Abstract
Aortopulmonary communication after percutaneous pulmonary valve implantation is an uncommon complication. Associated risk factors include acute trauma to the homograft in the setting of aggressive balloon dilatation. A high level of suspicion is required and aortography may be necessary for diagnosis. The presentation can be acute or chronic, and percutaneous approach is possible.
Key Words: congenital heart defect, pulmonic valve, valve replacement
Graphical Abstract
History of Presentation
We present the case of a 21-year-old man after Ross operation qualified for percutaneous pulmonary valve implantation (PPVI) because of pulmonary homograft degeneration. In recent months, he developed worsening dyspnea and decreased exercise tolerance. Physical examination revealed manifestations indicative of hypervolemia, including lower-limb edema, jugular distension, and hepatomegaly. Routine transthoracic echocardiography (TTE) demonstrated signs of restricted pulmonary valve opening associated with significant regurgitation.
Take-Home Messages
-
•
Aortopulmonary fistula formation after TPVR is a are but potentially life-threatening condition where percutaneous management is possible.
-
•
Abnormal diastolic flow in the pulmonary artery on TTE and increase in PA pressure early after intervention should prompt aortic angiography to establish diagnosis and timely intervention.
Past Medical History
The patient was born with congenital aortic valve dysplasia. Within the first day after birth, percutaneous balloon valvuloplasty was required. Subsequently, at the age of 2 years, the first open heart surgery was performed, entailing aortic homograft implantation, which was subsequently replaced the following year with another homograft. At the age of 9 years, the patient underwent a Ross procedure, incorporating the implantation of a 22-mm homograft, there was enlargement of the right ventricular outflow tract with a pericardial patch, and there was no modification of the aortic root in the implemented technique. On the third day after surgery, he underwent pacemaker implantation.
Differential Diagnoses
Our main differential diagnoses included stenosis from arterial conduit wall retraction or at the distal anastomosis, and structural valve deterioration. Patient-prosthesis mismatch was unlikely, because post-procedure gradients were normal.
Investigations
His TTE showed normal left ventricular ejection fraction, restricted of pulmonary homograft opening with significant regurgitation, right ventricle (RV) with increased dimensions and reduced function. Invasive manometry demonstrated increased systolic pressure of 96 mm Hg in the RV and the presence of a gradient of 48 mm Hg between the RV and pulmonary artery (PA) (Figures 1A and 1B). Pulmonary angiography demonstrated the presence of significant pulmonary valve regurgitation. Computed tomographic angiography (CTA) showed a calcification and diffuse reduction in the caliber of the pulmonary homograft with a minimum diameter of 13 mm (Figures 1C and 1D, Video 1). The reconstructed image of the autograft demonstrated the presence of focal calcification and a small saccular aneurysm located above the origin of the left coronary ostium and below the suture line of the autograft in the aorta (Video 2).
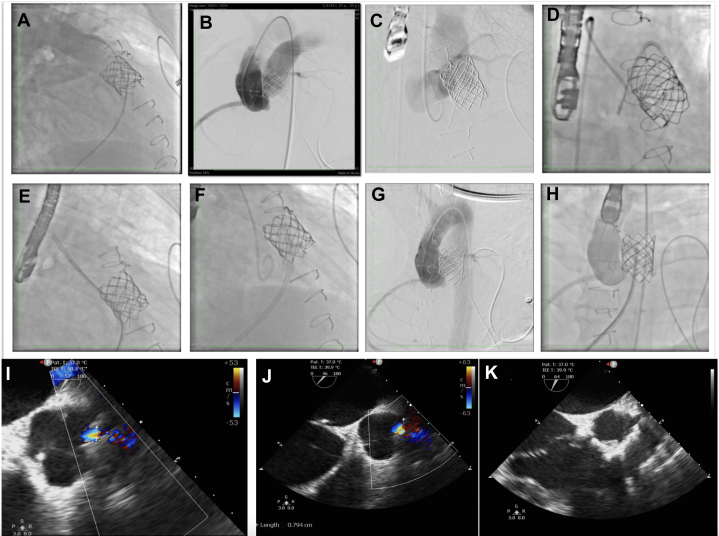
Figure 1.
Percutaneous Pulmonary Valve Implantation
(A) Pulmonary artery manometry. (B) Right ventricular manometry. (C) Computed tomographic angiography demonstrating the interaction between aorta and pulmonary homograft with calcification. (D) Three-dimensional reconstruction image of aortic root and pulmonary homograft. (E) Balloon predilation. (F) Balloon coronary compression testing demonstrating absence of left coronary involvement. (G) Stent predeployment within the right ventricular outflow tract. (H) Balloon postdilation. (I) Sapien valve after deployment.
