Graphical Abstract

Key words: anatomy, left atrial appendage
Physiological roles of the left atrial appendage include decompression of left atrial pressure (reservoir) via its increased distensibility compared with other parts of the left atrium and production of atrial natriuretic polypeptide in response to atrial stretch. In addition to these mechanical and hormonal functions, the left atrial appendage is likely to be involved in neuroregulatory circuits that control systemic blood pressure via the catecholamine and renin-angiotensin-aldosterone system; the detailed mechanism has not been fully elucidated.1,2 Because of its blind-end nature with multiple pectinate muscles along with variations in its shape and size,3,4 the resultant relative stasis of blood flow can play a significant role in thrombus formation, especially in atrial fibrillation. From the viewpoint of anatomy, in addition to its peculiar shape and size, the left atrial appendage exhibits unique relationships with surrounding structures as it protrudes anterosuperiorly from the superolateral region of the left atrial vestibule. Multiple invasive approaches, including catheter ablation of atrial and ventricular arrhythmias, left superior ganglionated plexus ablation, endocardial or epicardial and percutaneous or surgical left atrial appendage closure, and ethanol injection into the vein of Marshall, currently target the left atrial appendage or its adjacent structures. Thus, detailed knowledge of the clinical structural anatomy of the left atrial appendage is integral for procedural success without inadvertent complications.5, 6, 7, 8, 9, 10, 11 However, resources showing 3-dimensional nondistorted anatomy around the left atrial appendage have been limited. We herein provide comprehensive anatomical images of the left atrial appendage obtained from the pressure-perfused and fixed hearts of the University of California-Los Angeles, Wallace A. McAlpine and Amara-Yad Project Collections,12, 13, 14 with relevant clinical images (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11).
Take-Home Messages
-
•
The left atrial appendage exhibits wide variations in shape, size, and relationships with adjacent structures.
-
•
Detailed real dissection images of the nondistorted heart with corresponding clinical images offer a unique opportunity to review left atrial appendage anatomy.
-
•
Comprehensive 3-dimensional understanding of individual left atrial appendage anatomy is crucial for procedural success and minimizing procedural complications.
Figure 1.
Anatomy of the Left Atrial Appendage Within the Chest
(A) When viewed from the superior direction, the right lung, right atrial appendage, ascending aorta, pulmonary trunk, left atrial appendage, and left lung align from right to left. (B) When viewed from the anterior direction, only the tip of the left atrial appendage can be observed at the left lateral margin of the heart above the left ventricle. (C) A close-up view of the left atrial appendage shows its close topographic relationship to the pericardium, left phrenic nerve, parietal pleura, and left lung. (D,E) When the pressure-perfused and fixed hearts placed in the physiological position are viewed from the right posterosuperior direction after the removal of a substantial part of the atria, the images show the superolateral location of the left atrial appendage orifice relative to the mitral valve orifice. In (E), the left atrial appendage orifice is skewed to the superior direction relative to the mitral valve orifice in comparison with the orientation of the orifice depicted in (D) (Figure 6). The left atrial appendage extends toward the superoanterior direction.
Figure 2.
Multidirectional Views and Progressive Dissection of the Left Atrial Appendage
The frontal (A), left lateral (B), and left anterior oblique and cranial (C) views of the heart are presented. The left atrial appendage is located lateral to the pulmonary trunk and covers the left ventricular summit15 at the junction of anterior interventricular and left atrioventricular grooves. (D-F) Progressive dissections of the heart viewed from the left lateral direction are useful to understand the topographic relationship between the left atrial appendage, left ventricular summit, pulmonary trunk, left superior pulmonary vein, left upper bronchus, left pulmonary artery, and left coronary arteries. (E) The left atrial appendage is retracted with a pin.
Figure 3.
