Abstract
Background
Acute myocardial infarction (AMI) and cancer are leading causes of death worldwide. However, the relationship between AMI and hematologic malignancies remains unclear.
Objectives
The authors aimed to investigate the association between AMI and the subsequent risk of incident hematologic malignancies.
Methods
This retrospective cohort study included 103,686 patients with AMI and no history of hematologic malignancies, and 103,686 age- and sex-matched individuals with no history of AMI or hematologic malignancies, diagnosed between January 1, 2003, and December 31, 2021. Data were obtained from the Korean National Health Insurance claims database. We compared the cumulative incidence of hematologic malignancies between groups using Gray’s method. HRs and 95% CIs were calculated using Gray’s competing risk regression model, with death treated as a competing risk.
Results
During follow-up (AMI, 7.9 years [Q1-Q3: 5.2-11.4 years]; control group, 17.8 years [Q1-Q3: 14.8–17.9 years]), 1,043 and 1,479 individuals in the AMI and control groups, respectively, were newly diagnosed with hematologic malignancies (incidence rate per 1,000 person-years: 1.21 vs 0.93). Competing risk analysis revealed that the AMI group had a higher risk of hematologic malignancy than the control group (HR: 1.49; 95% CI: 1.31-1.69). Findings were consistent in sensitivity and standardized incidence ratio analyses.
Conclusions
Patients with AMI had a higher risk of hematologic malignancies than those without AMI. These findings suggest an association between AMI and hematologic malignancies, and underscore the importance of considering hematologic malignancy development in patients with AMI.
Key Words: acute coronary syndrome, bi-directional cardio-oncology, epidemiology, hematologic malignancy, myocardial infarction, outcomes, reverse cardio-oncology
Central Illustration
Acute myocardial infarction (AMI) remains a leading cause of death worldwide, and AMI-related mortality is projected to continue rising in the coming years.1 However, the advent of pharmacotherapy and timely coronary revascularization has substantially improved AMI prognosis over the past several decades.2 According to an Australian study, more than 80% of individuals aged ≥65 years who undergo percutaneous coronary intervention for AMI are expected to survive beyond 7 years.3
Although AMI-related mortality is highest in the early phase after the event, noncardiac causes—particularly cancer—account for a growing proportion of deaths during long-term follow-up.4 Cancer is also a leading cause of death, with rising prevalence attributed to increasing life expectancy.5 AMI and cancer share several common risk factors, including smoking, obesity, and comorbidities.6 As AMI survival improves, interest in the subsequent cancer burden in this population has grown. A large population-based study revealed that patients with AMI had higher short- and long-term cancer incidence than those without a history of AMI.7 However, findings remain inconsistent regarding the timing of cancer risk. Some studies suggest that increased cancer incidence may occur only in the early phase after AMI, potentially reflecting a paraneoplastic thrombotic event.8,9
Hematologic malignancies represent a substantial component of the global tumor burden, and their absolute numbers have increased over the past 3 decades.10 In the Republic of Korea, the incidence of hematologic malignancies has also risen significantly over the past 20 years.11 Despite these trends, limited data are available on the association between AMI and hematologic malignancy risk. Most prior studies have treated hematologic malignancies as a subset of overall cancer incidence rather than a primary outcome.8,12 Therefore, a focused investigation of this association is warranted. In this nationwide, population-based cohort study, we aimed to examine the risk of hematologic malignancies in patients with AMI.
Methods
Data source
We conducted a retrospective cohort study using data from the Korean National Health Insurance Service (NHIS) and National Health Screening databases.13 The Korean NHIS provides mandatory health insurance coverage for the entire Korean population (approximately 51 million people) and includes data on demographics, diagnoses, procedures, prescriptions, and examinations. Diagnoses were coded using the International Classification of Diseases, Tenth Revision (ICD-10). Additional information was obtained from the National Health Screening database, which is linked to the NHIS.
The Korean NHIS offers a free biennial health examination to all beneficiaries aged >40 years and to all employees regardless of age. Workers in physically demanding occupations receive annual screenings. These examinations include anthropometric and blood pressure measurements, laboratory testing, and a self-reported questionnaire on health-related behaviors such as smoking, alcohol consumption, and physical activity.14
This study adhered to the principles of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital (IRB No. SCHUH 2022-06-018-01-001). Informed consent was waived because all patient records were anonymized and deidentified prior to analysis.
