Abstract
A cuff-type electrode that curls and self-immobilizes upon exposure to moisture was developed entirely from organic materials. The device consists of polyvinyl alcohol hydrogel sheets and a sandwiched carbonized knit electrode, with one hydrogel layer pre-stretched. In its dry, flat state, the electrode allows easy insertion beneath tissue structures and curls within 30 s upon application of saline, thereby improving the safety and efficiency of electrode placement during surgery. By tuning the osmotic pressure, thickness, and pre-strain of the hydrogel layer, the curled inner diameter was reduced to as small as 0.4 mm. The swelling-induced folding force ensured secure fixation while accommodating dynamic changes in tissue size. In vivo experiments in porcine and mouse models demonstrated successful vagus nerve and muscle stimulation, confirming the viability and functional performance of the proposed electrode design.
Keywords: Cuff electrode, Hydrogel, Water-triggered folding, Carbon fabric, Nerve stimulation
Graphical abstract
1. Introduction
In advanced medicine, a wide range of implanted electrodes serve as powerful tools to support or restore the functions of the brain, central and peripheral nervous systems (PNS), cardiovascular system, musculoskeletal system, and urogenital system [[1], [2], [3], [4]]. Among these, the vagus nerve, one of the primary components of the PNS, has emerged as a key target for implantable devices, as vagus nerve stimulation (VNS) plays a crucial role in the treatment of refractory epilepsy and chronic treatment-resistant depression [5]. However, conventional implantable electrodes, typically composed of polymer substrates and metal conductors [[1], [2], [3], [4]], suffer from mechanical mismatch with biological tissues, chronic immune responses, and long-term instability in physiological environments. This challenge has been addressed in recent reports through materials engineering, with the goal of constructing stable mechanical and electrical biointerfaces [[6], [7], [8], [9]]. Among these approaches, several studies have focused on the use of hydrogels, intrinsically soft and stretchable materials, which have attracted increasing attention due to their tissue-like mechanical properties (ranging from a few to several tens of kilopascals [10]) [[11], [12], [13], [14], [15], [16]]. For instance, Bao et al. developed a hydrogel-based cuff electrode with promising performance [17]; however, its ability to achieve secure immobilization around nerve bundles has not been fully addressed.
Achieving stable immobilization of electrodes on organs immersed in tissue fluids remains a critical challenge. Cuff electrodes are commonly attached to nerves through manual methods such as suturing or clipping [[18], [19], [20]]. Some designs incorporate pre-folded structures, which that require manual unfolding during placement and subsequently self-wrap around the nerve upon release [[21], [22], [23], [24]]. It was previously reported that bilayer polyvinyl alcohol (PVA) hydrogels can exhibit self-curling behavior, enabling the immobilization of cuff electrodes [25]. The swelling-induced folding force generated by the hydrogel was demonstrated to be both safe and sufficient to securely attach the electrode to nerve bundles. However, this design relies on a pre-curled configuration, which increases the risk of nerve damage during surgical manipulation, particularly when targeting smaller, more delicate nerves. A promising strategy to overcome this limitation is to employ water-triggered, in situ deformation and folding of electrodes. For example, Yu et al. reported a silicone rubber-based electrode whose shape was fixed using a water-soluble material; upon contact with water, the material dissolved, allowing the electrode to deform and fix in place [26]. Hiendlmeier et al. used hydrogel-based hinges that swell upon hydration to induce the closing motion of cuff electrodes [27].
In this study, we present practical advancements in both materials and folding mechanisms for a hydrogel-based, fully organic cuff electrode. Specifically, a custom-fabricated carbon fiber knit electrode was developed, exhibiting sufficient softness and stability to avoid interfering with the curling behavior of the PVA substrate. Notably, water-triggered folding was successfully implemented, significantly simplifying electrode placement during surgery (Fig. 1). Both the inner diameter and folding speed of the cuff can be tuned by adjusting the internal stress and osmotic pressure of the hydrogel layers. The swelling-induced immobilization readily accommodates variations in tissue thickness as well as dynamic changes in tissue size. Finally, in vivo experiments in porcine and mouse models demonstrated successful VNS and muscle stimulation, confirming the viability and functional performance of the proposed electrode design.
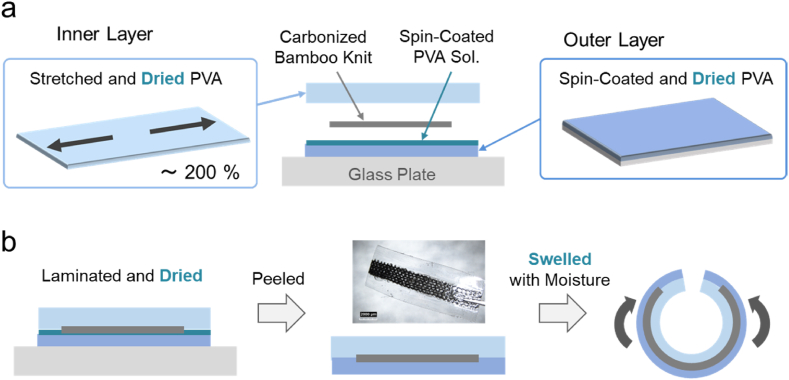
Fig. 1.
Schematic illustration of the water-triggered self-immobilization process: (a) easy insertion of the dry, flat electrode beneath a tissue structure, such as the vagus nerve or a muscle bundle; (b) swelling of the hydrogel triggered by moisture from a saline solution; (c) self-wrapping and immobilization of the electrode around the tissue for stimulation.
