Abstract
Investigations using the fruit fly Drosophila melanogaster have shown that the circadian clock gene period (Per) can influence behavioral responses to cocaine. Here we show that the mouse homologues of the Drosophila Per gene, mPer1 and mPer2, modulate cocaine sensitization and reward, two phenomena extensively studied in humans and animals because of their importance for drug abuse. In response to an acute cocaine injection mPer1 and mPer2 mutant mice as well as wild-type mice exhibited an approximately 5-fold increase in activity compared with saline control levels, showing that there is no initial difference in sensitivity to acute cocaine administration in Per mutants. After repeated cocaine injections wild-type mice exhibited a sensitized behavioral response that was absent in mPer1 knockout mice. In contrast, mPer2 mutant mice exhibited a hypersensitized response to cocaine. Conditioned place preference experiments revealed similar behavioral reactions: mPer1 knockout mice showed a complete lack of cocaine reward whereas mPer2 mutants showed a strong cocaine-induced place preference. In another set of experiments, we tested C57/BL6J mice at different Zeitgeber times and found that cocaine-induced behavioral sensitization and place preference are under the control of the circadian clock. In conclusion, we demonstrate that processes involved in cocaine addiction depend on the circadian rhythm and are modulated in an opposing manner by mPer1 and mPer2 genes.
In mammals, physiological and hormonal processes as well as behavioral reactions follow circadian rhythms that are driven by an endogenous master clock. The master clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and produces self-sustaining circadian rhythms that are synchronized by external cues. Components of the endogenous master clock were first identified in the fruit fly Drosophila melanogaster. The first genetically identified circadian mutant, period (Per) (1), encodes one of the essential elements involved in the transcription/translation-based autoregulatoy loop of the endogenous master clock (reviewed in ref. 2). Three homologues of Drosophila Per genes were subsequently identified in mice (mPer1, mPer2, and mPer3) (reviewed in ref. 3), leading to great progress in elucidation of the molecular mechanism underlying circadian rhythm in the SCN. Recent studies showed that the targeted mutation of the mPer1 gene leads to a shorter circadian rhythm under constant darkness in comparison to wild-type mice (4, 5). mPer2 mutant mice showed a complete loss of their rhythm when kept in constant darkness (5–7). The expression of mPer genes, however, is not restricted to the SCN, but they also are expressed in various brain regions as well as in peripheral tissues (8, 9). Although the importance of mPer1 and mPer2 genes in the SCN has been demonstrated (4–7), the relevance of mPer1 and mPer2 gene expression outside the SCN has not been fully elucidated.
Recently, it has been shown that psychostimulants such as methamphetamine affect the expression of Per genes outside of the SCN. Thus repeated administration of methamphetamine caused behavioral sensitization as well as sensitized expression of mPer1 but not of mPer2 in the striatum (10). These data implicate a role for Per genes in drug-induced behavioral sensitization processes. This suggestion is supported by investigations using Drosophila flies. Flies mutant in the Per gene did not sensitize after repeated exposure to volatilized free-base cocaine (11, 12). To further understand the role of Per genes in mediating behavioral responses to cocaine we first studied cocaine-induced behavioral sensitization and reward—two processes known to be involved in cocaine addiction—in mPer1 and mPer2 mutant mice. In addition, we investigated whether cocaine-induced behavioral sensitization and reward depend on the circadian time.
Materials and Methods
Animals.
Eight- to 10-week-old male wild-type mPer1 and mPer2 mutant animals, described in refs. 5 and 7, were used in our experiments. Mice were housed in groups of three or four and provided with food and water ad libitum. Artificial light was provided daily from 6 a.m. [Zeitgeber time (ZT)] to 6 p.m. (= ZT12) (12 h of light/12 h of darkness = LD12:12 cycle) with room temperature and humidity kept constant (temperature: 22 ± 1°C; humidity: 55 ± 5%). The experiments were approved by the Committee on Animal Care and Use of the relevant local governmental body and carried out following the German Law on the Protection of Animals.
Behavioral Sensitization.