Variation in the Shape and Size of the Left Atrial Appendage
Similar to a human fingerprint, each left atrial appendage exhibits unique shape and size. Given such variable morphology, the size of its orifice, direction of the tip, pattern and number of lobulation, and relationship with surrounding structures also vary widely.3,4 The central image highlights a view of the left atrial appendage from the superior direction. The surrounding images showcase the left atrial appendage of different hearts viewed from the left lateral direction.
Figure 4.
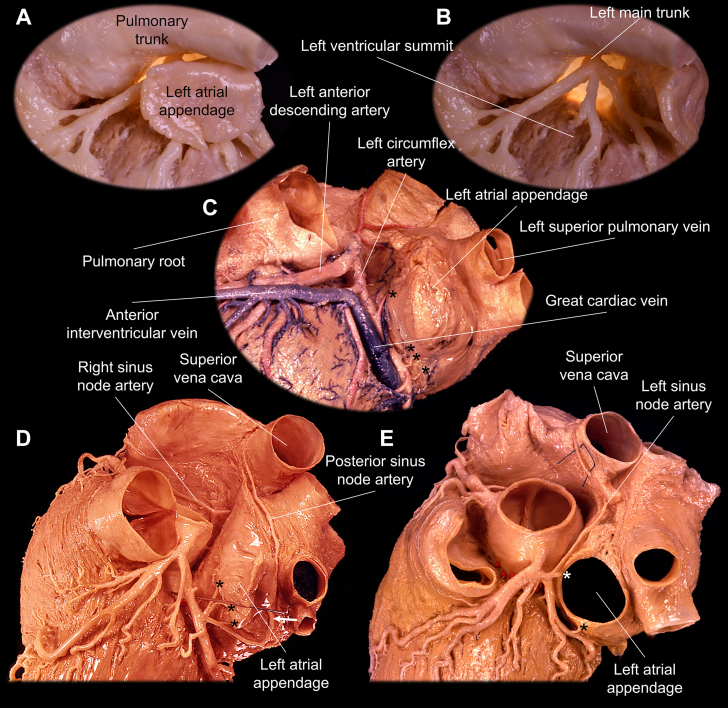
Relationship Between the Left Atrial Appendage With the Coronary Vessels
(A to C) Distal to the orifice of the left atrial appendage, the wall is freely mobile within the pericardial space. It generally covers the proximal left coronary artery and great cardiac vein–anterior interventricular vein junction located within the epicardial fat. Epicardial fat is removed in A and B, and the left atrial appendage is retracted in B to D. The left atrial appendage is removed in E to show its orifice. The posterior (D) and left (E) sinus node arteries run posterior and anterior to the left atrial appendage, respectively.16 The asterisks denote multiple tiny left atrial appendage arteries found at the base of the left atrial appendage. All these vessels can be jeopardized during invasive procedures targeting the left atrial appendage.
Figure 5.
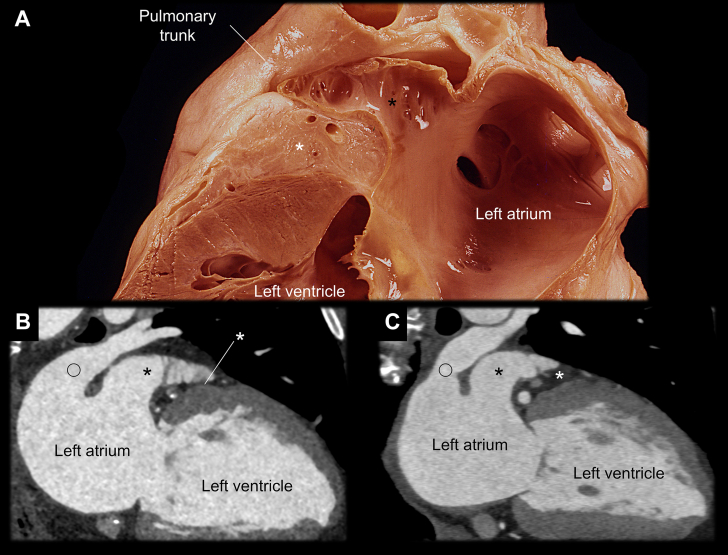
Variation in the Thickness of the Atrioventricular Groove Between the Left Atrial Appendage and Left Ventricle
(A) The 2-chamber view of the left heart shows the thick epicardial fat (white asterisks) of the left atrioventricular groove sandwiched between the basal anterior left ventricle and left atrial appendage (black asterisks). (B,C) The fat envelops coronary vessels (Figure 4), and its thickness is variable. When it is thin (B), the left atrial appendage is closer to the basal anterior left ventricle. The open circles indicate the left superior pulmonary veins. This variation affects the utility and feasibility of left ventricular epicardial mapping and ablation from the left atrial appendage.17
Figure 6.
