation and prostate cancer presented to our hospital with several recent episodes of syncope and progressive dyspnea, orthopnea, and peripheral edema.
Take-Home Messages
-
•
The case highlights the need for a timely, streamlined multidisciplinary approach to monitoring and managing drug interactions of uncertain clinical significance, particularly in the field of cardio-oncology.
-
•
Consider alternatives prior to initiation of cancer- or cardiac-related therapies, in consultation with the patient's broader multidisciplinary team when dealing with theoretical drug interactions of unknown clinical significance.
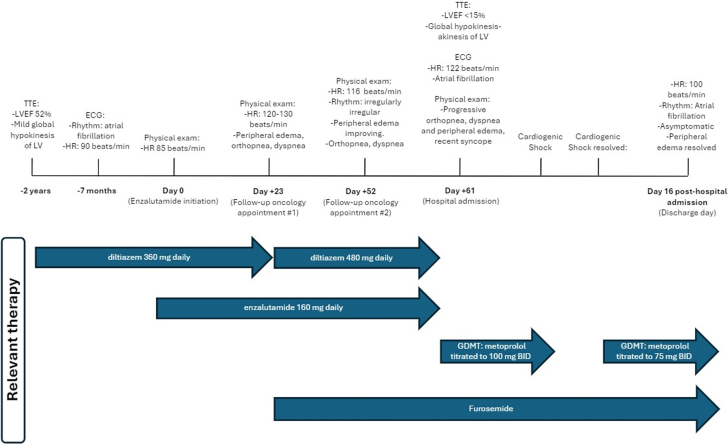
Two months before admission, the patient began enzalutamide therapy for castration-sensitive metastatic prostate cancer (Figure 1). At that time, a drug interaction between enzalutamide, a strong cytochrome P 3A4 (CYP3A4) inducer, and the patient’s long-standing rate control medication, diltiazem, a CYP3A4 substrate, was noted. However, no modifications to rate control therapy were made, and the patient continued to take diltiazem, 360 mg daily, which he had been stable on for over 6 years. Before enzalutamide initiation, the patient’s heart rate was 85 beats/min. His most recent electrocardiogram, completed approximately 7 months before starting enzalutamide, showed atrial fibrillation with a ventricular response rate of 90 beats/min. His most recent transthoracic echocardiogram (TTE), completed approximately 2 years before enzalutamide initiation, showed severe left ventricular dilatation, a mildly reduced left ventricular ejection fraction (LVEF) of 52%, and mild global left ventricular hypokinesis. His anticoagulation regimen was changed from apixaban to dabigatran before enzalutamide initiation, in recognition of the potential interaction between enzalutamide and apixaban that may reduce apixaban effectiveness. He was also taking escitalopram, which, when combined with enzalutamide, may result in decreased effectiveness because of CYP3A4 induction; however, no changes were made. The patient was not taking any other known CYP3A4 substrates, inducers, or inhibitors.
Figure 1.
Clinical Course and Time Frame
Relevant information related to the patient’s clinical history and events are highlighted. ECG = electrocardiogram; GDMT = guideline-directed medical therapy; HR = heart rate; LV = left ventricle; LVEF = left ventricular ejection fraction; TTE = transthoracic echocardiography.
Twenty-three days after enzalutamide initiation, at his follow-up oncology appointment, his heart rate was elevated at 120 to 130 beats/min, he had bilateral pitting edema, and his jugular venous pressure was 4 to 5 cm. The patient also reported that over the past couple of weeks he became progressively more dyspneic and orthopneic. His blood pressure remained well controlled near his baseline, measuring 124/79 mmHg. At this time, the patient’s diltiazem dose was increased from 360 mg daily to 480 mg daily, and furosemide, 20 mg daily, was started.
At his next follow-up appointment with his oncologist, 52 days after initiating enzalutamide, the patient was still in rapid atrial fibrillation, with a heart rate of 116 beats/min. Symptoms related to dyspnea and fluid overload were improved, and the decision was made to continue current therapy. However, the patient’s signs and symptoms ultimately worsened, and he was admitted with heart failure 11 days later.
Past Medical History
The patient had a medical history that included metastatic castration-sensitive prostate cancer, permanent atrial fibrillation, mild left ventricular dysfunction, and well-controlled hypertension. He did not smoke and drank on average 2 glasses of wine per week.
Differential Diagnosis
The initial differential diagnosis for the patient’s decompensated heart failure included tachycardia-induced cardiomyopathy secondary to a drug interaction, cardiomyopathy resulting from enzalutamide, tachycardia-induced cardiomyopathy of other causes, valvular heart disease, and ischemic heart disease.
Investigations
Telemetry showed atrial fibrillation with a rapid ventricular response and a heart rate of 122 beats/min. A TTE showed a dilated left ventricle, with severe systolic dysfunction, with an LVEF of <15%. Global left ventricular wall motion abnormalities were noted, ranging from hypokinesis to akinesis. There was no evidence of valvular heart disease. His N-terminal pro–B-type natriuretic peptide level was 6,476 ng/L, and his troponin level was mildly elevated at 22 ng/L (reference range: <14 ng/L). A cardiac stress test performed 2 years earlier showed no evidence of ischemia; his coronary arteries appeared patent on chest computed tomography 8 months before admission, and cardiac catheterization was not pursued.