After the measurement of basal locomotor activity, mice from each genotype (mPer1 mutant, mPer2 mutant, and wild type) were divided into three treatment groups (n = 9–15 per group). During the first day of the experiment, all animals were injected with saline at ZT4 (habituation phase). In the next 5 days, groups 1 and 2 continued to be injected with saline, whereas group 3 was injected with cocaine at ZT4. During the subsequent 3 days all groups were left untreated, and on the last day groups 2 and 3 received a cocaine injection and group 1 received a saline injection at ZT4. The mice were injected and immediately exposed to the activity chambers (True Scan, Coulborn, Allentown, PA) where locomotor activity was measured over a period of 30 min. For the measurement of long-term sensitization group 3 was challenged again with cocaine after 3 weeks. Cocaine was provided by Sigma and administered to the mice via i.p. injections in the HCl form. In pre-experiments different doses of cocaine (2.5, 5.0, and 10.0 mg/kg, i.p.) were tested in the appropriate wild-type mice. A dose of 10 mg/kg cocaine was chosen for all experiments because this dose produced a strong sensitized response.
Conditioned Place Preference (CPP).
To avoid the introduction of systematic errors, the CPP experiment was carried out in a light- and sound-controlled environment. To provide conditioned stimuli, the floors of six identical boxes (each with dimensions 32 × 16 × 22 cm) consisted of two sections into which tiles of two different surface textures were placed: the first of which had a “perforated” texture and the second had a “bar” texture of rods aligned in one direction. After each session, the boxes were cleaned with a damp cloth and carefully dried. The CPP was carried out continuously over 10 days and consisted of three different phases: habituation (one session), conditioning (eight sessions), and drug-free test (one session). For habituation all animals were injected with saline at ZT4 and immediately placed in the conditioning boxes for 30 min on a smooth wooden floor. Over the 8-day period of the conditioning phase, the mice of each genotype (n = 12–19; n = 3–4 per cage) were alternately injected with cocaine and saline solution at ZT4, and the floor type (i.e., perforated or bar) was alternated each day. For the drug-free test, on the final test day, the mice were left untreated and placed for 5 min in the conditioning boxes that had the two different floor types. The time that each mouse stayed on the floor type associated with the drug was measured.
Diurnal Differences in Behavioral Sensitization and Reward.
Male C57/BL6J mice 8 weeks old were divided into six experimental groups (n = 8–14 per group with 3–4 animals per cage). Artificial light was provided daily from 6 a.m. to 6 p.m. Three of these groups were tested for locomotor activity after injection of saline or cocaine, respectively, during the light phase at ZT4 (groups 1–3). The other three groups were tested during the dark phase at ZT12 (groups 4–6). Following the same protocol used for the mutant mice the animals were injected either with saline at the corresponding ZT (groups 1 and 4; n = 8) or cocaine (groups 3 and 6; n = 14) for 5 days and also after a 3-day withdrawal period. The animals in groups 2 and 5 (n = 10) were injected the first 5 days with saline and after a 3-day withdrawal period with cocaine (10 mg/kg).
For the place conditioning experiments male C57/BL6J mice 8 weeks old were divided into two experimental groups (n = 12 per group; n = 3 animals per cage). Conditioning sessions were conducted during the light phase at ZT4 (group 1) and during the dark phase at ZT12 (group 2) following the same protocol used for the mutant mice.
Data Analysis.
Statistical differences in locomotor activity were tested with a two-way ANOVA. Differences among individual means were verified subsequently by Newman–Keuls post hoc tests. The increase in locomotor activity after repeated cocaine injections was also calculated as percentage difference in activity to acute cocaine injection and was statistically tested by one-way ANOVA followed by Newman–Keuls post hoc tests. The diurnal rhythms of sensitization and CPP were analyzed with an unpaired t test.
Results
Cocaine-Induced Behavioral Sensitization.