2. Results and discussion
2.1. Fabrication of cuff electrodes
The fabrication process of the cuff electrode is illustrated in Fig. 2. The device consists of a laminate composed of inner and outer PVA layers and a carbonized bamboo knit electrode (RT-01). For the inner layer, a PVA hydrogel sheets (typically 0.5 mm in thickness) were stretched under uniaxial strain and subsequently dried to obtain stretched PVA xerogel plates. To accelerate the curling speed, some hydrogel sheets were immersed in 0.3 M glucose for 12 h prior to stretching. For the outer layer, a PVA aqueous solution was spin-coated to form a thin PVA film (0.4 mm in thickness), which was then dried to prepare PVA xerogel films. A PVA solution was further spin-coated as an adhesive to sandwich a strip of the carbonized knit electrode between the inner and outer PVA layers. The adhesive PVA infiltrates the internal structure of the carbon fiber knit, forming an interlocking configuration that prevents slippage during swelling-induced curing. The resulting laminate was once again frozen and dried, followed by peeling from the glass substrate to obtain a cuff electrode that undergoes curling upon swelling, driven by internal stress in the pre-stretched inner layer. Similar approaches using mechanical strain to generate three-dimensional structures have been reported for polydimethylsiloxane (PDMS) elastomers [28,29]; here, we apply this strategy to PVA hydrogel to achieve self-immobilization of the electrode around nerve and muscle bundles in a cuff-type configuration.
Fig. 2.
The illustrations of the construction (a) and the curing behavior (b) of the cuff electrode that is a laminate of inner and outer dried PVA xerogels and a carbonized bamboo knit electrode (RT-01). Upon hydration, the internal stress of the pre-stretched inner layer induces curling, enabling self-wrapping around a target tissue.
2.2. Characterization of cuff electrode device
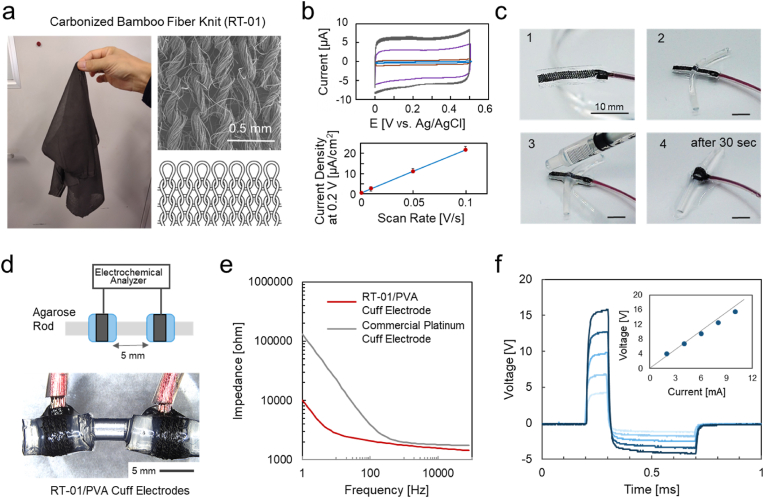
A key feature of the cuff electrode developed in this study is its entirely organic composition, which makes the device compatible with magnetic resonance imaging (MRI), as demonstrated in our previous studies [25,30,31]. Conductive polymers such as PEDOT/polyurethane (PU) composites, previously explored for cuff electrodes [25], have shown promise as organic materials but suffer from instability in aqueous environments, limiting their suitability for long-term implantation [14]. Conventional carbon fiber fabrics (CF) offer a stable and flexible organic electrode platforms, and have demonstrated satisfactory performance in hydrogel-based intracranial electrode for electroencephalogram (EEG) recordings [15]. However, due to their inherent stiffness, CF materials are not well-suited for cuff electrodes, which require high deformability and conformal contact with curved tissue surfaces. To address these limitations, we employ a carbonized knit fabric (RT-01) as the electrode material in this study, leveraging its organic composition, structural stability, and mechanical softness. As shown in Fig. 3a, RT-01 (thickness: 0.22 mm) consists of horizontally knitted bamboo fibers that are subsequently carbonized. This material exhibited significantly greater softness and stretchability compared to conventional plain-weave CF, as demonstrated in Supplementary Fig. S1. In addition, RT-01 maintained stable conductivity, remaining unchanged upon 10 % stretching (Supplementary Fig. S2). Cyclic voltammograms of the RT-01 electrodes (0.3 cm2) in PBS at scan rates of 0.1, 0.05, 0.01, and 0.001 V s−1 (Fig. 3b) showed a linear relationship between the scan rate and the current density, indicating capacitive behavior reflecting the charge/discharge of the electrical double layer at the electrode surface [25]. The slope of the linear plot corresponds to a capacitance density of approximately 0.22 mF cm−2 for RT-01 electrode.
Fig. 3.
(a) Photograph, SEM image, and schematic illustration of the RT-01 carbonized bamboo fiber knit (thickness: 0.22 mm). (b) Cyclic voltammograms of RT-01 electrodes (0.3 cm2) in PBS at scan rates of 0.1, 0.05, 0.01, 0.001 V s−1, and a plot of the current density at 0.2 V in the forward scan versus the scan rates. Data are presented as mean ± standard error (n = 3 individual tests). (c) Sequential snapshots showing the application of the RT-01/PVA cuff electrode. The electrode is inserted beneath an agarose rod and self-wraps upon hydration with PBS, completing the wrapping process within 30 s. (d) Setup of the RT-01/PVA cuff electrodes around a 2.0 mm-diameter agarose hydrogel rod (15 wt% agarose in PBS) with an inter-electrode distance of 5 mm.(e) AC impedance spectra measured at 5 mVpp for a pair of cuff electrodes wrapped around the agarose hydrogel rod, comparing the RT-01/PVA cuff electrode (red) and a commercial platinum cuff electrode (gray). (f) Voltage response measured across the cuff electrodes during the application of square DC current pulses (2–10 mA), using the same waveform as employed in the in vivo animal experiments of VNS. Inset: linear relationship between the applied current and the developed voltage. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The application of the PVA-based cuff electrode with the RT-01 (RT-01/PVA cuff electrode) is presented in Fig. 3c through a sequence of snapshots. The electrode was first inserted beneath a thin rod of agarose (2.0 mm in diam.) (steps 1 → 2), then moistened with phosphate-buffered saline (PBS) (step 3), which triggered self-wrapping around the rod within approximately 30 s (step 4).
