Abstract
Sexual maturity significantly impacts poultry production efficiency, yet data on testicular development and regulatory mechanisms in indigenous breeds remain limited. In this study, we examined Xianghuang chickens, a indigenous early-maturing breed, to investigate the hypothalamus-pituitary-testis (HPT) axis. Hypothalamus, pituitary, and testis tissues were collected at 80 and 120 days post-hatch (dph) for analysis. Results showed that testicular weight and index were significantly higher in 120dph compared to 80dph chickens. Histologically, the testes at 120dph contained multi-layered spermatocytes and mature sperm while the testes at 80dph only had 1–2 layers of spermatocytes. Weighted Gene Co-expression Network Analysis of transcriptome data clustered gene sets into 11 modules including those with testis-specific high expression, 80dph testis-specific high expression, and 120dph testis high expression. Differential expression analysis identified 26, 681, and 11,999 differentially expressed genes (DEGs) in the hypothalamus, pituitary, and testes respectively between the two age groups with 16 DEGs shared among the three tissues. DEGs in all three tissues were enriched in pathways such as cytoskeleton in muscle cells, ECM-receptor interaction, and focal adhesion highlighting the importance of HPT axis tissue remodeling during this period. Hormone-related pathways (GnRH signaling, melanogenesis, and progesterone-mediated oocyte maturation) were enriched with DEGs in the pituitary and testes between 80dph and 120dph . Concurrently the pituitary and testes exhibited distinct lipid metabolic adaptations through enrichment of PPAR, adipocytokine, and insulin signaling pathways. These adaptations provided substrates for sperm membrane formation and testosterone synthesis while supporting spermatogenesis and cellular functions. Collectively these findings clarify the morphological, histological, and molecular regulatory mechanisms underlying testicular development and sexual maturation in Xianghuang chickens and offer a theoretical basis for optimizing poultry breeding strategies.
Keywords: Chicken, Sexual maturity, Testis, HPT axis
Introduction
Reproductive development in vertebrates is hierarchically regulated by the hypothalamus-pituitary-gonadal (HPG) axis, with testicular maturation constituting the terminal effector stage that critically determines spermatogenic efficiency and male fertility (Kaprara and Huhtaniemi, 2018; Sharma et al., 2022). As economically important poultry species, the timing of sexual maturity in domestic fowl has garnered significant attention from breeders due to its direct impact on production efficiency (Qi, et al., 2024; Shi, et al., 2021). The Xianghuang chicken (also called Huanglang chicken), a distinctive indigenous breed in China, demonstrates desirable traits including early sexual maturity and consistent reproductive performance. However, the molecular mechanisms governing testicular development in this breed remain incompletely characterized.
Although transcriptomic advances enable tissue-specific gene network mapping, particularly for developmental dynamics (Ouyang, et al., 2021b; Wang, et al., 2023), current research disproportionately focuses on mammalian models or isolated tissues—creating a critical gap in developmental stage-specific transcriptomes of hypothalamus-pituitary-testis (HPT) axis coordination in male poultry. Existing avian studies emphasize environmental modulators over fundamental developmental mechanisms (Akhtar, et al., 2019; Bartman, et al., 2021; Ouyang, et al., 2021a). Preliminary identification of testicular development genes (Guo, et al., 2024; Liu, et al., 2024; Yan, et al., 2024) relies predominantly on static timepoints or single-tissue approaches, neglecting hypothalamus-pituitary upstream regulation and multi-tissue crosstalk. This contrasts markedly with integrative female HPG axis research on broodiness and oviposition (He, et al., 2024; Tong, et al., 2025; Xin, et al., 2024). Crucially, two knowledge deficits persist: uncharacterized dynamic gene expression during sexual maturation initial stage and absent system-level analyses of sexual maturity regulation in indigenous breeds. These limitations impede poultry breeding optimization and obscure avian reproductive evolution.
To address these gaps, we investigate the Xianghuang chicken HPT axis by collecting hypothalamus, pituitary, and testis tissues at two developmental milestones: 80 days post-hatch (dph) and 120dph. Through a combination of multi-tissue RNA-seq, weighted gene co-expression network analysis (WGCNA), and cross-tissue pathway integration, this study investigated how the multi-tissue network of the hypothalamus-pituitary-testis (HPT) axis dynamically regulates the transition of testicular development during sexual maturation initial stage in Xianghuang chickens.