Management
Following evaluation by the heart team, transcatheter intervention was chosen owing to the patient’s complex medical background and heightened surgical risk. We crossed the pulmonary valve with a pigtail diagnostic catheter and a hydrophilic guidewire. Proper positioning of the Lunderquist guide in the left pulmonary artery and balloon predilation was performed with a 22-mm and a 24-mm Atlas Gold (Bard Peripheral Vascular) (Figure 1E). After demonstrating that there was no coronary obstruction with the inflated balloon, a covered stent, CP 8 Zig 45 (Cordis Corp), mounted on an 24-mm Althosa balloon was implanted (Figures 1F and 1G). A 23-mm Sapien 3 valve was placed followed by postdilation (Figure 1H). Valve function was confirmed by angiography and TTE (Figures 1I and 2A). Control manometry showed an increase in PA pressure early after intervention. Aortic root angiography revealed left-to-right flow filling the distal PA (Figure 2B, Video 3). We decided to evaluate the fistula by means of transesophageal echocardiography which shows abnormal flow observed in the ascending aorta, 8 mm from the origin of the left coronary artery (LCA) (Figures 2I and 2J). The fistula was in the neo-ascending aorta, at approximately 18 mm from the virtual annular plane, at the topography of the left coronary sinus of valsalva (Figure 3, Video 4). To address this, transcatheter closure of the fistula was performed using a 6-mm Occlutech muscular ventricular septal defect device, selected based on fistula size, to minimize valve interference and avoid obstruction of the LCA ostium. The fistula was accessed via an aortic approach using a 6-F AL1 diagnostic catheter and a 0.014-in hydrophilic guidewire (Figure 2C). Subsequently, an arteriovenous loop was created to facilitate sheath advancement and device deployment (Figure 2D). Despite encountering difficulty in retrograde advancement of a 7-F sheath owing to unfavorable angulation, successful deployment was achieved via femoral vein, followed by placement of a supportive hydrophilic guidewire (Videos 5 and 6, Figures 2E and 2F). The procedure was completed without complications, achieving interruption of communication without affecting the origin of the LCA (Videos 7 and 8, Figures 2G and 2H). Hemostasis was reached with the use of a double Perclose technique at the main access site and manual compression at other vascular sites. The patient left the hemodynamic room stable and on invasive mechanical ventilation. In the first days in the intensive care unit, progressive worsening of kidney function associated with oliguria was observed. Therefore, there was difficulty in weaning from mechanical ventilation owing to pulmonary congestion. Hemodialysis was performed for 3 consecutive days, resulting in significant improvement in hypervolemia. Associated with the condition, tracheobronchitis was diagnosed and treated with culture-guided antibiotic therapy. The patient was discharged home after 14 days.
Figure 2.
Aortopulmonary Fistula Closure
(A) Control pulmonary angiography showing absence of regurgitation through the implanted pulmonary valve. (B) Aortography demonstrating contrast filling in the pulmonary artery. (C) This image demonstrates contrast injection and mild opacification of the pulmonary artery with the AL1 catheter immediately before crossing the aortopulmonary fistula with the PT2 coronary guidewire. (D) Guidewire captured with a snare catheter to create an arteriovenous loop for support. (E, F) Positioning and implantation of the device via anterograde approach. (G, H) Final angiography showing absence of residual flow through the fistula. (I, J) Transesophageal echocardiography (TEE) demonstrating the presence of unusual flow in the aortic root close to the left coronary ostium. (K) Postdeployment TEE evaluation.
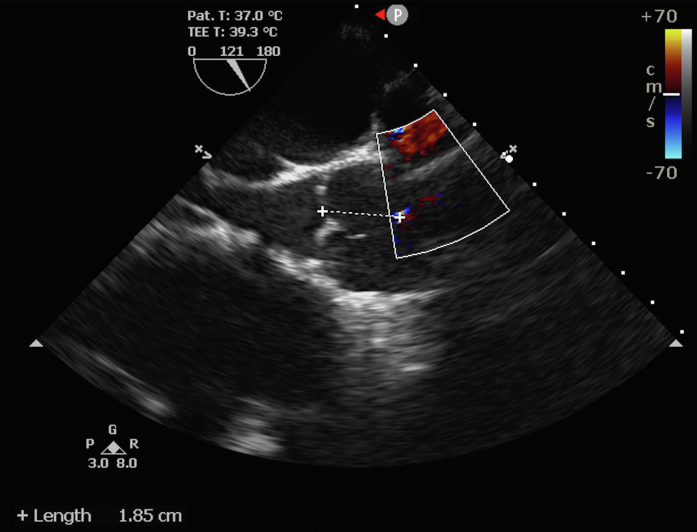
Figure 3.