Two RT-01/PVA cuff electrodes were mounted around a 2.0 mm-diameter agarose hydrogel rod (15 wt% agarose in PBS) with an inter-electrode distance of 5 mm (Fig. 3d) for AC impedance and voltage response measurements. Fig. 3e shows the AC impedance spectra for the RT-01/PVA cuff electrodes (red line) compared with a conventional platinum (Pt) cuff electrode (gray line). At high frequencies (>200 Hz), the impedance of the RT-01/PVA was comparable to that of the commercial Pt electrodes, indicating similar DC resistance for the systems, including reliable connections to the lead wires. At the lower frequencies, the RT-01/PVA impedance was markedly lower, attributable to the large double-layer capacitance of the RT-01 electrode. The larger capacitance is a notable advantage for safe stimulation, as it reduces the risk of tissue damage caused by Faradaic reactions, such as cytotoxic gas generation from water electrolysis [32,33]. Fig. 3f shows the voltage response over time between the cuff electrodes during the application of square DC current pulses (2–10 mA), using the same waveform as that employed in the subsequent animal experiments [34,35]. As shown in the inset, the voltage increased proportionally with the stimulation current, confirming that Faradaic polarization was negligible, again a result of the high capacitance of the RT-01 electrode. This not only ensures tissue safety but also contributes to the long-term electrochemical stability of the electrode itself.
2.3. Fabrication of cuff electrode with small inner diameter
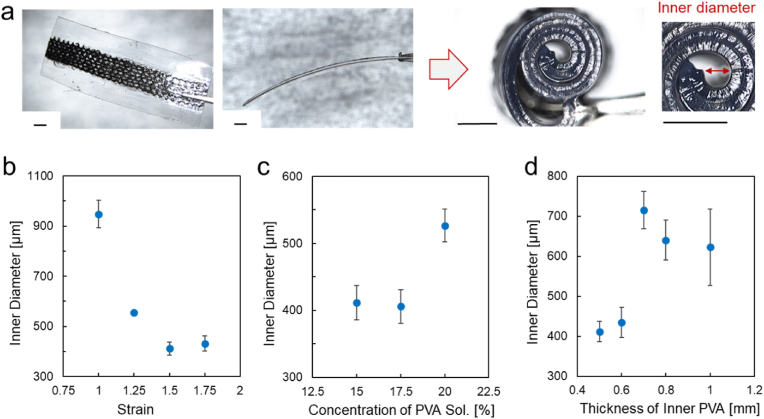
As shown in Fig. 4a, the RT-01/PVA cuff electrode curls upon swelling of the inner PVA layer, which had been dried under a pre-applied tensile strain. The inner diameter in the curled state was measured from side-view images, as indicated by the red bar in the photograph. This curling behavior enables spontaneous wrapping around cylindrical tissues such as VN, which typically has a diameter of approximately 2 mm in both humans and pigs [36,37]. To achieve the smallest possible inner diameter, enabling wrapping around fine nerves, we examined three fabrication parameters of the inner PVA layer, which was stretched and then dried: the applied strain, the concentration of the PVA solution, and the thickness before stretching. These parameters are key factors in the equation for the radius of curvature of a curled bilayer [25,27], and were therefore systematically varied to determine their effect on the final inner diameter. Among the tested combinations, a strain of 1.5, a PVA concentration of 15 wt%, and an inner layer thickness of 0.5 mm produced the smallest inner diameter (∼400 μm), as detailed below.
Fig. 4.
(a) Photographs of the RT-01/PVA cuff electrode before and after curling. Scale bars: 1.0 mm. (b–d) Dependence of the inner diameter of the curled cuff electrodes on the parameters in the inner PVA preparation; (b) the applied strain, (c) the concentration of the PVA solution, and (d) the thickness before stretching. The data represent the mean ± standard error (n = 3 individual tests).
First, larger pre-strain values (1.5 and 1.75) resulted in smaller inner diameters, likely due to stronger contraction forces upon rehydration of the hydrogel (Fig. 4b). Further miniaturization was limited by the mechanical stiffness of both the hydrogel and the carbonized bamboo knit structure. Second, as shown in Fig. 4c, increasing the PVA concentration to 20 wt% led to larger inner diameters, likely due to an increase in Young's modulus, which reduces flexibility and impairs curling. In contrast, a 12.5 wt% PVA hydrogel could not withstand a 1.5 strain and fractured during fabrication. Third, reducing the thickness of the inner PVA layer was effective in minimizing the inner diameter (Fig. 4d). Thicker inner layers (≥0.8 mm) were found to hinder curling due to increased mechanical resistance. Taken together, these results indicate that an inner diameter as small as ∼400 μm can be reliably achieved under the optimized fabrication conditions: 1.5 strain, 15 wt% PVA, and 0.5 mm inner layer thickness.
2.4. Self-immobilization properties of the cuff electrodes
Fig. 5a shows cross-sectional images of the developed RT-01/PVA cuff electrodes (15 mm length) wrapped around agarose rods with diameters of 1, 2, 3, and 4 mm. When wrapped around the 1 mm rod, the electrode exhibited a curvature that closely matched the surface of the rod, resulting in uniform adhesion. As the rod diameter increased, the curvature mismatch became more pronounced, leading to slight detachment at the electrode edges.