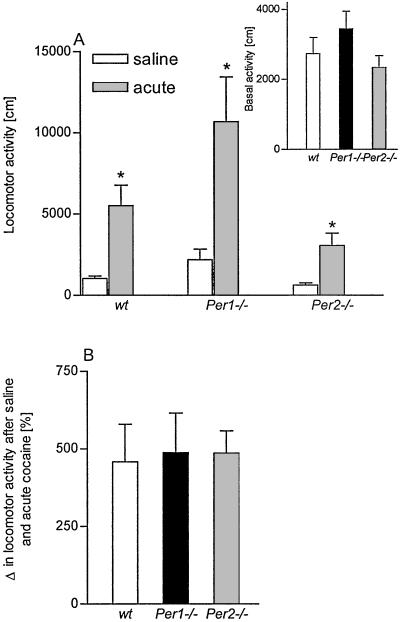
First, we measured basal locomotor activity in the activity boxes. mPer1 mutant mice exhibited a slightly enhanced locomotor response compared with wild-type and mPer2 mutants (Fig. 1A Inset). We further determined the locomotor activity in groups of mice that had received repeated saline injections. Although a tendency to increase the locomotor activity in mPer1 mutant mice was observed it was not significantly different from wild-type mice (P > 0.2) (Fig. 1A). In response to acute i.p. cocaine injections (10 mg/kg) wild-type, mPer1, and mPer2 mutant mice showed a ≈5-fold increase in activity from saline control levels (Fig. 1A). The relative induction of locomotor activity in response to acute cocaine administration was similar in all three genotypes (Fig. 1B).
Figure 1.
Basal locomotor activity and acute cocaine response in Per mutants. Locomotor responses to saline and single cocaine injections in wild-type (wt) mice or mice carrying a mutation in mPer1 or mPer2 genes are shown. (A) The graph shows the activity after saline and acute cocaine injections (* indicates a significant difference to saline treatment P < 0.05). (Inset) The basal locomotor response during a period of 30 min [F(2,36) = 1.38. P = 0.26]. (B) The increase in locomotor activity after an acute cocaine injection was also calculated as percentage difference in activity to a saline injection. One-way ANOVA did not show statistical differences between the three genotypes [F(2,39) = 0.046, P = 0.9]. All data are presented as mean ± SEM.
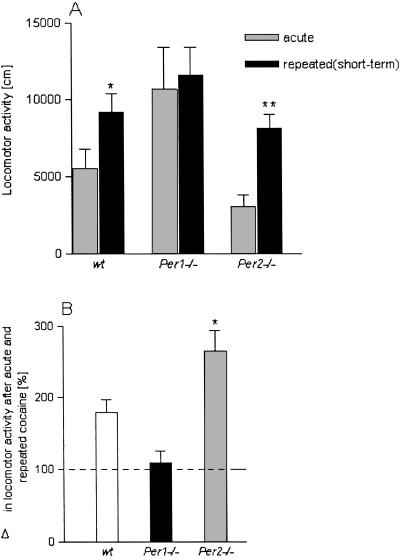
To assess the expression of cocaine sensitization in mPer1 and mPer2 mutant animals we measured the difference in locomotor activity after a cocaine challenge (10 mg/kg; i.p.) in mice that were treated repeatedly with saline or cocaine. After 3 days of withdrawal (short-term sensitization) wild-type mice exhibited a sensitized response whereas mPer1 mutant mice showed no difference after acute and repeated cocaine injections. mPer2 mutant animals displayed an enhanced expression of a sensitized behavioral response (Fig. 2A). Two-way ANOVA revealed that cocaine sensitization differed significantly for the different genotypes [interaction: treatment × genotype: F(2,71) = 7.57, P = 0.005]. Post hoc analysis is given in Fig. 2A. In addition, all three pairs of genotypes were tested for differences in mean increase after repeated vs. acute treatment. These second-order differences are shown in Fig. 2B.
Figure 2.
Short-term sensitization in Per mutants. Locomotor responses to acute and repeated saline/cocaine injections in wild-type (wt) mice or mice carrying a mutation in mPer1 or mPer2 genes after a 3-day withdrawal period are shown. (A) Mean locomotor activity ± SEM after acute cocaine and repeated cocaine injections (wt, n = 13–16; Per1 mutant, n = 9–10; and Per2 mutant, n = 14–15). Two-way ANOVA revealed that cocaine sensitization differed significantly for the different genotypes (see text). Further statistical comparisons between acute and repeated treatment showed the following differences for each genotype: wt, P = 0.04; Per1 mutant, P = 0.78; and Per2 mutant, P = 0.002. * indicates a significant difference to acute cocaine P < 0.05; **, P < 0.01. (B) The increase in locomotor activity after repeated cocaine injections was also calculated as percentage difference in activity to acute cocaine injection. The dashed line indicates mean activity after acute cocaine injection. One-way ANOVA revealed a genotype effect [F(2,36) = 9.5, P = 0.005]. * indicates a significant difference to wt mice, P < 0.05.