Materials and methods
Animals and sample collection
The Xianghuang chicken used in this study were sourced from the Hunan Xiangjia Husbandry Limited by Share Ltd Company (Changde, Hunan, China). A cohort of 880 fertilized Xianghuang chicken eggs were incubated under standard conditions. From the resulting hatch, 400 male chicks with comparable body weights were randomly selected and reared in controlled-environment cages with ad libitum access to water and standard diet. At 80dph and 120dph, twenty male chickens per age group were fasted for 12 h, anesthetized with sodium pentobarbital, euthanized by cervical dislocation, and immediately necropsied. Bilateral testes were excised and weighed using a precision balance, with the right testis flash-frozen in liquid nitrogen within 5 min of excision for transcriptomic analysis, while the left testis was immersion-fixed in 4 % paraformaldehyde at 4°C for 48 h for histological processing. Concurrently, hypothalamus and pituitary tissues were collected, snap-frozen in liquid nitrogen, and stored at −80°C. All procedures were approved by the Animal Ethics Committee of Hunan Agricultural University (Protocol No. HAU ACC 2024DKJQ022) following ARRIVE guidelines.
Testicular morphological analysis
Following dehydration, clearing, and paraffin embedding, testicular tissues (n = 10 in each group) were sectioned at 3-5 μm thickness using a rotary microtome. Sections were floated on a 40°C water bath for expansion, mounted onto adhesive glass slides, and dried overnight at 37°C. After deparaffinization and rehydration, sections underwent standard hematoxylin and eosin (H&E) staining, followed by graded alcohol dehydration and DPX mounting. Histological examination was performed using a Nikon Eclipse E100 brightfield microscope, with images captured for subsequent analysis. Quantitative morphometric analysis was conducted in KFSlideOS digital pathology system, where three non-overlapping fields per section were systematically evaluated. Measurements included seminiferous tubule luminal perimeter, cross-sectional area, and germ cell counts, with mean values calculated. Comparative analysis between 80dph and 120dph groups assessed the effects of age on testicular morphological.
Transcriptome sequencing and data analysis
Total RNA was isolated from hypothalamus, pituitary, and testis tissues (n = 10 in each group/per tissue) using TRIzol reagent (Invitrogen) based on phenol-guanidine isothiocyanate methodology. Post-extraction, RNA pellets were dissolved in nuclease-free DEPC-treated water and quantified via Qubit 4.0 Fluorometer (Thermo Fisher Scientific) with the Broad Range RNA kit. RNA integrity was assessed using the Qsep400 Bio-Fragment Analyzer (BiOptic Inc.), where samples with RNA Integrity Number (RIN) ≥ 8.0 and 28S/18S ratios > 1.8 were considered suitable for library construction. The qualified RNA samples were used for library construction. The mRNA libraries were sequenced by Metware Co., Ltd. (Wuhan, China) using MGI sequencing platform. The raw data have been deposited into the NCBl SRA database under the BioProject Number: PRJNA1296760. Raw data was filtered using fastp to remove reads with adapters: when the number of N in any sequencing read exceeded 10 % of the length of that read, the paired reads were removed; when any sequencing read contained low-quality bases (Q ≤ 20) exceeding 50 % of the length of that read, the paired reads were also removed. Subsequent analyses were performed using clean reads. The reference genome and its annotation files were downloaded from https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_016699485.2/. HISAT was used to build an index, and clean reads were aligned to the reference genome. Novel gene prediction was performed using StringTie, which employs a network flow algorithm and optional de novo transcript assembly to assemble transcripts.FeatureCounts was used to calculate gene alignment statistics, and then FPKM (Fragments Per Kilobase Million) values for each gene were calculated based on gene length. DEseq2 was used to identify the differentially expressed genes (DEGs) among different groups, the screening criteria were |log2Foldchange| > 1, q-value < 0.05. Enrichment analysis was performed based on the hypergeometric test, with pathway-based hypergeometric distribution testing for KEGG. Analysis of protein interactions for DEGs was based on the STRING database (https://cn.string-db.org), Cytoscape is used to visualize the results of protein-protein interaction (PPI). WGCNA was performed for weighted gene co-expression network analysis.