Study design and study population
The participant selection flowchart is shown in Figure 1. We included patients newly diagnosed with AMI between January 1, 2003, and December 31, 2016 (N = 303,788). Only those who underwent a health examination within 2 years before the AMI diagnosis were eligible. Patients younger than 18 years and with missing health examination data were excluded. A birth year- and sex-matched control group was randomly selected from the NHIS database at a 1:1 ratio, using the index date of the matched AMI patient (greedy matching algorithm with a caliper of 0.15).15 Control individuals had no history of AMI between 2002 and 2021, and had completed a health examination within 2 years of the index date.
Figure 1.
Participants Selection and Follow-Up Flow Chart
Patients newly diagnosed with acute myocardial infarction (AMI) between 2003 and 2016 who had a health examination within 2 years before diagnosis were included. Age- and sex-matched controls without a history of AMI or hematologic malignancies were also selected. Incident hematologic malignancies were tracked through December 2021.
Definition of AMI
The ICD-10 codes for AMI are I21 and I22. However, using these codes alone yields a positive predictive value of 92% for a definite AMI diagnosis.16 To reduce potential misclassification and minimize false-positive inclusion, we applied a stricter definition. Patients were classified as having AMI only if they met all of the following criteria: 1) a primary diagnosis of AMI (diagnostic code: I21, I22) with hospitalization for more than 1 day; 2) coronary angiography performed during the index hospitalization (procedure code: HA670); and 3) cardiac enzyme levels measured at least twice during the index hospitalization (laboratory test codes for troponin I and troponin T: CY277, CY278, C3941, C3942, D4021, D4022, or D4023).
Measurements and definition of covariates
Baseline characteristics, including age, sex, and comorbidities, were obtained for all participants. Demographic data such as smoking status, alcohol consumption status, physical activity, and body mass index (BMI) were collected from health examination records. Health insurance premium payment data were used as a proxy for low income. Additional details are provided in the Supplemental Appendix (Supplemental Tables 1 and 2).
Study outcomes and follow-up
The primary outcome was the incidence of hematologic malignancies during follow-up. Definitions of outcomes are presented in Supplemental Table 3.11 Participants were followed from the index year until the earliest occurrence of hematologic malignancy diagnosis, death, or censoring at the end of the study period (December 31, 2021), whichever occurred first.
Statistical analysis
Data are presented as mean ± SD for continuous variables and as numbers and proportions for categorical variables. Non-normally distributed variables are presented as medians with 25th-75th percentiles (Q1-Q3). Baseline characteristics between the AMI and control groups were compared using the standardized mean difference. Incidence rates of hematologic malignancies were calculated as the number of newly diagnosed events divided by total person-years during follow-up. Cumulative incidence curves accounting for the competing risk of death were generated using Gray’s method. HRs and 95% CIs were estimated using Gray’s competing risk regression model, with death treated as a competing risk. The initial analysis compared the overall incidence of hematologic malignancies between the AMI and control groups.
Second, 9 subtypes of hematologic malignancies (non-Hodgkin lymphoma, myeloid leukemia, multiple myeloma, myeloproliferative neoplasms [MPNs], myelodysplastic syndrome, malignant immunoproliferative diseases, lymphoid leukemia, Hodgkin lymphoma, and leukemia-unspecified)11 were analyzed separately. Multivariable models were adjusted for potential confounders, including age, sex, smoking status, heavy alcohol consumption status, regular exercise, low-income status, residential area, diabetes mellitus, hypertension, chronic kidney disease, ischemic stroke or transient ischemic attack, chronic obstructive pulmonary disease (COPD), chronic liver disease, and obesity (BMI ≥25 kg/m2).
Subgroup analyses were conducted based on age (<65 vs ≥65 years), sex, smoking status, heavy alcohol consumption status, obesity, diabetes, hypertension, dyslipidemia, and chronic kidney disease. Interaction terms in Cox proportional hazards models were used to assess potential effect modification.
To minimize the inclusion of pre-existing hematologic malignancies incidentally detected during the acute evaluation of AMI and to evaluate the long-term effect of AMI , landmark analyses were performed at 90 days, 1 year, and 5 years after AMI diagnosis. Only patients who remained free of hematologic malignancy and survived beyond each landmark point were included, and outcomes occurring before the landmark point were excluded. In the landmark analyses, multiple malignancy events were treated as a composite endpoint, with the date of the first occurring event used as the endpoint. All P values were 2-sided, and statistical significance was defined as P < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Standard incidence ratios analysis
Standardized incidence ratios (SIRs) were calculated to compare the incidence of hematologic malignancies in patients with AMI with that of the general Korean population. Age-, sex-, cancer type– , and calendar year–specific incidence rates were obtained from Statistics Korea.17 The SIR was calculated by dividing the observed number of hematologic malignancies in the AMI group by the expected number, based on national age- and sex-specific incidence rates. The 95% CI was calculated using the Poisson distribution.
