Abstract
Background
Ablation of cavotricuspid isthmus (CTI)–dependent atrial flutter with interventional cardiovascular magnetic resonance (CMR) has demonstrated its efficacy and safety in the initial phase for achieving bidirectional block. However, limited long-term follow-up data exist for these patients.
Case Summary
Thirteen consecutive patients were included between 2021 and 2022. CMR-guided catheter ablation of CTI-dependent atrial flutter was completed successfully in 11 patients (85%). CTI ablation had to be completed in a conventional electrophysiology laboratory for 2 patients: one patient required general anesthesia due to pain during CTI ablation, and the other deteriorated into atrial fibrillation during ablation shots. Among 11 patients with procedural success, 2 (18%) were lost to follow-up after the ablation and 9 (82%) were free of recurrent isthmus-dependent atrial flutter at a median follow-up of 27 months (IQR: 24-29).
Conclusions
Interventional CMR-guided ablation of CTI-dependent atrial flutter demonstrates a good safety profile and efficacy at 2-year follow-up.
Key words: CMR-guided ablation, interventional cardiovascular magnetic resonance, outcomes, procedural success, radiofrequency atrial flutter ablation
Graphical Abstract
Visual Summary.
CMR-Guided Flutter Ablation
Background and Aims
Catheter ablation in an electrophysiology laboratory with the guidance of fluoroscopy is the first-line therapy for patients presenting with cavotricuspid isthmus (CTI)–dependent atrial flutter. Recently, several studies reported the feasibility of CTI-dependent atrial flutter ablation under interventional cardiovascular magnetic resonance (iCMR) guidance.1, 2, 3 Indeed, the development of specific catheters has allowed active catheter tracking in cardiac chambers in real time under iCMR. Compared with fluoroscopy, the advantage of iCMR is the real-time visualization of the tip of the catheter along with the anatomy of the inferior vena cava and cardiac chambers, thereby overcoming some anatomic variations such as a ridge at the junction between the inferior vena cava and the right atrium. Bijovet et al3 recently showed in a small sample of 15 patients that T1 mapping by cardiovascular magnetic resonance (CMR) could identify regions of acute reconnection directly after ablation. Some studies have demonstrated the efficacy and safety of the procedure in the initial phase for achieving bidirectional block;1, 2, 3 however, limited long-term follow-up data exist for these patients.
The aim of this study was to determine the safety and accuracy of this technique at 2-year follow-up.
Methods
Population
Between July 2021 and February 2022, consecutive patients with suspected CTI-dependent atrial flutter on electrocardiogram (ECG) were prospectively screened at our institution to undergo their first flutter ablation procedure using iCMR. Inclusion criteria were as follows: >18 years of age, suspected CTI-dependent atrial flutter on ECG, and signed informed consent. Exclusion criteria were a history of CTI-dependent flutter ablation, and contraindications to CMR (cerebral clip, metallic eye implant, and claustrophobia). All patients gave informed written consent for iCMR-guided flutter ablation and enrollment in the clinical research study. The study was approved by the local ethics committee of our institution and conducted in accordance with the Declaration of Helsinki. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies.
Ablation Protocol
The principles of the electrophysiology protocol under iCMR have been previously described in details.1, 2, 3 Briefly, the iCMR setup was similar to that previously described in previous studies.1, 2, 3, 4 The procedure was performed in a large bore 1.5-T MAGNETOM Aera Scanner (Siemens Healthineers) with 2 magnetic resonance-conditional, open-irrigated, steerable ablation catheters (VISION-MR; Imricor Medical Systems). The catheters were equipped with fiber optical temperature sensors and integrated MR tracking coils. They were connected to an electrophysiology workstation (Advantage-MR; Imricor Medical Systems) that provided monitoring and pacing capabilities, and to a radiofrequency (RF) generator operated from outside the CMR suite (Figure 1).
Figure 1.