Variation in the Location of the Left Atrial Appendage Orifice Relative to the Pulmonary Vein and Mitral Valve
(A) Virtual dissection of 4 hearts using clinical cardiac computed tomography data sets reveals variation in the location of the left atrial appendage orifice (asterisks) relative to the left superior (open circles) and inferior (closed circles) pulmonary veins. Generally, the left atrial appendage orifice is closer to the left superior pulmonary vein orifice than it is to the left inferior pulmonary vein orifice, and the left atrial (Coumadin or warfarin) ridge is more prominent between the left atrial appendage and left superior pulmonary vein. (B,C) Dissections viewed from the left lateral direction also demonstrate the same spatial relationship. (D,E) When observed from the left atrium using an endoscope, the variations in the location of the left atrial appendage orifice (asterisks) relative to the center of the mitral valve are observed. Arrowheads denote the superolateral commissure of the mitral valve. This variation is related to the individual variation in the optimal rotation angle of the sectional plane to visualize the left atrial appendage during transesophageal echocardiography.
Figure 7.
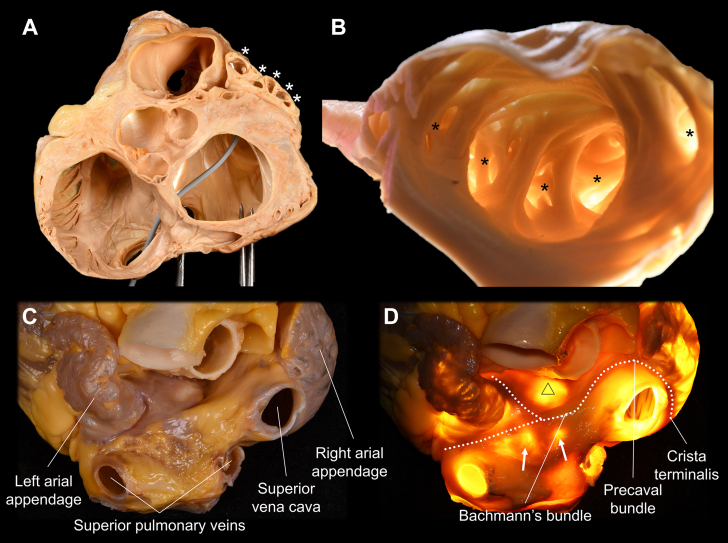
Multiple Lobulations and Epicardial Muscle Bundles of the Left Atrial Appendage
(A,B) The pectinate muscles within the left atrial appendage create multiple lobulations (asterisks).4 This is the main reason why thorough mapping of the left atrial appendage is almost impossible from the endocardial approach. (B,D) The wall between the pectinate muscle is thin enough to allow transillumination. (C,D) The heart viewed from the superior direction demonstrates how the Y-shaped myocardial bundles (white dotted lines) continue from Bachmann's bundle and surround the left atrial appendage orifice (D).11, 12, 13, 14 The triangle indicates the unprotected area at the anterior wall of the left atrium beneath Bachmann's bundle, located behind the aortic root.12 Arrows denote the left atrial diverticula on the roof of the left atrium (Figure 8).