To rule out the possibility that a dose of 10 mg/kg of cocaine produced a maximal effect that would preclude further sensitization, a lower dose of 5 mg/kg of cocaine was also tested. The findings with the lower dose were consistent with those at the higher dose: wild-type mice were sensitized whereas mPer1 mutants showed no sensitization and mPer2 mutants showed a hypersensitized response (data not shown).
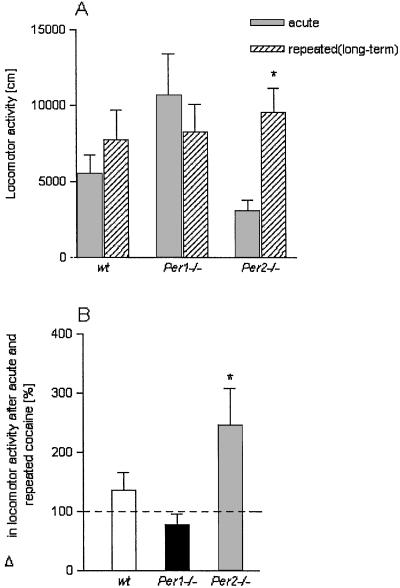
Sensitization to psychostimulants can remain present in rats and mice for weeks and even months (13). Therefore, we tested whether the sensitized response to cocaine was maintained in Per mutant mice after 3 weeks (long-term sensitization). We found that mPer1 mutants exhibited no sensitization whereas mPer2 mutant mice retained an enhanced sensitization in comparison with the group that received only an acute cocaine challenge injection (Fig. 3). Two-way ANOVA revealed that cocaine sensitization differed significantly for the different genotypes [interaction: treatment × genotype: F(2,52) = 4.16; P = 0.021]. Post hoc analysis is given in Fig. 3A. In addition, all three pairs of genotypes were tested for differences in mean increase after repeated vs. acute treatment. These second-order differences are shown in Fig. 3B.
Figure 3.
Long-term sensitization in Per mutants. Locomotor responses to acute and repeated saline/cocaine injections in wild-type (wt) mice or mice carrying a mutation in mPer1 and mPer2 genes after a withdrawal period of 3 weeks are shown. (A) Mean locomotor activity ± SEM after acute and repeated cocaine injections. Two-way ANOVA revealed that cocaine sensitization differed significantly for the different genotypes (see text). Further statistical comparison between acute and repeated (long-term) treatment for each genotype showed the following differences: wt, P = 0.37; Per1 mutant, P = 0.44; and Per2 mutant, P = 0.007. * indicates a significant difference to acute cocaine P < 0.01. (B) The increase in locomotor activity after repeated cocaine injections was also calculated as percentage difference in activity to acute cocaine injection. One-way ANOVA revealed a genotype effect [F(2,16) = 7.15, P = 0.007]. * indicates a significant difference to wt mice, P < 0.05.
Cocaine-Induced Reward.
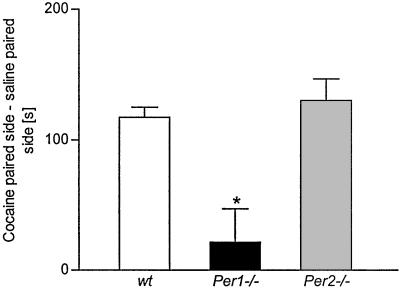
The CPP paradigm has been widely used to measure the rewarding properties of drugs of abuse such as cocaine (14). Therefore, we studied the response of the mPer1 and mPer2 mutant mice in the CPP paradigm to detect differences in cocaine-induced reward. As expected, wild-type animals showed a preference to the side that had been paired with cocaine administration (Fig. 4). However, the mPer1 mutant mice did not prefer the drug-paired side. In contrast, the mPer2 mutants spent more time on the drug-paired side although this response was not statistically different from wild-type animals. One-way ANOVA showed significant differences between genotypes [F(2,63) = 5.65, P = 0.005]. Post hoc analysis is given in Fig. 4.