Results
Baseline characteristics of the study population
A total of 103,686 patients in the AMI group and 103,686 individuals in the control group were included. The mean age of participants was 59.6 ± 10.3 years, and approximately 78.0% in both groups were men. Baseline demographics and comorbidities are presented in Table 1. The median follow-up duration was 7.9 years (Q1-Q3: 5.2-11.4 years) in the AMI group and 17.8 years (Q1-Q3:14.8-17.9 years) in the control group. Among patients diagnosed with hematologic malignancies, non-Hodgkin lymphoma was the most common subtype.
Table 1.
Baseline Characteristics of the Study Population
| Control Group (n = 103,686) | AMI Group (n = 103,686) | SMD | |
|---|---|---|---|
| Age, y | 59.6 ± 10.3 | 59.6 ± 10.3 | 0 |
| Male | 80,966 (78.1) | 80,966 (78.1) | 0 |
| Urban residence | 88,178 (85.0) | 91,978 (88.7) | −0.109 |
| Low income | 21,597 (20.8) | 19,230 (18.5) | 0.039 |
| Comorbidities | |||
| Diabetes | 10,637 (10.3) | 51,059 (49.2) | 0.943 |
| Hypertension | 21,967 (21.2) | 81,168 (78.3) | 1.391 |
| Dyslipidemia | 7,584 (7.3) | 82,781 (79.8) | 2.144 |
| Chronic kidney disease | 22 (0.2) | 3,450 (3.3) | 0.238 |
| Ischemic stroke/TIA | 1,581 (1.5) | 7,951 (7.7) | 0.297 |
| COPD | 6887 (6.6) | 14959 (14.4) | 0.256 |
| Chronic liver disease | 7136 (6.9) | 14765 (14.2) | 0.241 |
| Obesity, BMI >25 kg/m2 | 35,428 (34.2) | 48,231 (46.5) | 0.254 |
| Smoking status | |||
| Current smoker | 18,379 (17.7) | 13,453 (13.0) | 0.260 |
| Former smoker | 33,754 (32.6) | 43,319 (41.8) | |
| Never smoker | 56,479 (54.5) | 41,988 (40.5) | |
| Heavy alcohol drinker | 9,173 (8.8) | 16,771 (16.2) | 0.223 |
| Regular exercise | 21,187 (20.4) | 8,922 (8.6) | −0.341 |
Values are mean ± SD or n (%).
AMI = acute myocardial infarction; BMI = body mass index; COPD = chronic obstructive pulmonary disease; SMD = standardized mean difference; TIA = transient ischemic attack.
Risk of hematologic malignancies in patients with AMI
Table 2 presents the number of events, incidence rates, and both crude and adjusted HRs for hematologic malignancies in the AMI and control groups. During follow-up, 1,043 patients from the AMI group and 1,479 individuals in the control group were newly diagnosed with hematologic malignancies. The incidence rate was higher in the AMI group than in the control group (1.21% vs 0.93% per 1,000 person-years). In Gray’s competing risk regression analysis (Models 1 and 2 in Table 2), the risk of hematologic malignancies in the AMI group was 50.2% to 62.0% higher than in the control group (HR: 1.62; 95% CI: 1.48-1.76; and HR: 1.50; 95% CI: 1.32-1.71, respectively). After adjusting for all potential confounders (Model 3 in Table 2), the AMI group remained at higher risk, with a 49.0% increase in hematologic malignancies compared with the control group (HR: 1.49; 95% CI: 1.31-1.69) (Figure 2, Central Illustration).
Table 2.
Risk of Hematologic Malignancies in the AMI and Control Groups
| Groups | N | Events | Person-Years | Incidence Rate (1,000 Person-Years) | HR (95% CI) |
||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| Control | 103,686 | 1,479 | 1,592,069 | 0.93 (0.88-0.98) | Ref | Ref | Ref |
| AMI | 103,686 | 1,043 | 862,142 | 1.21 (1.14-1.28) | 1.62 (1.48–1.76) | 1.50 (1.32–1.71) | 1.49 (1.31–1.69) |
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking status, alcohol consumption status, regular exercise, low-income status, diabetes mellitus, hypertension, and dyslipidemia. Model 3 was adjusted for age, sex, smoking status, alcohol consumption status, regular exercise, low-income status, residential area, diabetes mellitus, hypertension dyslipidemia, chronic kidney disease, ischemic stroke/TIA, COPD, chronic liver disease, and obesity (BMI ≥25 kg/m2).