Fig. 5.
(a) Cross-sectional photographs showing the RT-01/PVA cuff electrode wrapped around agarose rods of varying diameters (1, 2, 3, and 4 mm). (b, c) Representative force–displacement curves for the RT-01/PVA cuff electrode during mechanical testing on agarose rods, with displacement applied at a constant speed of 5 mm/min in the peeling direction (b) and in the sliding direction (c). The accompanying bar graphs show the force at detachment (b) and the force during sliding (c) for agarose rods of different diameters. Data are presented as mean ± standard error (n = 3 individual tests). (d) The RT-01/PVA cuff electrode wrapped around a soft Ecoflex tube undergoing reversible deformation, with diameter changes from 1.5 mm to 0.8 mm. (e) Wrapping pressure exerted by the RT-01/PVA cuff electrode on a 2 mm sensor rod in saline. The red dashed line indicates the threshold (1300 Pa) associated with possible nerve damage. The data are presented as mean ± standard error (n = 3 individual tests). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To evaluate the immobilization force of the cuff electrodes, force–displacement measurements were conducted in both the peeling (Fig. 5b) and sliding (Fig. 5c) directions, with displacement applied at a constant speed of 5 mm/min. Importantly, in both directions, the measured forces significantly exceeded the self-weight of the electrode (∼1.2 mN), demonstrating its stable attachment under physiological conditions. Previous studies have similarly confirmed firm adhesion, even under vigorous shaking in saline [25]. The force-displacement curves in the peeling test (Fig. 5b) exhibited a gradual increase in force followed by a sudden drop, indicative of progressive detachment. Considering the electrode length (15 mm), it is reasonable that electrodes wrapped around rods with larger diameters detach earlier. For example, for a 4 mm-diameter rod (circumference, ca. 12.5 mm), when displaced by 6 mm, the remaining electrode length (9 mm) becomes shorter than the circumference, making detachment more likely. The bar graph plots the maximum load before detachment, showing a tendency for thinner rods to generate larger forces. This is likely because, in thicker rods, curvature mismatch prevents firm contact at the ends and reduces the detachment force, whereas in thinner rods (1 mm in diameter), curvature matching allows a catching force to be maintained until the end. In the sliding tests (Fig. 5c), the frictional force remained nearly constant at approximately 20 mN for rods with diameters of 2∼4 mm. For the 1 mm-diameter rod, the markedly smaller contact area and the relatively well-matched curvature resulted in a lower sliding force (about 6 mN). Nevertheless, this force was still sufficient to prevent passive displacement, indicating that the electrode can maintain its position without external fixation.
These results indicate that the immobilization performance of the RT-01/PVA cuff electrode is not only sufficient but also adaptable to dynamic changes in tissue size, such as those caused by muscle contraction and relaxation. This adaptability is further demonstrated in Fig. 5d, where the electrode maintained secure attachment to a soft, deformable rod made of Ecoflex, whose diameter was changed from 1.5 mm to 0.8 mm. These findings highlight the potential of the device for applications involving soft and deformable biological tissues, such as myoelectric signal acquisition and electrical stimulation therapies. This will be further demonstrated later through stimulation experiments on mouse muscle tissue.
Importantly, neural tissues are highly sensitive to mechanical stress, and excessive pressure must be avoided. Prior studies have reported that demyelination can occur at pressures as low as 10 mmHg (1.3 kPa), while significant axonal damage may arise at pressures exceeding 80 mmHg (10.6 kPa) [38]. As shown in Fig. 5e, the wrapping pressure exerted by the RT-01/PVA cuff electrode on a 2 mm sensor rod immersed in saline was only ∼200 Pa, far below thresholds associated with nerve damage. Only 2 mm sensor rods were available; however, based on the above immobilization results, it can be inferred that, for any of these diameters (1–4 mm), the generated wrapping force would remain within a safe range. Collectively, these results demonstrate that the self-wrapping PVA-based cuff electrode offers both secure immobilization and biomechanical safety, making it a promising candidate for chronic neuromodulation and neural interfacing applications.
2.5. Demonstrated animal experiments
The performance of the fabricated RT-01/PVA cuff electrode was evaluated by vagus nerve stimulation (VNS) in a porcine model and by muscle stimulation in the mouse leg. Compared to conventional pre-curled commercial electrodes and previously reported hydrogel-based cuff electrodes [25], the RT-01/PVA electrode demonstrated superior handling characteristics during surgical placement. As shown in Fig. 6a and Supplementary Movie S1, the electrode remained flat in its dry state, enabling smooth insertion beneath the vagus nerve. Upon application of a few drops of saline, the inner PVA layer swelled and triggered autonomous curling, firmly wrapping around the nerve. This delayed, on-demand curling mechanism not only simplifies placement but also has the potential to enhance surgical efficiency and precision.