Figure 8.
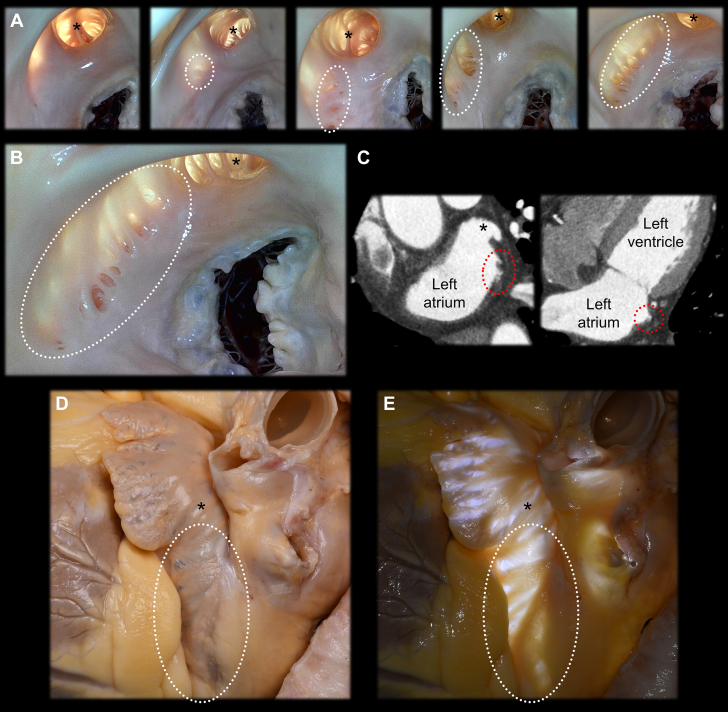
Diverticula Near the Left Atrial Appendage Orifice
(A,B) During real dissection, variable size and extent of the diverticula (dotted circles) along the lateral wall of the left atrium beneath the left atrial appendage orifice (asterisks) are commonly observed. They are associated with variable extent of the pectinate muscles and are also referred to as the accessory left atrial appendage,18,19 crevices and pits,9 or extrapectinate muscle trabeculations.10 (C) These diverticula can be detected by cardiac computed tomography in clinical settings under careful review. When the perfused fixed heart is viewed from the outside without (D) or with (E) transillumination, these structures are also easily observable. The multiple diverticula, ectopic pectinate muscles observed outside the left atrial appendage, and transilluminated thin wall of this region pose a risk for perforation, incomplete mapping or ablation, thrombogenesis, and entrapment of the catheters or devices. Additionally, these diverticula can be an ectopic arrhythmogenic substrate.20,21
Figure 9.
Histology of the Left Atrial Appendage
Histologic images of the apical (A to D) and basal (E to G) parts of the left atrial appendage are demonstrated. The wall of the left atrial appendage at the pectinate muscle exhibits multidirectional and multilayered myocardial fibers observed as coexisting longitudinal and cross-sectional myocardium (C). In some regions, the wall of the left atrial appendage is thinner than 40 μm (black arrows in D) and even devoid of any myocardium, indicating a risk for perforation. Within the epicardial and intramural layer of the basal section (G), multiple nerve fascicles (red arrows) are observed. These nerve fascicles can be jeopardized during an epicardial or surgical approach to the left atrial appendage. Further study is required to characterize the components of these nerve fascicles innervating the left atrial appendage.
Figure 10.