Results and analysis
Developmental progression of testicular morphology and spermatogenesis
As shown in Figs. 1A and 1C, the testis of 120dph chickens were significantly larger in size, heavier in weight, and exhibited a markedly higher gonadosomatic index compared to those of 80dph chickens (P < 0.0001). Subsequent histological analysis of the testes from both age groups (Fig. 1B) revealed that the seminiferous tubules in 80dph chickens contained fewer germ cell layers, typically only 1-2 layers. In contrast, the seminiferous tubules of 120dph chickens displayed a greater number and variety of germ cell layers (spermatogonia, spermatids, and spermatozoa), and mature spermatozoa were exclusively observed at this age. Morphometric analysis indicated that both the seminiferous tubule luminal perimeter and cross-sectional area increased significantly (P < 0.0001) during development from 80dph to 120dph. Furthermore, quantification of germ cells within the seminiferous tubule lumens demonstrated a significantly higher germ cell count in 120dph chickens (P < 0.0001). Additionally, the number of Sertoli cells also showed a significant increase in 120dph chickens (P < 0.001).
Fig. 1.
Comparative anatomy and histology of testis in Xianghuang chickens at 80dph and 120dph. (A) Representative gross anatomical images of testis. (B) H&E-stained histological sections of testis from 80dph (left) and 120dph (right) chickens. (C) Quantitative analysis of testicular parameters. ST: seminiferous Tubules, SC: Sertoli Cells, SM: spermatogonia, LC: Leydig Cells, SD: spermatids, SA: spermatozoa (Mature Sperm).
Data are presented as mean ± SD. **** P<0.0001 and *** P<0.001 by unpaired Student's t-test.
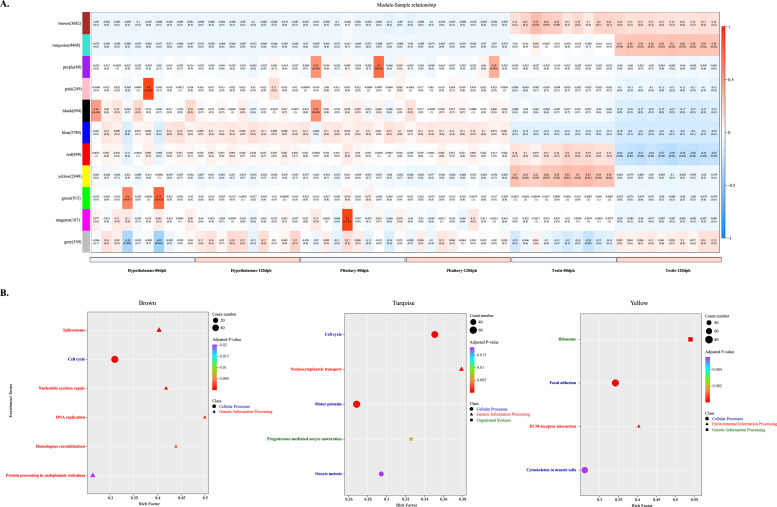
WGCNA analysis reveals tissue-specific module characteristics
A total of 3,714,074,594 raw reads were obtained through sequencing, and 3,660,042,648 clean reads remained after quality control. Meanwhile, the Q20 and Q30 values ranged from 99.09 % to 99.39 % and from 96.56 % to 97.84 %, respectively, and the average mapping rate of all samples was 94.89 % (Supplementary table.1). Co-expression networks were constructed using WGCNA with a minimum module size of 50, mergeCutHeight of 0.25, and soft-thresholding power of 11 to achieve scale-free topology. This partitioned expressed genes into 11 distinct modules (Fig. 2A), the gene list of all WGCNA modules was shown in Supplementary table.2. Three developmentally significant modules exhibited tissue- and age-specific expression patterns: The brown module (testis-specific expression) exhibited significant enrichment for spliceosome, cell cycle, nucleotide excision repair, DNA replication, homologous recombination, and endoplasmic reticulum protein processing. The turquoise module (120dph testis-enriched) showed enrichment in cell cycle, nucleocytoplasmic transport, motor proteins, progesterone-mediated oocyte maturation, and oocyte meiosis. Conversely, the yellow module (80dph testis-enriched) demonstrated enrichment for ribosome, focal adhesion, ECM-receptor interaction, and cytoskeleton regulation in muscle cells (Fig. 2B).