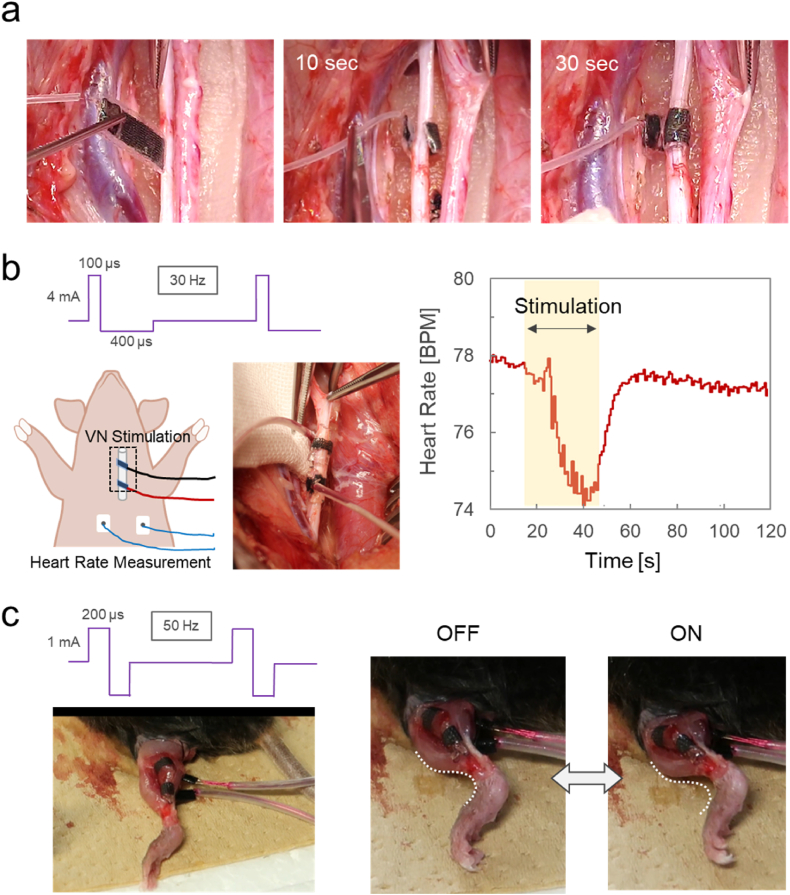
Fig. 6.
(a) Sequential snapshots showing the attachment process of the RT-01/PVA cuff electrode to the vagus nerve in a porcine model. The dry, flat electrode is first inserted beneath the nerve bundle. Upon application of saline drops, curling is initiated, and complete fixation is achieved within 30 s. (b) Photograph of the RT-01/PVA cuff electrodes attached to the vagus nerve, waveform of the electrical stimulation, and a representative heart rate response showing a transient decrease during 30 s of vagus nerve stimulation. (c) Photograph of the attached RT-01/PVA cuff electrodes on the lateral head of the exposed left gastrocnemius muscle, waveform of the electrical stimulation, and snapshots of the mouse leg in the off/on states of the stimulation taken from Supplementary Movie S2.
The curling speed was significantly enhanced by pre-immersing the inner PVA layer in a 0.3 M glucose solution prior to drying. The resulting osmotic effect increased water absorption upon rehydration, thereby accelerating the actuation process. As a result, whereas the untreated inner layer prepared with distilled water required approximately 90 s to complete curling, the glucose-treated electrode curled fully in approximately 30 s (Supplementary Fig. S3). This ∼30-s window was found to be optimal for electrode positioning, providing a practical balance between handling time and rapid immobilization.
As shown in Fig. 6b, two RT-01/PVA cuff electrodes were placed around the vagus nerve of a 12-week-old pig, and biphasic square pulses (0.1 ms pulse width, 4 mA, 30 Hz) were applied through the electrodes. The stimulation resulted in a reduction in heart rate from a baseline of 78 bpm to 74 bpm, followed by recovery to the baseline level after cessation of the stimulation. Previous studies have reported heart rate modulation via VNS in large animal models including pigs [39,40]. The observed reversible induction of bradycardia without signs of tissue injury further supports the utility of the developed hydrogel-based, fully organic cuff electrode for safe and effective in vivo neuromodulation. As another in vivo demonstration, the RT-01 cuff electrodes were immobilized on the lateral head of the exposed left gastrocnemius muscle of a mouse (Fig. 6c). Application of biphasic square pulses (0.2 ms pulse width, 1 mA, 50 Hz) through the electrode induced successive bending of the leg (Supplementary Movie S2). These results demonstrate the robust immobilization of the developed cuff electrode, which can maintain tight adhesion to tissue even during motions involving size changes, such as muscle contraction.
3. Conclusion
The cuff-type RT-01/PVA electrode developed in this study demonstrated safe and facile fixation to delicate nerve bundles, enabled by its moisture-triggered curling and self-immobilization capabilities. The deformation speed was tunable by adjusting the osmotic pressure of the hydrogel and was optimized to approximately 30 s based on performance during implantation in a porcine model. The swelling-induced fixation is expected to be applicable not only to nerves but also to muscle bundles, as it accommodates dynamic changes in tissue size. Ongoing developments include the integration of an insulating layer to enhance stimulation efficiency. Furthermore, to support long-term implantation, we are exploring the combination of this system with a hydrogel-based electro-osmotic pump [41] capable of delivering anti-inflammatory agents.
4. Material
4.1. Materials
Phosphate-buffered saline (PBS, 045–29795), D-glucose (049–31165), and dimethyl sulfoxide (DMSO, 043–07216) were purchased from FUJIFILM Wako Pure Chemical Corporation. Polyvinyl alcohol (PVA, Mowiol 28–99) was obtained from Sigma-Aldrich. Polydimethylsiloxane (PDMS, SILPOT184) was supplied by DuPont Toray Specialty Materials. Ecoflex® (00–30) was purchased from Smooth-On Inc. A commercial cuff electrode (VNS lead M304) was obtained from LivaNova. Carbonized bamboo fiber knits (RT-01, 0.22 mm thickness) were provided by Nakatsuyama Netsusyori Co. The bamboo fiber knits were carbonized by baking at 1200 °C in an N2 atmosphere. The purity of the carbonized bamboo fabric was examined by EDS elemental analysis: atomic content of C, O, Si were 91.76, 8.11, 0.13 %, respectively.