Clinical Procedures Associated With the Left Atrial Appendage
(A,C) The mitral isthmus (yellow dotted lines) is defined as the region between the left inferior pulmonary vein (closed circles) and the inferolateral mitral annulus. The open circles and asterisks indicate the left superior pulmonary vein and left atrial appendage orifice, respectively. (B to D) The vein of Marshall (white arrows), also referred to as the oblique vein of the left atrium, is a remnant of the left superior vena cava.22 It descends along the epicardial region between the left atrial appendage and left pulmonary veins, running across the mitral isthmus and draining into the coronary sinus.12,13 Either the vein of Marshall or the valve of Vieussens may be used as the anatomical marker to demarcate the margin between the coronary sinus and great cardiac vein.23 Thrombus (red arrows) in the left atrial appendage could be detected using transesophageal echocardiography (E) and contrast-enhanced computed tomography (F,G). A surgical and epicardial left atrial appendage occlusion device is shown in maximum-intensity projection (H), volume-rendered (I), and virtual dissection (J) images. Note the proximity of the superior edge of the occlusion clip to the pulmonary trunk.
Figure 11.
Percutaneous Endocardial Left Atrial Appendage Occlusion Device
(A to F) The percutaneous and endocardial left atrial appendage occlusion device (first generation) is observed in the images. (B) The device viewed through the floor of the fossa ovalis (opened). (C to E) The central and partial endothelialization of the device is observed. (F) Microperforation (arrowhead) is also noted. (G) A simulation of the coaxial direction between the left atrial appendage and the floor of the fossa ovalis. In this heart, the red direction offers more of a coaxial approach compared with the yellow direction.24 (H to J) Multiplanar and virtual dissection images reconstructed from the clinical cardiac computed tomography data sets after the placement of the next-generation endocardial left atrial appendage occlusion device show partial thrombi (white asterisks) and a focal gap (red asterisks) that measures 2.2 mm in length.
Funding Support and Author Disclosures
This work was made possible by support from National Institutes of Health grant P01 HL164311 to Dr Shivkumar and the UCLA Amara-Yad Project (https://www.uclahealth.org/medical-services/heart/arrhythmia/about-us/amara-yad-project). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the individual who donated their body for the advancement of education and research. The authors are grateful to the OneLegacy Foundation and the National Institutes of Health (grant P01 HL164311 to Dr Shivkumar), which formed the basis for obtaining donor hearts for research and for funding this effort, respectively. The authors also thank the University of California-Los Angeles (UCLA), Amara-Yad Project for supporting this work. The authors are grateful to Prof Warwick J. Peacock, Mr Nestor J. Juarez, and Mr Edwin Ng with the UCLA Surgical Sciences Laboratory as well as Mr Travis G. Siems, Mr Alex Rodriguez, and Ms Melissa Demers with the UCLA Donated Body Program for their support in dissections. The authors thank Dr Olujimi A. Ajijola for establishing and maintaining an organ procurement pipeline for research. The authors deeply appreciate their research operations managers, Ms Amiksha S. Gandhi and Ms Hunter N. Strause, for their dedication and support for their projects.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Al-Saady N.M., Obel O.A., Camm A.J. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakkireddy D., Turagam M., Afzal M.R., et al. Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol. 2018;71:135–144. doi: 10.1016/j.jacc.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L., Santangeli P., Anselmino M., et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Veinot J.P., Harrity P.J., Gentile F., et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 5.Sepahpour A., Ng M.K., Storey P., McGuire M.A. Death from pulmonary artery erosion complicating implantation of percutaneous left atrial appendage occlusion device. Heart Rhythm. 2013;10:1810–1811. doi: 10.1016/j.hrthm.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Hanazawa K., Brunelli M., Saenger J., et al. Close proximity between pulmonary artery and left atrial appendage leading to perforation of the artery, tamponade and death after appendage closure using cardiac plug device. Int J Cardiol. 2014;175(2):e35–e36. doi: 10.1016/j.ijcard.2014.04.260. [DOI] [PubMed] [Google Scholar]