Figure 4.
CPP in wild-type (wt) mice or mice carrying a mutation in mPer1 and mPer2 genes. Cocaine-induced place conditioning engendered opposite responses in Per1 mutant and Per2 mutant mice. Data are given as mean ± SEM of difference between time on cocaine-paired and saline-paired side. One-way ANOVA revealed significant differences for the factor genotype: F(2,63) = 5.65, P = 0.005. Post hoc analysis showed differences between wt and Per1 mutant (P = 0.029) and no difference between wt vs. Per2 mutant (P = 0.45), Per1 mutant vs. Per2 mutant P = 0.026. * indicates a significant difference to wt mice, P < 0.05.
Diurnal Differences in Behavioral Sensitization and Reward.
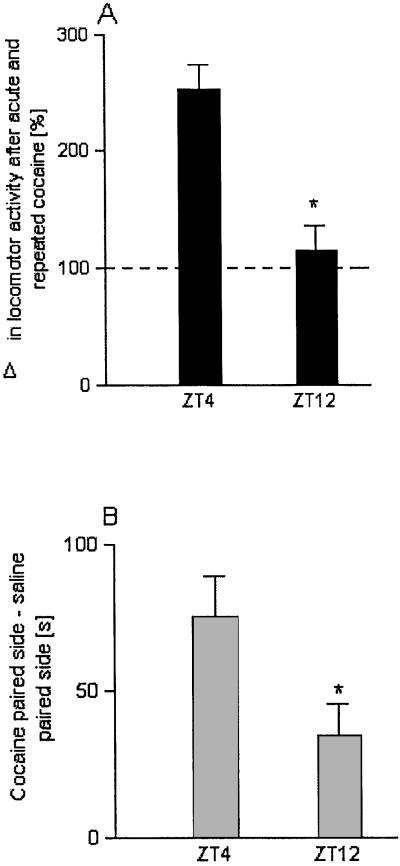
The same protocol used for the experiments with Per mutant mice was applied to study the expression of cocaine-induced sensitization at different time points in C57/BL6J mice. We have found that the expression of cocaine-induced behavioral sensitization depends on the time of day. A strong sensitization response was found in the morning hours during the light phase (ZT4), whereas poor sensitization was observed at the end of the light phase (ZT12) (Fig. 5A). This response was statistically different from the response at ZT4 (P = 0.0001).
Figure 5.
Diurnal differences in cocaine-induced behavioral sensitization and reward. (A) Locomotor activity was measured at different time points (ZT4 and ZT12) in C57/BL6J mice (n = 15 per group) after acute and repeated cocaine injections. The increase in locomotor activity after repeated cocaine injections was calculated as percentage difference in activity to acute cocaine injection. The dashed line indicates mean activity after acute cocaine injection. Unpaired t test reveals significant decrease in behavioral sensitization at the end of the light phase in comparison to sensitization at ZT4, P = 0.0001. (B) Cocaine-induced place preference measured at two different time points. At ZT4 place preference was significantly different from cocaine-induced place preference at ZT12, P = 0.028. * indicates significant difference to ZT4, P < 0.05.
Cocaine-induced CPP was also measured at different time points in C57/BL6J mice by using the same protocol as for the mutant mice. At ZT4 a clear place preference for cocaine could be detected whereas at ZT12 cocaine-induced CPP was only weakly expressed. This decrease in cocaine-induced place preference was significant (P = 0.0282) (Fig. 5B).
Discussion
Several clock genes including the Per genes have been cloned in the last few years (reviewed in ref. 3). Our current molecular understanding of how clock genes interact with each other and thereby influence a variety of physiological and behavioral responses is based on studies in mice carrying targeted mutations in these genes (4–7, 15, 16). In the present study we show that the lack of Per genes in mice results in significant alterations in behavioral responses to repeated cocaine administration.