Fig. 2.
WGCNA of HPT axis tissues. (A) Module-sample correlation analysis of HPT axis transcriptomes. Heatmap depicting Pearson correlations between gene co-expression modules (y-axis) and tissue samples (x-axis). Color gradient (blue-white-red) represents correlation coefficients from −1.0 (strong negative) to 1.0 (strong positive). (B) KEGG pathway enrichment of key modules. Bubble plots showing significantly enriched pathways for brown, turquoise, and yellow module.
HPT axis transcriptional dynamics during sexual maturation
As shown in Fig. 3A, the expression profiles of the hypothalamus and pituitary tissues clustered in close proximity within the PCA plot, while the testis tissue was distinctly separated from both brain tissues. Furthermore, testicular tissues from 120dph and 80dph Xianghuang chicken were completely separated in the PCA results, whereas hypothalamus and pituitary tissues could not be clearly distinguished by age. As shown in Supplementary table.3, 26, 681, and 11,999 DEGs were identified in the hypothalamus, pituitary, and testis, respectively, when comparing 120dph to 80dph Xianghuang chickens (Fig. 3B). Notably, the expression levels of LAMB1, NID1, APOLD1, KLF2, COL3A1, COL1A2, PLN, C7, LOC107052718, COL6A2, COL6A1, PI15, C8orf4, PDK4, COL4A5, and LOC121106447 were significantly downregulated in the hypothalamus, pituitary, and testis of 120dph chickens compared to 80dph chickens (Fig. 3C). These genes were enriched in the ECM-receptor interaction, Focal adhesion, and AGE-RAGE signaling pathway in diabetic complications KEGG pathways. Subsequently, these 16 genes were used to construct a PPI network, revealed that COL3A1, COL6A1, COL6A2, and LAMB1 occupied core positions within the network (Supplementary Fig..1).
Fig. 3.
Differential expression profiles within the HPT axis of Xianghuang chickens at 80dph and 120dph. (A) Principal component analysis (PCA) of all tissues. (B) Bar chart showing the number of DEGs in the hypothalamus, pituitary, and testis. (C) Venn diagram illustrating DEGs in the hypothalamus, pituitary, and testis.
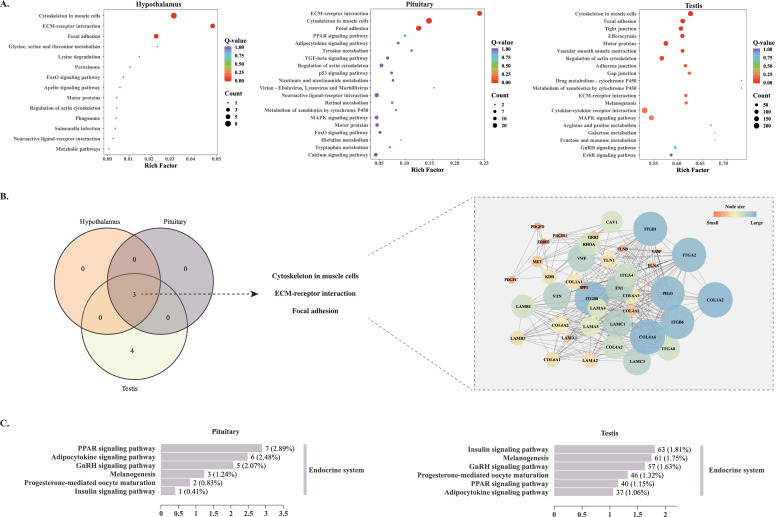
Functional enrichment reveals hierarchical pathway coordination in the HPT axis
The KEGG enrichment results of all genes are shown in Supplementary table.4. Bubble plots depicting the KEGG pathway enrichment of DEGs in the hypothalamus, pituitary, and testis are presented in Figs. 4A. For clarity, only the top 20 enriched KEGG pathways are visualized for the pituitary and testis. As shown in Fig. 4B, DEGs across all three tissues (hypothalamus, pituitary, and testis) were significantly enriched in the Cytoskeleton in muscle cells, ECM-receptor interaction, and Focal adhesion signaling pathways. Furthermore, DEGs specific to testis tissue showed significant enrichment in the Tight junction, Efferocytosis, Motor proteins, and Vascular smooth muscle contraction signaling pathways. To identify core regulatory elements, DEGs associated with the Cytoskeleton in muscle cells, ECM-receptor interaction, and Focal adhesion pathways were used to construct a PPI network. Subsequent analysis using the MCODE algorithm revealed ITGB1, ITGB8, ITGB6, ITGA2, PELO, COL1A2, and COL4A6 as core genes within this network. In addition, the DEGs in pituitary and testis tissues are enriched in endocrine related pathways, including PPAR signaling pathway, Adipocytokine signaling pathway, GnRH signaling pathway, Melanogenesis, Progesterone-mediated oocyte maturation and the Insulin signaling pathway (Fig. 4C).