Abbreviations as in Table 1.
Figure 2.
Cumulative Incidence of Hematologic Malignancies by AMI Status
The acute myocardial infarction (AMI) group had a 49.0% higher risk of hematologic malignancies compared with those without a history of AMI (HR: 1.49; 95% CI: 1.31-1.69; P < 0.0001). A multivariable Gray’s subdistribution hazard model was used to account for competing risks, adjusting for age, sex, smoking status, drinking status, regular exercise, low-income status, residential area, diabetes mellitus, hypertension, ischemic stroke or transient ischemic attack, chronic obstructive pulmonary disease, chronic liver disease, obesity (body mass index ≥25 kg/m2), and chronic kidney disease.
Central Illustration.
Risk of Hematologic Malignancies in Patients With Acute Myocardial Infarction
A total of 103,686 patients diagnosed with acute myocardial infarction (AMI) from the Korean National Health Insurance Service database between 2003 and 2016 were compared to 103,686 age- and sex-matched non-AMI control subjects. The incidence rate of hematologic malignancies was higher in the AMI group than in the control group (1.21% vs 0.93% per 1,000 person-years). After adjusting for all potential confounding variables, the risk of hematologic malignancies in the AMI group was 49.0% higher than that in the control group (HR: 1.49; 95% CI: 1.31-1.69).
To determine whether the association was driven by relatively indolent conditions such as MPNs, we conducted a sensitivity analysis excluding all individuals with MPN diagnosis codes. Results were consistent with the primary analysis, showing a higher risk of hematologic malignancies in the AMI group (HR: 1.38; 95% CI: 1.21-1.58) (Model 3 in Supplemental Table 4).
Landmark analysis
Results of the landmark analyses are presented in Table 3. To minimize surveillance bias, we performed a landmark analysis starting 90 days after AMI diagnosis. The AMI group had a higher risk of hematologic malignancies than the control group (Model 3: HR: 1.40; 95% CI: 1.23-1.59). Although the magnitude of risk attenuated over time, the association remained statistically significant. At 1 year post-AMI, patients had a 36% higher risk of hematologic malignancies (Model 3: HR: 1.36; 95% CI: 1.19-1.56), and at 5 years, a 26% higher risk (Model 3: HR: 1.26; 95% CI: 1.08-1.48).
Table 3.
Risk of Hematologic Malignancies at 90 Days, 1 Year, and 5 Years
| Control Group | AMI Group | |
|---|---|---|
| 90 d | ||
| Number | 103,504 | 99,265 |
| Events | 1,454 | 975 |
| Duration, person-years | 1,592,050 | 861,950 |
| Time to event, y | 10.47 (6.22-14.11) | 5.37 (2.45-8.44) |
| Incidence rate | 0.9 [0.87-0.96] | 1.1 [1.06-1.20] |
| Model 1 | Reference | 1.58 (1.45-1.72) |
| Model 2 | Reference | 1.41 (1.24-1.61) |
| Model 3 | Reference | 1.40 (1.23-1.59) |
| 1 y | ||
| Number | 103,058 | 97,493 |
| Events | 1,413 | 894 |
| Duration, person-years | 1,591,780 | 860,905 |
| Time to event, y | 10.74 (6.59-14.17) | 5.84 (3.17-8.71) |
| Incidence rate | 0.9 (0.84-0.93) | 1.0 (0.97-1.11) |
| Model 1 | Reference | 1.55 (1.42-1.70) |
| Model 2 | Reference | 1.38 (1.20-1.57) |
| Model 3 | Reference | 1.36 (1.19-1.56) |
| 5 y | ||
| Number | 98,996 | 80,646 |
| Events | 1,182 | 517 |
| Duration, person-years | 1,578,150 | 795,440 |
| Time to event, y | 12.05 (8.51-14.69) | 12.05 (8.51-14.69) |
| Incidence rate | 0.65 [0.59-0.71] | 0.75 [0.71-0.79] |
| Model 1 | Reference | 1.45 (1.30-1.62) |
| Model 2 | Reference | 1.28 (1.09-1.49) |
| Model 3 | Reference | 1.26 (1.08-1.48) |
Values are median [Q1-Q3], n, or adjusted HR (95% CI).