Acute treatment of cocaine leads to psychomotoric stimulation in mice. After repeated administration this behavioral response is further increased. This phenomenon is known as behavioral sensitization and has been implicated in cocaine craving and relapse (17, 18). mPer1 and mPer2 mutant mice exhibited opposing behavioral responses to repeated administration of cocaine. However, stimulating effects seen after a single acute cocaine administration in mPer1 and mPer2 mutant mice did not differ from that in wild-type animals, demonstrating that acute and chronic effects of cocaine are under the control of different sets of genes. After repeated cocaine administration a lack of behavioral sensitization in mPer1 mutant mice was observed. A similar behavioral response in terms of stimulation of locomotor activity has been reported in Drosophila flies that carry a mutation in the period (pero) gene. These mutant flies did not sensitize after repeated exposures either to 75 or 100 μg of volatilized free-base cocaine (11). In contrast to mPer1 mutants, mice carrying a mutation in the mPer2 gene exhibited a great difference in response to repeated cocaine administration. Thus mPer2 mutant mice showed a hypersensitized response to cocaine in comparison to wild-type mice. Consistent with these results, mPer1 mutants animals did not show a preference to the cocaine-paired side in the CPP paradigm in comparison with the wild-type mice. In contrast, mPer2 mutants displayed a pronounced preference to the cocaine-paired side. These experiments indicate that cocaine sensitization is modulated in an opposing manner by Per1 and Per2 genes and that these genes also influence rewarding properties of cocaine. It should be pointed out that behavioral sensitization and place conditioning induced by cocaine involve learning processes. Thus the observations made in our experiments also could be explained by differences in the learning abilities of the three genotypes. However, mPer1 and mPer2 mutants did not differ from wild-type animals in a fear conditioning paradigm, demonstrating that Per mutants do not differ in their learning abilities from wild-type animals (unpublished observations).
Per genes and proteins are expressed in the SCN and other brain areas in a diurnal manner (19–21). Hence, we tested whether cocaine sensitization and reward depends on the diurnal rhythm. We found that the degree of response to cocaine-induced behavioral sensitization and reward are under the influence of the diurnal state of the animal (Fig. 5). Thus the expression of a sensitized response to cocaine is time dependent with the most robust sensitization and reward occurring during the early light phase when the level of mPer1 gene expression is maximal (ZT4) (22, 23). Poor sensitization and reward were observed at the end of the light phase (ZT12) when mPer1 gene expression is low (22, 23). A similar time-dependent profile was reported in methylphenidate-sensitized animals (ref. 24 but see also ref. 25). These results indicate that cocaine sensitization and reward seem to be influenced at least partially by the expression of mPer1.
A major question that remains to be solved is: which are the brain areas that modulate cocaine sensitization and also reward and express Per genes? There is no indication from the literature that the SCN is involved in cocaine responses. The recent finding that repeated injections of methamphetamine induces mPer1 gene expression in the striatum but not in the SCN (10) suggests that expression of Per genes in brain areas mediating drug-induced sensitization and reward influence responsiveness to cocaine.
At the present it is not known which molecular mechanisms are involved in the different responses to cocaine in mice carrying a mutation in the mPer1 and mPer2 genes. However, it is known that different components of the midbrain dopamine system and the glutamatergic system play a critical role in cocaine-induced sensitization and reward (26–28). The dopamine transporter (DAT) is the primary target of cocaine, and changes in DAT function caused by the Per mutations might explain the observed alterations in sensitization and reward. However, because we have not found differences in acute cocaine-induced locomotor activity in the three genotypes we conclude that neither a knockout of the mPer1 nor mutation of the mPer2 gene leads to functional changes of the DAT. Dopamine D1 and D2 receptors are other components of the midbrain dopamine system that are critically involved in cocaine sensitization and reward (26–28). D1 binding studies revealed no differences in Kd and Bmax values in mPer1 and mPer2 mutants in comparison to wild-type animals (unpublished data), but it has been found that D2 receptor responsiveness in Drosophila is modulated by per-dependent molecular oscillators (29). In addition N-methyl-d-aspartate (NMDA) receptor function also might be influenced by Per genes. It has been demonstrated that methamphetamine-induced increase in mPer1 mRNA was antagonized by pretreatment with a NMDA receptor antagonist (10), suggesting a link between NMDA receptors and mPer1 gene function and possibly vice versa.