Fig. 4.
Functional enrichment analysis of DEGs in the HPT axis of Xianghuang chickens at 80dph and 120dph. (A) Bubble plots of KEGG pathway enrichment for DEGs in the hypothalamus, pituitary, and testis. (B) Venn diagram of significantly enriched KEGG pathways shared among hypothalamus, pituitary, and testis DEGs, with the core PPI network of key pathways. (C) KEGG pathways related to the endocrine system enriched by DEGs in the pituitary and testis.
Discussion
Testicular morphology findings confirm the accelerated testicular maturation trajectory in Xianghuang chickens during the sexual maturation initial stage (80dph-120dph). The significant increases in testis weight and testis index (P < 0.0001) align with prior reports linking these metrics to functional sexual maturity in avian species (Qi, et al., 2024). Histologically, the progression from limited spermatogenic layers (1–2 cells) at 80dph to multi-layered organization with spermatid formation at 120dph demonstrates complete activation of the spermatogenic (Mfoundou, et al., 2022; Udoumoh, et al., 2021). Notably, the sperm began to appear in the testis of 120dph Xianghuang chickens, which is consistent with the early maturity trait selected for this breed. Collectively, these structural benchmarks establish a phenotypic foundation for our transcriptomic analysis.
Our transcriptomic landscape analysis identified three developmentally significant modules (brown, turquoise, yellow) that illuminate stage-specific regulatory mechanisms during testicular maturation in Xianghuang chickens. The brown module's testis-specific enrichment of spliceosome, DNA repair, and cell cycle pathways aligns with the high transcriptional activity and genomic integrity requirements during spermatogenesis. Spliceosome hyperactivity maintains transcriptome plasticity through precise processing of germ cell-specific alternative splicing events, ensuring exon selection fidelity across spermatogenic stages from spermatogonial proliferation to spermiogenic transformation (Ma et al., 2024; Qin, et al., 2023). DNA repair pathway dominance addresses inherent genomic vulnerability from meiosis-induced double-strand breaks and elevated oxidative stress, preventing transgenerational mutation transmission via timely lesion repair (Bisht, et al., 2017; Hashemi Karoii, et al., 2022). Furthermore, testis-enriched cyclin expression sustains the periodic regeneration rhythm of spermatogenesis, coordinating mitotic spermatogonia division, meiotic spermatocyte development, and spermatid differentiation to ensure daily production of millions of spermatozoa (Franca, et al., 1998; Roy Choudhury, et al., 2010).
The hypothalamus serves as the central hub of the neuroendocrine system, responsible for maintaining homeostasis in key physiological processes such as feeding and reproduction (Clarke, 2015). Consistent with previous findings in avian species (Lin, et al., 2023; Tang, et al., 2022), our differential expression analysis revealed a significantly lower number of DEGs in the hypothalamus compared to the pituitary and testis between the two developmental stages. This indicates that its core regulatory network may be largely established or relatively stable during this period. Its regulatory role likely manifests more in transmitting instructions to the pituitary or performing fine-tuning adjustments, rather than undergoing large-scale transcriptional reprogramming itself. Studies in human have similarly shown that the HPG axis becomes relatively inactive after the mini-puberty phase until the onset of puberty (Becker and Hesse, 2020), suggesting that the HPG axis in poultry exhibits spatiotemporal activity patterns analogous to those in mammals. Concurrently, the testis undergoes key physiological changes during puberty, including seminiferous tubule maturation, blood-testis barrier (BTB) formation, and the initiation of spermatogenesis. The identification of 11,999 DEGs reflects the dramatic shift of the testis from an "immature" to a "functionally active" state. The complete separation by age in the PCA plot further confirms the stage-specific transcriptional remodeling of testicular development.