Abnormal Flow Observed in the Ascending Aorta
Distance from the aortic autograft annulus to the aortopulmonary fistula.
Discussion
Iatrogenic aortopulmonary fistula after PPVI is a rare complication and can occur immediately as well as weeks after the procedure. In the literature we found 6 cases of this complication after PPVI, half of which occurred in patients who had previously undergone Ross surgery.1, 2, 3, 4, 5 This relationship may be related to architectural distortion as a result of the incision and enlargement of the aortic ring, which leads to anterior displacement off the neo-aorta toward the infundibulum of the RV. Consequently, PPVI would result in a significantly greater risk of compression of the neo-aortic root and may generate erosive pressure on the autograft.
The diagnosis necessitates suspicion and can be conducted either at the time of the procedure or throughout the follow-up. Aortic root angiography and TTE after PPVI can identify acutely formed fistulas. CTA is a useful tool for identifying and evaluating the anatomy and location of the complication when the patient has already left the procedure. In the present patient, we observed an increase in pulmonary pressure at the end of PPVI, which made us suspect the complication. We performed angiography of the aortic root, which detected the presence of a left-right shunt tracing the pulmonary branches. TTE was also useful because a shunt in the aorta was visualized. Furthermore, information such as size, location, and proximity to the LCA ostium was information collected from the TTE and was fundamental in choosing the treatment strategy.
This complication can be treated percutaneously or surgically.1 In this case, we opted for transcatheter closure after detailed analysis of the fistula position, thinking that the prosthesis would not affect valve movement or obstruct the LCA. Using angiography and echocardiography, we assessed the fistula for size, guiding the selection of the appropriate prosthesis. To mitigate concerns regarding occlusion of the LCA ostium and potential interaction with the pulmonary prosthesis, a muscular ventricular septal defect prosthesis with smaller disc dimensions was implanted. A more supportive guidewire was required, leading to the use of the pulmonary route to advance the material. On completion of the implantation, complete cessation of fistula flow was observed, with no compromise to the LCA ostium or impairment of pulmonary prosthesis function.
This case presents a unique scenario where the fistula was promptly identified after pulmonary valve implantation, and a percutaneous correction intervention was executed concomitantly during the surgical procedure. The decision was made to preemptively close them before the onset of hemodynamic deterioration, given the potential for undiagnosed fistulas to expand progressively. Our conjecture posits that forceful expansion of the conduit or margins may have precipitated the defect. Thus, heightened awareness of this infrequent complication, particularly in the context of post–Ross surgery procedures, is warranted. We advocate for meticulous 3-dimensional assessment of the spatial interrelations between the RV outflow tract and the aortic root, alongside routine aortic root angiography subsequent to PPVI.
Follow-Up
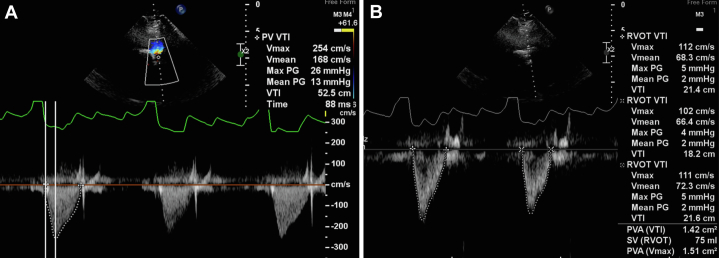
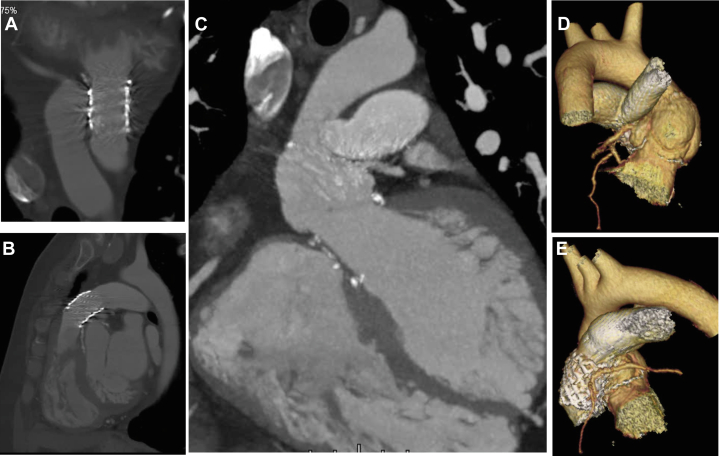
Six months later, the patient remained asymptomatic. Control TTE was performed which demonstrated aortic autograft with normal opening and trace regurgitation (Video 9). The device at the level of aortic-pulmonary fistula was stable and without residual shunt (Video 10). The pulmonary endoprosthesis was shown with normal opening and no regurgitation. The systolic gradients and acceleration time were low, and the effective orifice area was 1.4 cm2 (Figure 4). Control CTA demonstrated the presence of a stent in the pulmonary homograft with good appearance, absence of flow between the aorta and the pulmonary artery, and patency of the ostia of both coronary arteries (Figure 5).