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking status, alcohol consumption status, regular exercise, low-income status, diabetes mellitus, hypertension, and dyslipidemia. Model 3 was adjusted for age, sex, smoking status, alcohol consumption status, regular exercise, low-income status, residential area, diabetes mellitus, hypertension dyslipidemia, chronic kidney disease, ischemic stroke/TIA, COPD, chronic liver disease, and obesity (BMI ≥25 kg/m2).
Abbreviations as in Table 1.
SIRs of hematologic malignancies in patients with AMI
Compared with the general Korean population (Korean Statistical Information Service [KOSIS] data), patients with AMI had a higher overall risk of hematologic malignancies (Table 4). The relative risk was highest for MPNs (SIR: 5.57; 95% CI: 4.74-6.40) and lowest for malignant immunoproliferative diseases (SIR: 1.71; 95% CI: 1.20-2.22) (Table 4).
Table 4.
Age- and Sex-Standardized Incidence Rates of Specific Hematologic Malignancies in Patients With AMI
| Type of Hematologic Malignancy | Observed | Expected | Person-Years | SIR (95% CI) |
|---|---|---|---|---|
| Non-Hodgkin lymphoma | 328 | 123.1 | 864,486 | 2.66 (2.38-2.95) |
| Myeloid leukemia | 147 | 56.5 | 865,166 | 2.60 (2.18-3.02) |
| Multiple myeloma | 206 | 49.5 | 865,058 | 4.16 (3.60-4.73) |
| Myeloproliferative neoplasms | 172 | 30.9 | 864,646 | 5.57 (4.74-6.40) |
| Myelodysplastic syndrome | 133 | 28.9 | 865,201 | 4.60 (3.82-5.38) |
| Malignant immunoproliferative diseases | 43 | 25.1 | 865,342 | 1.71 (1.20-2.22) |
| Lymphoid leukemia | 29 | 12.8 | 865,421 | 2.27 (1.45-3.10) |
| Hodgkin lymphoma | 27 | 5.5 | 865,410 | 4.95 (3.09-6.82) |
| Leukemia, unspecified | 47 | 9.7 | 865,353 | 4.86 (3.47-6.25) |
AMI = acute myocardial infarction; SIR = standardized incidence ratio.
Incidence rate of specific hematologic malignancies in patients with AMI
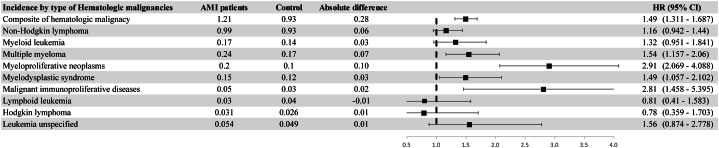
We examined the association between AMI and 9 specific hematologic malignancies. Increased risk was observed for multiple myeloma (MM), MPNs, myelodysplastic syndrome, and malignant immunoproliferative diseases. No significant association was observed between AMI and the other hematologic malignancies (Figure 3). Detailed subtype distributions and P values are presented in Supplemental Table 5.
Figure 3.
Association Between AMI and Incidence of Specific Hematologic Malignancies
A Gray’s competing risk regression model adjusted for age, sex, smoking status, and alcohol consumption status, regular exercise, low-income status, residential area, diabetes mellitus, hypertension, ischemic stroke or transient ischemic attack, chronic obstructive pulmonary disease, chronic liver disease, obesity (body mass index ≥25 kg/m2), and chronic kidney disease was used. AMI = acute myocardial infarction.
Subgroup analyses
Across all subgroups, patients with AMI consistently demonstrated an elevated risk of hematologic malignancies (Figure 4).
Figure 4.
Subgroup Analysis of Hematologic Malignancy Risk in Patients With AMI
A Gray’s competing risk regression model adjusted for age (years), sex, smoking status, alcohol consumption status, regular exercise, low-income status, residential area, diabetes mellitus (DM), hypertension, ischemic stroke or transient ischemic attack, chronic obstructive pulmonary disease, chronic liver disease, obesity (body mass index [BMI] ≥25 kg/m2), and chronic kidney disease was used. AMI = acute myocardial infarction.
Discussion
In this study, patients with newly diagnosed AMI had a 49.0% higher risk of hematologic malignancies compared with those without a history of AMI, even after adjustment for multiple confounding variables. These findings were consistent with the SIR analysis using data from the KOSIS.