During sexual maturation, DEGs in the hypothalamus, pituitary and testis jointly enriched in the Cytoskeleton in muscle cells, ECM–receptor interaction and focal-adhesion pathways. The same three pathways are also significantly enriched among the genes that WGCNA pinpointed as specifically up-regulated in 80dph testis, suggesting that these tissues are undergoing an active phase of structural remodelling at this age (Kanchanawong and Calderwood, 2023; Ohanian, et al., 2015; Wlodarczyk, et al., 2011). Across the three organs we identified 16 shared hub genes (LAMB1, NID1, APOLD1, KLF2, COL3A1, COL1A2, PLN, C7, LOC107052718, COL6A2, COL6A1, PI15, C8orf4, PDK4, COL4A5 and LOC121106447) whose defining feature is a striking over-representation of extracellular-matrix (ECM) and cell–matrix adhesion processes. Several of these genes encode structural collagens (COL1A2, COL3A1, COL4A5, COL6A1, and COL6A2) or the laminin subunit LAMB1, while NID1 is a key basement-membrane component (Hohenester, 2019). APOLD1, linked to angiogenesis (Pays, 2024), and KLF2, a regulator of blood-flow and endothelial function (Kotlyarov and Kotlyarova, 2023), are likewise associated with vascular and stromal micro-environments. Within the PPI network, COL3A1, COL6A1, COL6A2, and LAMB1 emerge as central hubs, underscoring the pivotal role of ECM constituents among the co-down-regulated genes. Collectively, our data indicate that the 80dph testis (and, by extension, the hypothalamus and pituitary) mounts a robust ECM-synthetic programme to support tissue expansion and architectural assembly. By 120dph, the tissue framework has stabilised, ECM demand subsides, and expression of these genes declines accordingly.
Within the PPI network constructed from DEGs enriched in the Cytoskeleton in muscle cells, ECM–receptor interaction, and focal adhesion pathways, integrin family members (ITGB1, ITGB8, ITGB6, ITGA2) play pivotal roles. As a universal subunit of the β1 family, ITGB1 mediates adhesion between spermatogonial stem cells (SSCs) and Sertoli cells, as well as between Sertoli cells and the basement membrane. This interaction maintains BTB integrity and activates the PI3K-Akt pathway, thereby balancing SSC self-renewal and differentiation (Wang, et al., 2019). Furthermore, given the conserved role of ITGB6 in mediating tripartite regulation of ECM remodeling, immune response, and vascular function in organs such as the lung (Puthawala, et al., 2008), it is plausible that ITGB6 contributes to basement membrane maturation and modulation of immune cell infiltration during gonadal maturation.
DEGs in the pituitary and testis between 80dph and 120dph were enriched in hormone-related pathways, including the GnRH signaling pathway, Melanogenesis, and Progesterone-mediated oocyte maturation. This suggests that during sexual maturation initial stage (80dph-120dph), the pituitary gland relays hormonal signals, while the testes act as executors of these signals. In contrast, the hypothalamus maintains relatively stable function throughout this period. In the pituitary, sensitivity to hypothalamic GnRH is low at 80dph. By 120dph, the upregulation of GNRH1 (the initiating signaling molecule of the GnRH pathway) enhances responsiveness to GnRH signals, thereby increasing gonadotropin secretion to drive sexual maturation (Duittoz, et al., 2022). Notably, the expression of CGA, the common α-subunit of glycoprotein hormones such as LH and FSH (Bieche, et al., 2002), is significantly downregulated in the 120dph pituitary. This reduction may reflect a shift in gonadotropin secretion regulation, where secretion becomes more dependent on the specific β-subunits rather than the total abundance of the α-subunit. The GnRH pathway regulates testicular function indirectly: pituitary-secreted LH and FSH act on the testis, while local paracrine/autocrine regulation of the GnRH pathway may also occur within the testis. The differential gene expression in the 120dph testis reflects enhanced responsiveness to pituitary gonadotropin signals, alongside maturation of spermatogenesis and steroidogenic functions. Compared to 80dph, PLCB1/PLCB4 (phospholipase Cβ) and ADCY1/ADCY8 (adenylate cyclase) are significantly upregulated in the 120dph testis. The upregulation of these enzymes, which are key downstream effectors of G protein-coupled receptors (Gresset, et al., 2012; Jaiswal and Conti, 2003), signifies an increased responsiveness of testicular cells to LH/FSH signals. This allows for more efficient conversion of hormonal signals into intracellular second messengers providing impetus for testosterone synthesis and spermatogenic support. As downstream effectors