Figure 4.
Doppler Mode of the Parasternal Short Axis View
(A) Pulmonary prosthesis velocity time integral. (B) Right ventricular outflow tract velocity time integral.
Figure 5.
Control Computed Tomographic Angiography
(A, B) Pulmonary homograft with stent in its conduit and percutaneous pulmonary valve prosthesis and absence of contrast passage connecting the aorta with the pulmonary artery. (C to E) Patent ostium of the right coronary artery and left main coronary artery.
Conclusions
Aortopulmonary fistula after PPVI is a rare complication. A high level of suspicion is required, and aortic angiography helps to diagnose this unusual complication earlier. Percutaneous treatment is possible in cases with favorable anatomy.
Funding Support and Author Disclosures
Dr Rômulo is a proctor for Lifetech. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Evaluation of pulmonary homograft with computed tomographic angiography reconstruction.
Evaluation of aortic root morphology with computed tomographic angiography reconstruction.
Aortic root angiography demonstrating left-to-right flow filling the distal pulmonary artery.
Anatomic assessment of aortopulmonary fistula by transesophageal echocardiography.
Arteriovenous loop performed after capture of the guidewire at the distal pulmonary artery by the snare catheter.
Difficulty in retrograde advancement of a 7-F sheath owing to unfavorable angulation.
Advancement of the 7-F sheath via anterograde route with the support of the arteriovenous loop and extra supportive hydrophilic guidewire.
Final aortic root angiography demonstrating absence off left-to-right flow.
Doppler of the autograft in aortic position.
Absence of residual shunt through the device shown in short-axis view.
References
- 1.Torres A., Sanders S.P., Vincent J.A., et al. Iatrogenic aortopulmonary communications after transcatheter interventions on the right ventricular outflow tract or pulmonary artery: pathophysiologic, diagnostic, and management considerations. Catheter Cardiovasc Interv. 2015;86:438–452. doi: 10.1002/ccd.25897. [DOI] [PubMed] [Google Scholar]
- 2.Peer S.M., Sinha P. Percutaneous pulmonary valve implantation after Ross-Konno aortoventriculoplasty: a cautionary word. J Thorac Cardiovasc Surg. 2014;147:e74–e75. doi: 10.1016/j.jtcvs.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Taggart N.W., Hagler D.J., Connolly H.M. Melody valve erosion into the ascending aorta. Congenit Heart Dis. 2013;8:e64. doi: 10.1111/chd.12021. [DOI] [PubMed] [Google Scholar]
- 4.Kenny D., Holoshitz N., Turner D., et al. Aortopulmonary fistula after transcatheter pulmonary valve replacement. Circ Cardiovasc Interv. 2013;6:e67–e68. doi: 10.1161/CIRCINTERVENTIONS.113.000654. [DOI] [PubMed] [Google Scholar]
- 5.Loureiro P., Martins J.F., Fraisse A., et al. Iatrogenic fistula between the aorta and the right ventricular outflow tract after Melody valve implantation: case report and literature review. Rev Port Cardiol (Engl Ed) 2020;39(9):545.e1–545.e4. doi: 10.1016/j.repc.2018.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of pulmonary homograft with computed tomographic angiography reconstruction.
Evaluation of aortic root morphology with computed tomographic angiography reconstruction.
Aortic root angiography demonstrating left-to-right flow filling the distal pulmonary artery.
Anatomic assessment of aortopulmonary fistula by transesophageal echocardiography.
Arteriovenous loop performed after capture of the guidewire at the distal pulmonary artery by the snare catheter.
Difficulty in retrograde advancement of a 7-F sheath owing to unfavorable angulation.
Advancement of the 7-F sheath via anterograde route with the support of the arteriovenous loop and extra supportive hydrophilic guidewire.
Final aortic root angiography demonstrating absence off left-to-right flow.
Doppler of the autograft in aortic position.
Absence of residual shunt through the device shown in short-axis view.