- 7.Suwalski G., Wojnowski A., Mizerski J., Gryszko L. Delayed pulmonary artery perforation with left atrial appendage occluder hooks. Ann Thorac Surg. 2016;101:e37–e39. doi: 10.1016/j.athoracsur.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Miller W.H., Dhruvakumar S., Owlia M.C., D'Onofrio G.R., Hsi D. Late presentation of pulmonary artery-left atrial appendage fistula formation after left atrial appendage device closure. JACC Case Rep. 2020;2:814–818. doi: 10.1016/j.jaccas.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera J.A., Ho S.Y., Climent V., Sánchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera J.A., Saremi F., Sánchez-Quintana D. Left atrial appendage: anatomy and imaging landmarks pertinent to percutaneous transcatheter occlusion. Heart. 2014;100:1636–1650. doi: 10.1136/heartjnl-2013-304464. [DOI] [PubMed] [Google Scholar]
- 11.Ho S.Y., Cabrera J.A., Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol. 2012;5:220–228. doi: 10.1161/CIRCEP.111.962720. [DOI] [PubMed] [Google Scholar]
- 12.McAlpine W.A. Springer-Verlag; 1975. Heart and Coronary Arteries: An Anatomical Atlas for Clinical Diagnosis, Radiological Investigation, and Surgical Treatment. [Google Scholar]
- 13.Mori S., Shivkumar K. Cardiotext Publishing; 2022. Atlas of Cardiac Anatomy: Anatomical Basis of Cardiac Interventions1. [Google Scholar]
- 14.Tung R., Mori S., Shivkumar K. Cardiotext Publishing; 2024. Atlas of Interventional Electrophysiology: Anatomical Basis of Cardiac Interventions2. [Google Scholar]
- 15.Mori S., Bradfield J.S., Fukuzawa K., Shivkumar K. Comprehensive anatomy of the summit of the left ventricle. JACC Clin Electrophysiol. 2024;10:168–184. doi: 10.1016/j.jacep.2023.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Esrailian D.L., Mori S., Shivkumar K. Understanding cardiac anatomy and imaging to improve safety of procedures: the sinus node artery. JACC Case Rep. 2023;28 doi: 10.1016/j.jaccas.2023.102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakubov A., Salayev O., Hamrayev R., Sultankhonov S. A case of successful ablation of ventricular tachycardia focus in the left ventricular summit through the left atrial appendage: a case report. Eur Heart J Case Rep. 2018;2 doi: 10.1093/ehjcr/yty110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee W.J., Chen S.J., Lin J.L., Huang Y.H., Wang T.D. Images in cardiovascular medicine. Accessory left atrial appendage: a neglected anomaly and potential cause of embolic stroke. Circulation. 2008;117:1351–1352. doi: 10.1161/CIRCULATIONAHA.107.744706. [DOI] [PubMed] [Google Scholar]
- 19.Lee W.J., Chen S.J., Wang T.D. Multiple accessory left atrial appendages along a semi-circular path. Eur Heart J. 2008;29:2447. doi: 10.1093/eurheartj/ehn144. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi H. Left atrial diverticula present in the right lower pulmonary vein thrombus attachment area. Cureus. 2024;16 doi: 10.7759/cureus.53422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji T., Hasegawa K., Tada H. Left atrial diverticulum as a rare but possible origin of a sustained atrial tachycardia: a case report. Eur Heart J. 2024;45:2679. doi: 10.1093/eurheartj/ehae317. [DOI] [PubMed] [Google Scholar]
- 22.Marshall J. VI. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of fœtal structure found in the adult, a comparative view of these great veins the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philosophical Trans R Soc Lond. 1850;140:133–170. [Google Scholar]
- 23.Silver M.A., Rowley N.E. The functional anatomy of the human coronary sinus. Am Heart J. 1988;115:1080–1084. doi: 10.1016/0002-8703(88)90080-4. [DOI] [PubMed] [Google Scholar]
- 24.Fukutomi M., Fuchs A., Bieliauskas G., et al. Computed tomography-based selection of transseptal puncture site for percutaneous left atrial appendage closure. EuroIntervention. 2022;17:e1435–e1444. doi: 10.4244/EIJ-D-21-00555. [DOI] [PMC free article] [PubMed] [Google Scholar]