of calcium signaling, CAMK2A/CAMK2D (calcium/calmodulin-dependent kinase 2) are involved in regulating cell proliferation, differentiation, and gene transcription (Ignotz and Suarez, 2005; Sun, et al., 2010). Their upregulation suggests potentiated calcium signaling in the testis, which may directly promote testosterone synthesis in Leydig cells or enhance nutrient supply from Sertoli cells to developing spermatogenic cells. Although named for its role in female oocyte maturation, the Progesterone-mediated oocyte maturation pathway exhibits highly conserved functionality in males. Core molecules such as CDK1 (Clement, et al., 2015), PLK1 (Wellard, et al., 2022), and PI3K/MAPK signaling components (Deng, et al., 2021; Maher, et al., 2014) are reused in regulating meiosis of male spermatocytes. By modulating cell cycle transitions and signal transduction, these molecules support the maturation of male germ cells, highlighting the evolutionary conservation of key regulatory mechanisms across sexes in reproductive development.
The pituitary-testis axis undergoes endocrine-regulated energy metabolic remodeling during sexual maturation initial stage, driven by PPAR, Adipocytokine, and Insulin signaling pathways. The pituitary gland appears to reduce its own energy consumption and redirect systemic metabolism toward supporting reproductive functions through coordinated adjustments: inhibiting its own fatty acid uptake and transport (as indicated by the downregulation of FABP4 (Lv, et al., 2023), CD36 (Li, et al., 2022), SLC27A6 (Zhang, et al., 2021), etc.), enhancing lipid catabolism and utilization (reflected by the upregulation of APOC3 and downregulation of LPL (Gaudet, et al., 2014)), and regulating energy intake (via upregulation of PPY (Perez-Frances, et al., 2021)). In the testis at 120dph, the upregulated genes ACSBG2 and ACSL3/4/6, which catalyze fatty acid activation—the initial step in fatty acid synthesis or degradation (Soupene and Kuypers, 2008)—suggest enhanced testicular lipid metabolism, potentially providing substrates for sperm membrane formation or testosterone biosynthesis. PPARG and RXRG, as core regulators of lipid metabolism, can activate genes involved in fatty acid uptake and storage; their upregulation may promote lipid accumulation in testicular Sertoli cells or Leydig cells, thereby supporting spermatogenesis (Esmaeili, et al., 2015). Conversely, the expression levels of genes associated with fatty acid oxidation, such as CPT1A (Ran, et al., 2023) and ADIPOQ (Lee, et al., 2013), were significantly downregulated in the testis at 120 days of age. As the central organ responsible for reproductive function, the observed shifts in testicular energy metabolism during this stage are likely tailored to support processes such as spermatocyte meiosis, spermatogenesis, and testosterone synthesis in Leydig cells.
Conclusion
In conclusion, our study provides a comprehensive molecular characterization of sexual maturation in Xianghuang chickens. We demonstrate that maturation of the HPT axis is driven by coordinated tissue remodeling, evidenced by the consistent enrichment of cytoskeleton and adhesion pathways across all three tissues. A pivotal finding is hormonal reprogramming in the pituitary and testes: the pituitary enhances its sensitivity to GnRH, while the testes amplify their intracellular signal transduction to it. Furthermore, we uncover a critical role for tissue-specific lipid metabolic shifts, mediated by PPAR and insulin signaling, in fueling the energetic demands of spermatogenesis and steroidogenesis. These insights move beyond morphology and histology to reveal the core molecular drivers of puberty in this breed. This work not only advances our understanding of avian sexual maturation but also provides a valuable genetic resource for optimizing breeding strategies in indigenous poultry.
CRediT authorship contribution statement
Qingyuan Ouyang: Writing – review & editing, Writing – original draft, Visualization, Funding acquisition, Formal analysis, Data curation. Jing Li: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Yuanbo Song: Writing – review & editing, Investigation, Formal analysis, Data curation. Jian Xiao: Methodology, Investigation. Zehe Song: Resources. Xi He: Resources, Project administration, Funding acquisition. Haihan Zhang: Writing – review & editing, Resources, Project administration, Methodology.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Yuelushan Laboratory Talent Program and Hunan Agriculture Research System of Poultry Industry (HARS-06) for the financial support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2025.105736.