Previous studies have reported an increased detection of malignancies immediately after AMI diagnosis, suggesting that AMI may occasionally present a paraneoplastic manifestation of an undiagnosed tumor.8 In such cases, prothrombotic conditions associated with cancer may precipitate AMI. Other studies have proposed surveillance bias, wherein malignancies are incidentally detected during hospitalization for AMI.9 In our cohort, the incidence of newly diagnosed hematologic malignancies was elevated within 90 days of AMI. To minimize the inclusion of pre-existing cases, we conducted a 90-day landmark analysis excluding events within this window. Notably, the risk of hematologic malignancies remained significantly higher in the AMI group at both short-term (90 days) and longer-term (1-year and 5-year) follow-up.
The mechanisms underlying this association are not well understood. It is possible that AMI and hematologic malignancies share biological pathways or predispositions that contribute to their co-occurrence. We outline 3 potential contributing factors below.
First, AMI and hematologic malignancies may share traditional risk factors, such as age and smoking, that may explain part of the association.18 In our study, patients in the AMI group had significantly different baseline characteristics than controls. Although we adjusted for multiple clinical covariates, residual confounding may persist. Differences in underlying health conditions, such as diabetes or dyslipidemia, could also influence malignancy risk. Prior studies have reported associations between diabetes,19 dyslipidemia,20 and physical activity21 and the risk of hematologic malignancies. In our cohort, the prevalence of diabetes, hypertension, hyperlipidemia, and obesity was higher in the AMI group than in the control group. These factors may have contributed, at least in part, to the increased risk observed in this population.
Second, chronic inflammation and oxidative stress may contribute to the development of both AMI and hematologic malignancies. Atherosclerosis—the primary cause of AMI—is a well-established chronic inflammatory disease.22 Inflammation is also implicated in tumor progression23 and plays a key role in regulating hematopoietic stem cell function.24 Chronic inflammation has been linked to the pathogenesis of both myeloid25 and lymphoid malignancy.26 In addition, AMI can alter the innate immune system, driving myeloid cells in hematopoietic reservoirs toward an immunosuppressive state that facilitates harmful cross-disease interactions and promotes cancer progression.27 These findings support the possibility of shared inflammatory and immune-related mechanisms underlying the association between AMI and hematologic malignancies.
Third, clonal hematopoiesis of indeterminate potential (CHIP) may provide a biological link between AMI and hematologic malignancies. CHIP is an age-related condition characterized by the acquisition of leukemogenic mutations in hematopoietic stem cells, resulting in the expansion of genetically distinct blood cell clones.28 It is considered a premalignant state, and individuals with CHIP have an elevated risk of developing hematologic malignancies.29 CHIP has also been associated with cardiovascular disease, likely through inflammation-driven processes that promote the formation or progression of atherosclerotic plaques.30 Among CHIP-related mutations, TET2 has been specifically linked to both AMI31 and hematologic malignancies.32 Although our study could not directly assess CHIP status due to the limitations of the NHIS data set, these findings raise the possibility that CHIP may predispose individuals to both AMI and hematologic malignancy through shared inflammatory and genetic mechanisms.
In our study, the hematologic malignancies most strongly associated with AMI were MM and MPNs. MPNs are commonly associated with cardiovascular disease33 and are characterized by chronic inflammation, with malignant clones producing cytokines that drive both disease symptoms and thrombosis.34 As anticipated, the incidence of MPNs was high within 90 days of AMI. In both the subtype and SIR analyses, MPNs exhibited the highest relative risk among all hematologic malignancy subtypes. Given their indolent and slow-growing nature,35 we considered the possibility that some MPNs were pre-existing and incidentally detected during the acute evaluation of AMI. To address this, we conducted a sensitivity analysis excluding patients with MPNs, and the results remained consistent, supporting the robustness of our primary findings.
The risk of MM also remained consistently elevated throughout the follow-up period. MM is a hematologic malignancy characterized by a pronounced inflammatory response36 and is well known to be associated with increased cardiovascular risk.37 Nearly all cases of MM arise from an asymptomatic premalignant condition known as monoclonal gammopathy of undetermined significance,38 which progresses to symptomatic MM at an estimated rate of 1% to 2% per year, reaching approximately 18% over 20 years.39 These features may explain the steady increase in MM diagnoses observed during post-AMI follow-up. In the case of both MPN and MM, it is plausible that these conditions existed in a subclinical state at the time of AMI diagnosis or that AMI-related inflammatory processes contributed to their subsequent development.
Study limitations
First, misclassification of diagnoses based on ICD codes is a potential limitation. However, the diagnosis of AMI using ICD-10 codes from the Korean NHIS database has been validated in a previous study, demonstrating a positive predictive value of 92%.15 To further reduce false-positive classifications, we applied a stricter definition of AMI based on hospitalization records, procedures, and laboratory testing.