Block Diagram of the Setup During Cardiovascular Magnetic Resonance–Guided Ablation of Isthmus-Dependent Atrial Flutter
DAS = digital amplifier stimulator; ECG = electrocardiogram: EP = electrophysiology; MR = magnetic resonance; MRI = magnetic resonance imaging; PDI = patient device interface; RF = radiofrequency.
Patients were prepped on a dedicated and dockable CMR table in a preparation room just outside of the CMR suite. Patients were placed under conscious sedation. Midazolam (2 mg) was injected intravenously at the time of the venous puncture. Then, sufentanil (5 μg) was administered for analgesia. Two 9-F introducers were placed in the femoral vein under echography guidance, and patients were transferred to the CMR suite for flutter ablation.
Oxygen saturation and blood pressure were continuously monitored during the procedure in all patients. Catheters were tracked using receiver coils placed at the tip of the catheter under compressed sensing real-time cine CMR imaging (Video 1). The diagnostic catheter was introduced into the coronary sinus to serve as a reference catheter, and an irrigated ablation catheter was introduced in the right atrium to perform ablation shots at the CTI (the power of each shot was 50 W during 60 seconds). RF ablation was performed using a point-by-point method across the CTI until the block was confirmed.
Procedural Success
Procedural success was defined as the achievement of a bidirectional conduction block across the CTI. Then, patients were monitored in a dedicated room during at least 4 hours after the procedure. Patients were discharged on the same day after a follow-up ECG and in the absence of focal venous complications.
Follow-Up
Patients were followed-up at 1 and 2 years by a clinical visit and an ECG. In case of palpitations, a 24-hour Holter ECG was performed.
Statistical Analysis
The data are presented as mean ± SD and median (IQR) depending on the variable distribution. Descriptive statistical analyses were performed using PRISM 6 (GraphPad Software Inc).
Results
Patients’ characteristics are given in Table 1. Overall, 13 patients were included. The median age was 76 years (IQR: 73-78, range: 49-90). Among them, 1 patient had a history of myocarditis, 4 had valvular heart disease, 2 had ischemic heart disease, and 6 had no known heart disease (Table 1). All patients had a left ventricular ejection fraction >50%. All patients were under oral anticoagulant therapy for >1 month, and 10 patients received at least 1 antiarrhythmic drug. Eleven patients had atrial flutter during the procedure.
Table 1.
Patient Characteristics (N = 13)
| Age, y | 76 (73-78) |
| Male | 8 (62) |
| Heart disease | |
| None | 6 (46) |
| Ischemic | 2 (15) |
| Valvular heart disease | 4 (31) |
| Myocarditis | 1 (8) |
| LVEF, % | 60 (60-60) |
| Antiarrhythmic drugs | |
| None | 3 (23) |
| Beta-blockers | 5 (38) |
| Amiodarone | 5 (38) |
| Flecainide | 3 (23) |
Values are median (IQR) or n (%).
LVEF = left ventricular ejection fraction.
Procedural Data
Ablation success was achieved in 11 patients (85%), as confirmed by the presence of a bidirectional conduction block across the CTI. The CTI ablation had to be completed in a conventional electrophysiology laboratory in 2 patients: 1 had severe chest pain during RF shots and needed general anesthesia, and the other deteriorated into atrial fibrillation during the procedure.
No procedural or periprocedural complications (pericardial effusion or tamponade, vascular complications, or thromboembolic events) occurred.