4.2. Fabrication of cuff electrodes
For the inner layer, a 15 wt% PVA solution was prepared using a 4:1 (v/v) mixture of DMSO and distilled water and sterilized by autoclaving at 120 °C for 30 min. DMSO was used only in the inner PVA layer to impart elasticity for accommodating additional strain. The PVA solution was poured into a mold fabricated using a 3D printer and gelled via a freeze-thaw method, consisting of three cycles of freezing at −28 °C for 10 min and thawing at room temperature for 10 min [25]. After DMSO removal by immersion in distilled water for 30 min, the resulting PVA hydrogel sheets (typical thickness: 0.5 mm) were stretched under uniaxial strain and dried in an food dehydrator (KWASYO, DSC-06B) at 25 °C for 12 h to obtain stretched PVA xerogel plates. The dehydration ratio for this drying process was approximately 95 % (Supplementary Fig. S4), which is comparable to that achieved using a vacuum desiccator (−0.1 MPa, 12 h). For the outer layer, a 15 wt% aqueous PVA solution was spin-coated (ACT-220DⅡ, Active Co.) onto a glass plate at 700 rpm for 30 s to form a thin PVA film. After freezing at −28 °C for 10 min, the films were dried in an food dehydrator at 25 °C for 12 h to obtain PVA xerogel sheets. The same precursor solution (15 wt% PVA) was further spin-coated at 1500 rpm for 30 s to serve as an adhesive to sandwich a strip of bamboo knit electrode between the inner and outer PVA layers. The laminate was once again frozen and dried, followed by peeling from the glass substrate to prepare a cuff electrode which has a character to cure on swelling due to the inner stress of the inner layer.
4.3. Electrical measurements of electrode materials
Cyclic voltammetry (CV) was performed in phosphate-buffered saline (PBS) using an electrochemical analyzer (ALS 7082E; BAS, Tokyo, Japan) with a gold counter electrode and an Ag/AgCl reference electrode. The AC impedance of the Pt and carbon knit electrodes was measured over a frequency range of 1–10,000 Hz with an amplitude of 5 mVpp using the electrochemical analyzer. The DC resistance of the electrodes was measured using the four-point probe method with a resistivity meter (MCP-T370; Nittoseiko Analytech, Kanagawa, Japan) at room temperature.
4.4. Evaluation of transformation behavior and immobilization properties of the cuff electrodes
The microstructure of the carbon knit electrodes was observed using scanning electron microscopy (SEM; SU-70, Hitachi High-Tech Corporation). To evaluate the transformation behavior and inner diameter of the cuff electrodes, the fabricated flat xerogel electrodes were immersed in phosphate-buffered saline (PBS) at room temperature. Their lateral transformation was then observed and photographed using a microscope (HRX-01, Hirox Co.).
The immobilization forces in the sliding and peeling directions were evaluated according to a previously reported method [25]. Briefly, the swollen cuff electrodes were wrapped around agarose hydrogel rods with diameters of 1, 2, 3, and 4 mm. The immobilization force was measured by applying displacement was applied vertically using a motorized test stand (EMX-1000N; IMADA Co., Ltd., Aichi, Japan) in combination with a digital force gauge (ZTA-5N; IMADA Co.), as explained in Supplementary Fig. S5. Also the wrapping pressure of the cuff electrodes was measured as previously reported [25] using a thin-walled silicone tube (2 mm diameter) capped with a pressure gauge (GP-M001; Keyence) (Supplementary Fig. S6).
4.5. Animal experiments
All animal procedures were approved by the Center for Laboratory Animal and Gene Research at Tohoku University (Approval No. 2019MdA-324-04 and 2020BeA-006-02) and were conducted in accordance with the Regulations for Animal Experiments and Related Activities at Tohoku University.
The VSN experimental protocol followed the methodology in a previous study [25]. As illustrated in Supplementary Fig. S7(a), under general anesthesia, the carotid sheath of a 12-week-old male mixed-breed pig (cross-bred from Yorkshire and Landrace; body weight: 46 kg) was surgically exposed to access the internal jugular vein and the vagus nerve (VN). The fabricated cuff electrode was positioned beneath the VN, and a few drops of phosphate-buffered saline (PBS) were applied to initiate wrapping of the nerve. The procedure was performed by a neurosurgeon with clinical experience in vagus nerve stimulation (VNS) surgery. Biphasic square-wave pulses (500 μs pulse width, 4 mA, 30 Hz) were applied for 30 s using an electronic stimulator (SEN-3401; Nihon Kohden Co.) and isolator (SS-203J; Nihon Kohden Co.), with signal acquisition through a recording unit (PowerLab 8/35; ADInstruments Japan Inc., Nagoya, Japan). Electrocardiograms (ECGs) were recorded using surface electrodes (NM-31; Nihon Kohden Co.) placed on the left and right thoracic regions. The signals were amplified with a dual bio amplifier (FE135 Dual Bio Amp; ADInstruments Japan Inc.) and processed by the recording unit. ECG waveforms filtered in the 5–18 Hz range were converted to heart rate data using LabChart v8 software (ADInstruments Japan Inc.).
For the muscle stimulation experiments, 12-week-old male mice (C57BL/6J, Japan CLEA) were used. The RT-01 cuff electrode were placed on the lateral head of the exposed left gastrocnemius muscle as illustrated in Fig. S7(b), and electrical stimulation with current pulses was delivered using the same setup as in the above pig experiments. Stimulation was applied in cycles of 1 s on-time and 1 s off-time. During the on-time, pulses with a width of 400 μs in a 50 % biphasic waveform at 50 Hz and an intensity of 1 mA were delivered.
4.6. Statistical analysis
The data are presented as mean ± standard error, n = 3 (individual tests). Statistical analysis was carried out using Microsoft 365 Excel.