In summary, the finding that the circadian clock modulates cocaine responses in flies can be extended to mammals. Our data show that the mPer1 and mPer2 genes influence cocaine-induced sensitization and reward in a opposite manner. This finding is comparable to the opposing roles of mPer1 and mPer2 in light-mediated phase shifting of the circadian clock (30). The lack or dysfunction of the mPer1 gene abolishes cocaine sensitization and reward whereas the dysfunction of the mPer2 gene induces a hypersensitized response to cocaine. This finding indicates that mPer1 and mPer2 can control distinct sets of target genes (5).
Acknowledgments
We thank J. Hirsh for helpful discussions and J. L. Dreyer, F. A. Henn, and M. Cowen for helpful comments on the manuscript. This work was supported by the Max-Planck-Society (F. Holsboer and G. Eichele), Bundesministerium für Bildung und Forschung Grant FKZ 01GS0117 (to R.S.), and Deutsche Forschungsgemeinschaft Grant Al 549/1-1 (to U.A.).
Abbreviations
- CPP
conditioned place preference
- Per
period
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Konopka R J, Benzer S. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert S M, Weaver D R. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht U. J Appl Physiol. 2002;92:1348–1355. doi: 10.1152/japplphysiol.00759.2001. [DOI] [PubMed] [Google Scholar]
- 4.Cermakian N, Monaco L, Pando M P, Dierich A, Sassone-Corsi P. EMBO J. 2001;20:3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun Z S, Eichele G, Bradley A, Lee C C. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 6.Bae K, Jin X, Maywood E S, Hastings M H, Reppert S M, Weaver D R. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 7.Zheng B, Larkin D W, Albrecht U, Sun Z S, Sage M, Eichele G, Lee C C, Bradley A. Nature (London) 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 8.Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, Honma K. Neurosci Lett. 1999;267:69–72. doi: 10.1016/s0304-3940(99)00324-9. [DOI] [PubMed] [Google Scholar]
- 9.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 10.Nikaido T, Akiyama M, Moriya T, Shibata S. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- 11.Andretic R, Chaney S, Hirsh J. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 12.Hirsh J. Pharmacogenomics J. 2001;1:97–100. doi: 10.1038/sj.tpj.6500020. [DOI] [PubMed] [Google Scholar]
- 13.Vanderschuren L J, Schmidt E D, De Vries T J, Van Moorsel C A, Tilders F J, Schoffelmeer A N. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzschentke T M. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 15.van der Horst G T J, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker A P M, van Leenen D, et al. Nature (London) 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 16.Vitaterna M H, Selby C P, Todo T, Niwa H, Thompson C, Fruechte E M, Hitomi K, Thresher R J, Ishikawa T, Miyazaki J, et al. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman A, Gilman A. In: The Pharmacological Basis of Therapeutics. Hardman J, Limbird L, Molinoff P, Ruddon R, editors. New York: McGraw–Hill; 1996. pp. 557–577. [Google Scholar]
- 18.Robinson T E, Berridge K. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 19.Takumi T, Matsubara C, Shigeyoshi Y, Taguchi K, Yagita K, Maebayashi Y, Sakakida Y, Okumura K, Takashima N, Okamura H. Genes Cells. 1998;3:167–176. doi: 10.1046/j.1365-2443.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 20.Hastings M H, Field M D, Maywood E S, Weaver D R, Reppert S M. J Neurosci. 1999;19:RC11–RC15. doi: 10.1523/JNEUROSCI.19-12-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field M D, Maywood E S, O'Brien J A, Weaver D R, Reppert S M, Hastings M H. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- 22.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Nature (London) 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z S, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee C C. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 24.Gaytan O, Lewis C, Swann A, Dafny N. Eur J Pharmacol. 1999;374:1–9. doi: 10.1016/s0014-2999(99)00243-5. [DOI] [PubMed] [Google Scholar]
- 25.Gaytan O, Yang P, Swann A, Dafny N. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 26.Spanagel R, Weiss F. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 27.Vanderschuren L J, Kalivas P W. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 28.Cornish J L, Kalivas P W. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- 29.Andretic R, Hirsh J. Proc Natl Acad Sci USA. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albrecht U, Zheng B, Larkin D, Sun Z S, Lee C C. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]