Management
The patient’s enzalutamide was held on admission, and diltiazem was discontinued once TTE results were reported. He was subsequently started on guideline-directed medical therapy (GDMT) with metoprolol, dapagliflozin, spironolactone, and perindopril. Rapid up-titration of GDMT resulted in sinus bradycardia and cardiogenic shock. Dobutamine, epinephrine, and norepinephrine were initiated, and he was transferred to the cardiac intensive care unit. Once the patient was titrated off the infusions and was hemodynamically stable, GDMT was restarted and titrated slowly.
Outcomes and Follow-Up
At the time of discharge, 16 days after admission, the patient was taking GDMT with metoprolol, 75 mg twice daily, dapagliflozin, 10 mg daily, spironolactone, 25 mg daily, and perindopril, 2 mg daily. His rhythm was atrial fibrillation, his heart rate was maintained at ∼100 beats/min, and his dyspnea had improved. He was euvolemic, and diuretic agents were downtitrated. Enzalutamide was held until further evaluation by the oncologist. Patient consent was obtained for this case report.
Discussion
Enzalutamide is a known strong CYP3A4 inducer and was shown in a pharmacokinetic study to reduce the area under the curve of midazolam, a CYP3A4 substrate by 86%.1 To our knowledge, there are no known pharmacokinetic or clinical studies describing the impact of enzalutamide use in conjunction with diltiazem; however, given the theoretical interaction through a common metabolic pathway, various resources suggest avoiding the combination.2,3
Enzalutamide’s time to steady state, assuming 5 half-lives, is 14 to 51, days and maximal CYP3A4 induction occurs approximately 14 days after inducers are initiated.4,5 The onset of the patient’s reported signs and symptoms was consistent with these time frames, thus making the drug interaction a likely reason for the loss of rate control and the ensuing tachycardia-induced cardiomyopathy and decompensated heart failure. Assessing the drug interaction by using the Naranjo Adverse Drug Reaction Probability Scale resulted in a possible rating.
Although the oncology service anticipated the drug interaction and then subsequently noted the clinical effects of the drug interaction with preliminary management, cardiac investigations and interventions related to rate control were deferred to the patient’s cardiologist and family doctor. However, follow-up was not able to be done in a timely manner, and rate control was subsequently lost despite an increase in diltiazem dose. Together, the case provides a cautionary tale of the complications that may arise with gaps in drug interaction management and timely follow-up.
Although the overall rates of heart failure associated with enzalutamide are low in phase 3 clinical trials, a recent case report suggested that enzalutamide may exhibit direct cardiotoxic effects.6,7 In this study, the patient presented to the emergency department with new onset shortness of breath and was found to have new cardiomyopathy, an LVEF of 20%, and severe diffuse hypokinesis.7 The left ventricular dysfunction resolved on removal of the drug.7 Given our patient’s underlying cardiac dysfunction, it is unclear whether there were direct cardiotoxic effects that in fact worsened his underlying left ventricular dysfunction. Therefore, the decision was made to hold enzalutamide until it could be re-evaluated by the oncology team after discharge.
Additionally, enzalutamide has been associated with atrial fibrillation. A systematic review found that enzalutamide was associated with a higher risk of grade ≥3 atrial fibrillation compared with other androgen receptor pathway inhibitors and prostate cancer standard of care.8 It is unclear whether this increased risk was through direct cardiotoxicity or through indirect means, such as drug interactions or worsening hypertension. In our case, it is also unclear whether the underlying atrial fibrillation was indeed worsened by intrinsic activity of the drug. It is of course possible that a combination of all these effects in our patient resulted in heart failure.
Conclusions
In patients with atrial fibrillation, there is a predictable and clinically significant drug interaction between enzalutamide and diltiazem. As in this case, the interaction may result in loss of rate control and subsequent tachycardia-induced cardiomyopathy and decompensated heart failure. It theoretically may also pose risks in patients who use diltiazem for hypertension or angina. Consequently, this combination should be used only when there are no safer alternatives and when frequent monitoring and management by interdisciplinary teams are feasible, to ensure patient safety.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Gibbons J.A., de Vries M., Krauwinkel W., et al. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54(10):1057–1069. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Canada. Xtandi (Enzalutamide) Product Monograph. https://pdf.hres.ca/dpd_pm/00078033.PDF
- 3.Lennep B.W., Mack J., Poondru S., et al. Enzalutamide: understanding and managing drug interactions to improve patient safety and drug efficacy. Drug Saf. 2024;47(7):617–641. doi: 10.1007/s40264-024-01415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DrugBank Online. Enzalutamide https://go.drugbank.com/drugs/DB08899
- 5.Bettonte S., Berton M., Stader F., Battegay M., Marzolini C. Management of drug interactions with inducers: onset and disappearance of induction on cytochrome P450 3A4 and uridine diphosphate glucuronosyltransferase 1A1 substrates. Eur J Drug Metab Pharmacokinet. 2023;48(4):353–362. doi: 10.1007/s13318-023-00833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis I.D., Martin A.J., Stockler M.R., et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/nejmoa1903835. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A., Reddy A., Sekar A. Enzalutamide induced non-ischemic cardiomyopathy. A case report and review of literature on anti-androgen therapy-related cardiovascular events. CardioOncology. 2023;9(1):9. doi: 10.1186/s40959-023-00160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsukawa A., Yanagisawa T., Parizi M.K., et al. Cardiovascular events among men with prostate cancer treated with androgen receptor signaling inhibitors: a systematic review, meta-analysis, and network meta-analysis. Prostate Cancer Prostatic Dis. 2025;28:298–308. doi: 10.1038/s41391-024-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]