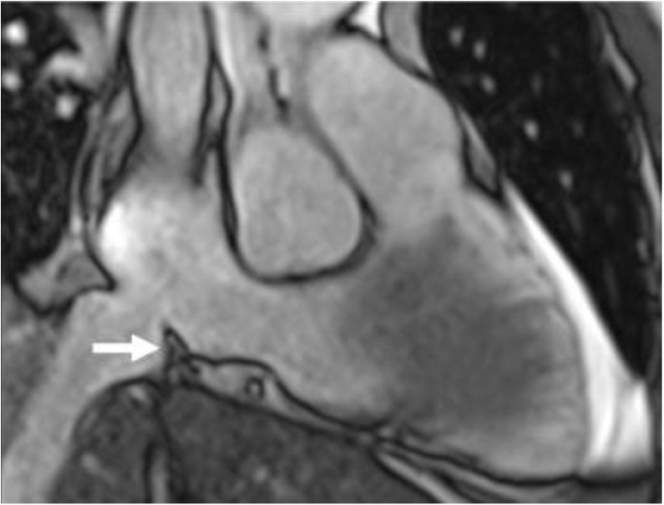
In all patients, T2-weighted short-tau inversion recovery sequences centered on the CTI were acquired before and after ablation. All patients showed a clear hypersignal of the isthmus after ablation compared with baseline images, indicating the presence of focal myocardial edema (Figure 2). A prominent ridge was identified at the junction of the inferior vena cava and the right atrium in 1 patient (Figure 3).
Figure 2.
Short-Tau Inversion Recovery Images of the Right Heart Chambers
(A) Before radiofrequency ablation and (B) after radiofrequency ablation, showing an acute lesion of the cavotricuspid isthmus (hypersignal, arrow).
Figure 3.
Identification of a Prominent Cavotricuspid Isthmus Ridge During Planning Cardiovascular Magnetic Resonance
The arrow indicates the cavotricuspid isthmus.
Follow-Up
Among 11 patients with procedural success, 9 (82%) had no recurrence of CTI-dependent atrial flutter at median follow-up of 27 months (IQR: 24-29) (Table 2). Two patients (18%) were lost to follow-up after ablation because they moved and were not reached despite several attempts.
Table 2.
Cavotricuspid Isthmus Ablation Parameters and Follow-Up
| Interventional procedure duration, min | 60 (60-120) |
| Total radiofrequency pulse duration, s | 478 (206-577) |
| Obtention of bidirectional isthmus block, % | 11 (85) |
| Double potentials, ms | 90 (77-120) |
| Procedural complications | |
| Pericardial effusion/tamponade | 0 (0) |
| Vascular complications | 0 (0) |
| Thromboembolic events | 0 (0) |
| Outpatients | 13 (100) |
| Lost to follow-up | 2 (18) |
| Follow-up, mo | 27 (24-29) |
| Recurrence of isthmus-dependent atrial flutter | 0 (0) |
Values are median (IQR) or n (%).
Discussion
This study demonstrates the accuracy and safety of iCMR-guided ablation of CTI-dependent atrial flutter at 2-year follow-up. Primary procedural success rate of iCMR-guided ablation was 85%, which is concordant with other studies.1 It is worth noting that 2 procedural failures were due either to sedation issues in the magnetic resonance suite, which can be overcome with proper anticipation, or to the need for electrical cardioversion. In the current study, no patient experienced recurrence of common atrial flutter during a 2-year follow-up. Nevertheless, the procedural success rate is lower than that with catheter ablation in an electrophysiology laboratory with fluoroscopy.5 The procedure was stopped in 1 patient because the flutter deteriorated into atrial fibrillation and required electrical cardioversion. A CMR-compatible defibrillation device could overcome this issue and would facilitate the procedures. In the magnetic resonance environment, a patient's pain is more difficult to manage than in a conventional electrophysiology laboratory, because of suite accessibility, the need of CMR compatible syringe pump, and appropriate ventilation support devices. Overall, no complication occurred, and all procedures were performed on an outpatient basis. Besides the absence of ionizing radiation, several advantages may be highlighted such as the visualization of the CTI before ablation. Indeed, the presence of a CTI ridge, the visualization of the Eustachian valve, and its interaction with the CTI may help accelerate catheter access to the isthmus.
This technique also allows the visualization of post-RF endocardial damage through CMR tissue characterization. Bijvoet et al3 recently demonstrated that tissue characterization of acute lesions during CMR-guided CTI-dependent atrial flutter ablation demonstrates edema, perfusion defects, and necrosis with a core of microvascular damage. It can be assumed that these lesions progress to fibrosis and focal scar; however, in this study, CMR follow-up was not performed at 2 years.