CRediT authorship contribution statement
Shin-ichiro Osawa: Writing – review & editing, Investigation. Fumiya Imamura: Writing – original draft, Investigation, Data curation, Conceptualization. Natsuki Nozaki: Investigation, Conceptualization. Tenyo Ko: Investigation. Shuto Osaki: Investigation, Data curation. Soichiro Tottori: Investigation. Atsuhiro Nakagawa: Writing – review & editing, Supervision. Matsuhiko Nishizawa: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors express appreciation to Nakatsuyama Netsusyori Co., Ltd. for donating the carbonized knit fabrics, and to Professor Makoto Kanzaki for his valuable cooperation in the experiments using mice. This work was supported by Grant-in-Aids for Scientific Research S (22H04956) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2025.102248.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Lee J.H., Kim H., Kim J.H., Lee S.H. Soft implantable microelectrodes for future medicine: prosthetics, neural signal recording and neuromodulation. Lab Chip. 2016;16:959. doi: 10.1039/c5lc00842e. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B., Xie R., Jiang J., Hao S., Fang B., Zhang J., Bai H., Peng B., Li L., Liu Z., Fu Li. Implantable neural electrodes: from preparation optimization to application. J. Mater. Chem. C. 2023;11:6550–6572. [Google Scholar]
- 3.Wang Y., Yang X., Zhang X., Wang Y., Pei W. Implantable intracortical microelectrodes: reviewing the present with a focus on the future. Microsys. Nanoengin. 2023;9:7. doi: 10.1038/s41378-022-00451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X., Azizl A., Sheng X., Wang L., Yin L. Bioresorbable neural interfaces for bioelectronic Medicine. Curr. Opin. Biomed. Eng. 2024;32 [Google Scholar]
- 5.Goggins E., Mitani S., Tanaka S. Clinical perspectives on vagus nerve stimulation: present and future. Clin. Sci. (Lond) 2022;136:695–709. doi: 10.1042/CS20210507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seong D., Choi Y., Choi I.C., Lee J., Choi J.H., Park J.H., Nam J.J., Ju J., Ryoo H.J., Kwak D., Lee J., Kim S.-G., Kim D.H., Park J.W., Shin M., Son D. Adv. Mater. 2024;36 doi: 10.1002/adma.202307810. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y., Lee H., Lee J., Shin M., Son D. ACS Nano. 2025;19 doi: 10.1021/acsnano.5c07150. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y., Kang K., Koo J.H., Jeong Y., Lee S., Jung D., Seong D., Kim H., Han H.-S., Suh M., Kim D.-H., Son D. Adv. Mater. 2025;37 doi: 10.1002/adma.202503413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon H., Park B., Chou N., Park K.-S., Lee S., Kim S. Adv. Mater. 2025;37 doi: 10.1002/adma.202409942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Liu J., Lin S., Zhao X. Hydrogel machines. Mater. Today. 2020;36:102. [Google Scholar]
- 11.Cheng S., Zhu R., Xu X. Hydrogels for next generation neural interfaces. Commun. Mater. 2024;5:99. [Google Scholar]
- 12.C Horn C., Forssell M., Sciullo M., Harms J.E., Fulton S., Mou C., Sun F., Simpson T.W., X G., Fisher Lee E., Bettinger C., Fedder G.K. Hydrogel-based electrodes for selective cervical vagus nerve stimulation. J. Neural. Eng. 2021;18 doi: 10.1088/1741-2552/abf398. [DOI] [PubMed] [Google Scholar]
- 13.Zhou T., Yuk H., Hu F., Wu J., Tian F., Roh H., Shen Z., Gu G., Xu J., Lu B., Zhao X. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023;22:895–902. doi: 10.1038/s41563-023-01569-2. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M., Karikkineth B.C., Nagamine K., Kaji H., Torimitsu K., Nishizawa M. Highly conductive stretchable and biocompatible electrode-hydrogel hybrids for advanced tissue engineering. Adv. Healthcare Mater. 2014;3:1919–1927. doi: 10.1002/adhm.201400209. [DOI] [PubMed] [Google Scholar]
- 15.Oribe S., Yoshida S., Kusama S., Osawa S., Nakagawa A., Iwasaki M., Tominaga T., Nishizawa M. Hydrogel-based organic subdural electrode with high conformability to brain surface. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura A., Suwabe R., Ogihara Y., Yoshida S., Abe H., Osawa S., Nakagawa A., Tominaga T., Nishizawa M. Totally transparent hydrogel-based subdural electrode with patterned salt bridge. Biomed. Microdevices. 2020;22:57. doi: 10.1007/s10544-020-00517-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Li J., Song S., Kang J., Tsao Y., Chen S., Mottini V., McConnell K., Xu W., Zheng Y.-Q., Tok J.B.-H., George P.M., Bao Z. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020;38:1031–1036. doi: 10.1038/s41587-020-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otchy T.M., Michas C., Lee B., Gopalan K., Nerurkar V., Gleick J., Semu D., Darkwa L., Holinski B.J., Chew D.J., White A.E., Gardner T.J. Printable microscale interfaces for long-term peripheral nerve mapping and precision control. Nat. Commun. 2020;11:4191. doi: 10.1038/s41467-020-18032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowan C.C., Graudejus O., Otchy T.M. A microclip peripheral nerve interface (μcPNI) for bioelectronic interfacing with small nerves. Adv. Sci. 2022;9 doi: 10.1002/advs.202102945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noller C.M., Levine Y.A., Urakov T.M., Aronson J.P., Nash M.S. Vagus nerve stimulation in rodent models: an overview of technical considerations. Front. Neurosci. 2019;13:911. doi: 10.3389/fnins.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur R., Aplin F.P., Fridman G.Y. A hydrogel-based microfluidic nerve cuff for neuromodulation of peripheral nerves. Micromachines. 2021;12:1522. doi: 10.3390/mi12121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G., Choi Y.W., Lee T., Lim K.S., Shin J., Kim T., Kim H.K., Koo B.-K., Kim H.B., Lee J.-G., Ahn K., Lee E., Lee M.S., Jeon J., Yang H.S., Won P., Mo S., Kim N., Jeong M.H., Roh Y., Han S., Koh J.-S., Kim S.M., Kang D., Choi M. Nature-inspired rollable electronics. NPG Asia Mater. 2019;11:67. [Google Scholar]