Second, this study included only individuals from the Korean population. Because the incidence and causes of hematologic malignancies may vary across different socioeconomic and ethnic groups, the generalizability of our findings to other populations may be limited.
Third, although patients with AMI are likely to engage with the health care system more frequently over time, potentially increasing the risk of surveillance bias, several factors may have mitigated this concern. South Korea's universal health insurance system offers high health care accessibility and reports the highest number of medical visits per capita among OECD (Organisation for Economic Co-operation and Development) countries.40 Additionally, our study included only individuals with available national health screening data, and all Korean adults are eligible for biennial screenings.41 Therefore, both the AMI and control groups likely had comparable opportunities for hematologic malignancy detection. Furthermore, HR estimates did not change substantially after excluding hematologic malignancies diagnosed within the first 90 days in the landmark analysis. Lastly, as this was an observational study, a causal relationship between AMI and hematologic malignancies cannot be established.
Conclusions
In this large population-based cohort study, individuals with AMI had a higher risk of developing hematologic malignancies compared with those without a history of AMI. These findings suggest a potential shared pathogenesis between AMI and hematologic malignancies. Although routine screening is not indicated, increased clinical awareness of this association may be warranted in the care of patients with AMI. Further studies are needed to clarify the underlying mechanisms and to determine whether targeted surveillance strategies may benefit selected patient populations.
Perspectives.
COMPETENCY IN PATIENT CARE: Patients with AMI appear to be at higher risk of developing hematologic malignancies than those without AMI. Clinical awareness of this potential association may be important when managing patients after AMI.
TRANSLATIONAL OUTLOOK: Further basic research, including studies validating clonal hematopoiesis of indeterminate potential, is needed to clarify the biological links between AMI and hematologic malignancies. In addition, prospective cohort studies are needed to identify predictors of hematologic malignancies and to determine whether selected patients with AMI may benefit from targeted screening strategies.
Data Sharing Statement
Because of the sensitive nature of the database, requests to access the data from qualified researchers may be sent to the National Health Insurance Service at https://nhiss.nhis.or.kr.
Funding Support and Author Disclosures
This research was supported by the Working Group on Cardio-Oncology of the Korean Society of Cardiology (Grant No., 2023-02-01) and the Soonchunhyang University Research Fund. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orozco-Beltran D., Cooper R.S., Gil-Guillen V., et al. Trends in mortality from myocardial infarction. A comparative study between Spain and the United States: 1990-2006. Rev Esp Cardiol (Engl Ed) 2012;65:1079–1085. doi: 10.1016/j.recesp.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Nadlacki B., Horton D., Hossain S., et al. Long term survival after acute myocardial infarction in Australia and New Zealand, 2009-2015: a population cohort study. Med J Aust. 2021;214:519–525. doi: 10.5694/mja2.51085. [DOI] [PubMed] [Google Scholar]
- 4.Doost Hosseiny A., Moloi S., Chandrasekhar J., Farshid A. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open Heart. 2016;3 [Google Scholar]
- 5.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 6.Johnson C.B., Davis M.K., Law A., Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32:900–907. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Rinde L.B., Småbrekke B., Hald E.M., et al. Myocardial infarction and future risk of cancer in the general population-the Tromsø Study. Eur J Epidemiol. 2017;32:193–201. doi: 10.1007/s10654-017-0231-5. [DOI] [PubMed] [Google Scholar]
- 8.Leening M.J.G., Bouwer N.I., Ikram M.A., et al. Risk of cancer after ST-segment-elevation myocardial infarction. Eur J Epidemiol. 2023;38:853–858. doi: 10.1007/s10654-023-00984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N., Huang Z., Zhang Y., Sun H., Wang J., Zhao J. Increased cancer risk after myocardial infarction: fact or fiction? A systemic review and meta-analysis. Cancer Manag Res. 2019;11:1959–1968. doi: 10.2147/CMAR.S193658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N., Wu J., Wang Q., et al. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023;13:82. doi: 10.1038/s41408-023-00853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park W.J., Park J.H., Cho S., Shin M.G. Twenty-year incidence trend of hematologic malignancies in the Republic of Korea: 1999-2018. Blood Res. 2021;56:301–314. doi: 10.5045/br.2021.2021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell C.F., Lei X., Haas A., et al. Risk of cancer after diagnosis of cardiovascular disease. JACC CardioOncol. 2023;5:431–440. doi: 10.1016/j.jaccao.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi E.K. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seong S.C., Kim Y.Y., Park S.K., et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7 [Google Scholar]