The main limitations of the study are as follows. First, although endocardium, myocardium, cardiac chambers, and structures are remarkably depicted in real time, spatial resolution is lower and real-time tissue characterization is not available. Second, active catheter tracking is accurate but at a lower temporal resolution than in fluoroscopy. Third, significant interferences occur between the CMR field and the electrograms, requiring stepwise point-by-point ablation. Fourth, significant interferences may occur between RF and electrograms. Finally, the CMR environment requires technical adaptations (wires between the CMR suite and the pump, RF generator, EP recording system). Well-established safety and emergency procedures are needed and derived from stress CMR studies.
iCMR flutter ablation may pave the way to future ventricular tachycardia (VT) ablation procedures in the magnetic resonance environment. iCMR-guided VT ablation would enable RF ablation on scarred tissue without the need to induce VT and with direct visualization of myocardial substrates. The feasibility of CMR guidance and real-time assessment of ablation injury has been demonstrated in the porcine left ventricle.6 The VISABL-VT (Vision-MR Ablation Catheter 2.0 for the Treatment of Ventricular Tachycardia) trial is a prospective, single-arm, multicenter, interventional study investigating the safety and efficacy of RF ablation of VT associated with ischemic cardiomyopathy using the Vision-MR Ablation Catheter 2.0 in the iCMR environment (NCT05543798).
Conclusions
iCMR-guided ablation of CTI-dependent atrial flutter demonstrates a good safety profile and efficacy at 2-year follow-up.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Take-Home Messages
-
•
iCMR-guided flutter ablation is feasible, safe and accurate under tailored conscious sedation and in-room monitoring.
-
•
The study shows the accuracy of iCMR-guided flutter ablation over 2-year follow-up.
Acknowledgments
The authors thank the paramedical and administrative staff of the Institut Cardiovasculaire Paris Sud involved in the study. The authors also thank Imricor for providing and installing the specific equipment needed for iCMR flutter ablation and for their constant technical support during the study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Interventional Cardiovascular Magnetic Resonance-Guided Flutter Ablation
References
- 1.Paetsch I., Sommer P., Jahnke C., et al. Clinical workflow and applicability of electrophysiological cardiovascular magnetic resonance-guided radiofrequency ablation of isthmus-dependent atrial flutter. Eur Heart J Cardiovasc Imaging. 2019;20(2):147–156. doi: 10.1093/ehjci/jey143. [DOI] [PubMed] [Google Scholar]
- 2.Ulbrich S., Huo Y., Tomala J., et al. Magnetic resonance imaging-guided conventional catheter ablation of isthmus-dependent atrial flutter using active catheter imaging. Heart Rhythm O2. 2022;3(5):553–559. doi: 10.1016/j.hroo.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijvoet G.P., Nies H.M.J.M., Holtackers R.J., et al. Tissue characterization of acute lesions during cardiac magnetic resonance-guided ablation of cavo-tricuspid isthmus-dependent atrial flutter: a feasibility study. Eur Heart J Cardiovasc Imaging. 2023;29 doi: 10.1093/ehjci/jead334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijvoet G.P., Holtackers R.J., Smink J., et al. Transforming a pre-existing MRI environment into an interventional cardiac MRI suite. J Cardiovasc Electrophysiol. 2021;32(8):2090–2096. doi: 10.1111/jce.15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez F.J., Schubert C.M., Parvez B., Pathak V., Ellenbogen K.A., Wood M.A. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol. 2009;2(4):393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee R.K., Roujol S., Chubb H., et al. Epicardial electroanatomical mapping, radiofrequency ablation, and lesion imaging in the porcine left ventricle under real-time magnetic resonance imaging guidance-an in vivo feasibility study. Europace. 2018;20(FI2):f254–f262. doi: 10.1093/europace/eux341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interventional Cardiovascular Magnetic Resonance-Guided Flutter Ablation