- 23.Zhang Y., Zheng N., Cao Y., Wang F., Wang P., Ma Y., Lu B., Hou G., Fang Z., Liang Z., Yue M., Li Y., Chen Y., Fu J., Wu J., Xie T., Feng X. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng H., Zhang Z., Jiang S., Yan B., Shi X., Xie Y., Huang X., Yu Z., Liu H., Weng S., Nurmikko A., Zhang Y., Peng H., Xu W., Zhang J. A shape-memory and spiral light-emitting device for precise multisite stimulation of nerve bundles. Nat. Commun. 2019;10:2790. doi: 10.1038/s41467-019-10418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terutsuki D., Yoroizuka H., Osawa S., Ogihara Y., Abe H., Nakagawa A., Iwasaki M., Nishizawa M. Totally organic hydrogel-based self-closing cuff electrode for vagus nerve stimulation. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu M., Wang C., Cui H., Huang J., Yu Q., Wang P., Huang C., Li G., Zhao Y., Du X., Liu Z. Self-closing stretchable cuff electrodes for peripheral nerve stimulation and electromyographic signal recording. ACS Appl. Mater. Interfaces. 2023;15:7663–7672. doi: 10.1021/acsami.2c15808. [DOI] [PubMed] [Google Scholar]
- 27.Hiendlmeier L., Zurita F., Vogel J., Duca F.D., Boustani G.A., Peng H., Kopic I., Nikić M., Teshima T.F., Wolfrum B. 4D-Printed soft and stretchable self-folding cuff electrodes for small-nerve interfacing. Adv. Mater. 2023;35 doi: 10.1002/adma.202210206. [DOI] [PubMed] [Google Scholar]
- 28.Kalaitzidou K., Crosby A.J. Adaptive polymer particles. Appl. Phys. Lett. 2008;93 [Google Scholar]
- 29.Jin Y., Wang N., Yuan B., Sun J., Li M., Zheng W., Zhang W., Jiang X. Stress-induced self-assembly of complex three dimensional structures by elastic membranes. Small. 2013;9:2410. doi: 10.1002/smll.201300929. [DOI] [PubMed] [Google Scholar]
- 30.de Jonge J.C., Melis G.I., Gebbink T.A., de Kort G.A.P., Leijten F.S.S. Safety of a dedicated brain MRI protocol in patients with a vagus nerve stimulator. Epilepsia. 2014;55 doi: 10.1111/epi.12774. [DOI] [PubMed] [Google Scholar]
- 31.Carrette E., Tiège X.D., Beeck M.O.D., Herdt V.D., Meurs A., Legros B., Raedt R., Deblaere K., Roost D.V., Bourguignon M., Goldman S., Boon P., Bogaert P.V., Vonck K. Magnetoencephalography in epilepsy patients carrying a vagus nerve stimulator. Epilepsy Res. 2011;93:44. doi: 10.1016/j.eplepsyres.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida S., Sumomozawa K., Nagamine K., Nishizawa M. Hydrogel microchambers integrated with organic electrodes for efficient electrical stimulation of human iPSC-derived cardiomyocytes. Macromol. Biosci. 2019;19 doi: 10.1002/mabi.201900060. [DOI] [PubMed] [Google Scholar]
- 33.Cogan S.F., Stimulation N. Recording electrodes. Annu. Rev. Biomed. Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 34.Labiner D.M., Ahern G.L. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol. Scand. 2007;15:23. doi: 10.1111/j.1600-0404.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T. Vagus nerve stimulation therapy: indications, programing, and outcomes. Neurol. Med.-Chir. 2015;55:407. doi: 10.2176/nmc.ra.2014-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan H., Silberstein S.D. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part III. Headache. 2016;56:479. doi: 10.1111/head.12649. [DOI] [PubMed] [Google Scholar]
- 37.Pelot N.A., Goldhagen G.B., Cariello J.E., Musselman E.D., Clissold K.A., Ezzell J.A., Grill W.M. Quantified morphology of the cervical and subdiaphragmatic vagus nerves of human, pig, and rat. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuoco F.A., Jr., Durand D.M. Measurement of external pressures generated by nerve cuff electrodes. IEEE Trans. Rehabil. Eng. 2000;8:35. doi: 10.1109/86.830947. [DOI] [PubMed] [Google Scholar]
- 39.Buschman H.P., Storm C.J., Duncker D.J., Verdouw P.D., van der Aa H.E., van der Kemp P. Heart rate control via vagus nerve stimulation. Neuromodulation. 2006;9:214. doi: 10.1111/j.1525-1403.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolthuis A.M., Stakenborg N., D'Hoore A., Boeckxstaens G.E. The pig as preclinical model for laparoscopic vagus nerve stimulation. Int. J. Colorectal Dis. 2016;31:211. doi: 10.1007/s00384-015-2435-z. [DOI] [PubMed] [Google Scholar]
- 41.Terutsuki D., Miyazawa S., Takagi J., Yamada A., Sun Y., Abe H., Wang G., Nishizawa M. Spatiotemporally controllable chemical delivery utilizing electroosmotic flow generated in combination of anionic and cationic hydrogels. Adv. Funct. Mater. 2023;34 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.