- 15.Stuart E.A. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J., Kwon S., Choi E.-K., et al. Validation of diagnostic codes of major clinical outcomes in a National Health Insurance database. Int J Arrhythm. 2019;20(1):5. doi: 10.1186/s42444-019-0005-0. [DOI] [Google Scholar]
- 17.Korean Statistical Information Service Cancer Registration Statistics. Daejeon (KR): Statistics Korea. http://kosis.kr
- 18.Lau E.S., Paniagua S.M., Liu E., et al. Cardiovascular risk factors are associated with future cancer. JACC CardioOncol. 2021;3:48–58. doi: 10.1016/j.jaccao.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong I.Y., Cheung M.C., Read S., Na Y., Lega I.C., Lipscombe L.L. Association between diabetes and haematological malignancies: a population-based study. Diabetologia. 2021;64:540–551. doi: 10.1007/s00125-020-05338-7. [DOI] [PubMed] [Google Scholar]
- 20.Jeong S.M., Choi T., Kim D., et al. Association between high-density lipoprotein cholesterol level and risk of hematologic malignancy. Leukemia. 2021;35:1356–1364. doi: 10.1038/s41375-020-01081-5. [DOI] [PubMed] [Google Scholar]
- 21.Walter R.B., Buckley S.A., White E. Regular recreational physical activity and risk of hematologic malignancies: results from the prospective VITamins And lifestyle (VITAL) study. Ann Oncol. 2013;24:1370–1377. doi: 10.1093/annonc/mds631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf D., Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H., Wu L., Yan G., et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietras E.M. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130:1693–1698. doi: 10.1182/blood-2017-06-780882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stubbins R.J., Platzbecker U., Karsan A. Inflammation and myeloid malignancy: quenching the flame. Blood. 2022;140:1067–1074. doi: 10.1182/blood.2021015162. [DOI] [PubMed] [Google Scholar]
- 26.Smedby K.E., Ponzoni M. The aetiology of B-cell lymphoid malignancies with a focus on chronic inflammation and infections. J Intern Med. 2017;282:360–370. doi: 10.1111/joim.12684. [DOI] [PubMed] [Google Scholar]
- 27.Koelwyn G.J., Newman A.A.C., Afonso M.S., et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26:1452–1458. doi: 10.1038/s41591-020-0964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal S., Ebert B.L. Clonal hematopoiesis in human aging and disease. Science. 2019;366 [Google Scholar]
- 29.Genovese G., Kähler A.K., Handsaker R.E., et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Hu S., Luo X., et al. Prevalence and prognostic significance of DNMT3A- and TET2- clonal haematopoiesis-driver mutations in patients presenting with ST-segment elevation myocardial infarction. EBioMedicine. 2022;78 [Google Scholar]
- 32.Chiba S. Dysregulation of TET2 in hematologic malignancies. Int J Hematol. 2017;105:17–22. doi: 10.1007/s12185-016-2122-z. [DOI] [PubMed] [Google Scholar]
- 33.Leiva O., Hobbs G., Ravid K., Libby P. Cardiovascular disease in myeloproliferative neoplasms: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. 2022;4:166–182. doi: 10.1016/j.jaccao.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon S.Y., Won J.H. Novel therapeutic strategies for essential thrombocythemia/polycythemia vera. Blood Res. 2023;58:83–89. [Google Scholar]
- 35.Nangalia J., Green A.R. Myeloproliferative neoplasms: from origins to outcomes. Blood. 2017;130:2475–2483. doi: 10.1182/blood-2017-06-782037. [DOI] [PubMed] [Google Scholar]
- 36.de Jong M.M.E., Kellermayer Z., Papazian N., et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat Immunol. 2021;22:769–780. doi: 10.1038/s41590-021-00931-3. [DOI] [PubMed] [Google Scholar]
- 37.De Stefano V., Larocca A., Carpenedo M., et al. Thrombosis in multiple myeloma: risk stratification, antithrombotic prophylaxis, and management of acute events. A consensus-based position paper from an ad hoc expert panel. Haematologica. 2022;107:2536–2547. doi: 10.3324/haematol.2022.280893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landgren O., Kyle R.A., Pfeiffer R.M., et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyle R.A., Larson D.R., Therneau T.M., et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GBD 2019 South Korea BoD Collaborators Population health outcomes in South Korea 1990-2019, and projections up to 2040: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2023;8(8):e639–e650. doi: 10.1016/S2468-2667(23)00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin D.W., Cho J., Park J.H., Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 2022;6:9–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.