Appendix. Supplementary materials
References
- Akhtar M.F., Wei Q.., Zhu H., Chen Z., Ahmad E., Zhendan S., Shi F. The role of active immunization against inhibin alpha-subunit on testicular development, testosterone concentration and relevant genes expressions in testis, hypothalamus and pituitary glands in Yangzhou goose ganders. Theriogenology. 2019;128:122–132. doi: 10.1016/j.theriogenology.2019.01.039. [DOI] [PubMed] [Google Scholar]
- Bartman J., Zaguri S., Avital-Cohen N., Dishon L., Druyan S., Gumulka M., Rozenboim I. Targeted differential illumination improves reproductive traits of broiler breeder males. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M., Hesse V. Minipuberty: why does it happen? Horm. Res. Paediatr. 2020;93:76–84. doi: 10.1159/000508329. [DOI] [PubMed] [Google Scholar]
- Bieche I., Latil A., Parfait B., Vidaud D., Laurendeau I., Lidereau R., Cussenot O., Vidaud M. CGA gene (coding for the alpha subunit of glycoprotein hormones) overexpression in ER alpha-positive prostate tumors. Eur. Urol. 2002;41:335–341. doi: 10.1016/s0302-2838(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Bisht S., Faiq M., Tolahunase M., Dada R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017;14:470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- Clarke I.J. Hypothalamus as an endocrine organ. Compr. Physiol. 2015;5:217–253. doi: 10.1002/cphy.c140019. [DOI] [PubMed] [Google Scholar]
- Clement T.M., Inselman A..L., Goulding E.H., Willis W.D., Eddy E.M. Disrupting cyclin dependent kinase 1 in spermatocytes causes late meiotic arrest and infertility in mice. Biol. Reprod. 2015;93:137. doi: 10.1095/biolreprod.115.134940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C.Y., Lv M.., Luo B.H., Zhao S.Z., Mo Z.C., Xie Y.J. The role of the PI3K/AKT/mTOR signalling pathway in male reproduction. Curr. Mol. Med. 2021;21:539–548. doi: 10.2174/1566524020666201203164910. [DOI] [PubMed] [Google Scholar]
- Duittoz A.H., Forni P..E., Giacobini P., Golan M., Mollard P., Negron A.L., Radovick S., Wray S. Development of the gonadotropin-releasing hormone system. J. Neuroendocrinol. 2022;34 doi: 10.1111/jne.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili V., Shahverdi A.H., Moghadasian M.H., Alizadeh A.R. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3:450–461. doi: 10.1111/andr.12024. [DOI] [PubMed] [Google Scholar]
- Franca L.R., Ogawa T.., Avarbock M.R., Brinster R.L., Russell L.D. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol. Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Gaudet D., Brisson D., Tremblay K., Alexander V.J., Singleton W., Hughes S.G., Geary R.S., Baker B.F., Graham M.J., Crooke R.M., Witztum J.L. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 2014;371:2200–2206. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- Gresset A., Sondek J., Harden T.K. The phospholipase C isozymes and their regulation. SubCell Biochem. 2012;58:61–94. doi: 10.1007/978-94-007-3012-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Cong B., Zhu L., Zhang Y., Yang Y., Qi X., Wang X., Xiao L., Long C., Xu Y., Sheng X. Whole transcriptome sequencing of testis and epididymis reveals genes associated with sperm development in roosters. BMC. Genomics. 2024;25:1029. doi: 10.1186/s12864-024-10836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi Karoii D., Azizi H., Skutella T. Microarray and in silico analysis of DNA repair genes between human testis of patients with nonobstructive azoospermia and normal cells. Cell Biochem. Funct. 2022;40:865–879. doi: 10.1002/cbf.3747. [DOI] [PubMed] [Google Scholar]
- He Z., Ouyang Q., Chen Q., Song Y., Hu J., Hu S., He H., Li L., Liu H., Wang J. Molecular mechanisms of hypothalamic-pituitary-ovarian/thyroid axis regulating age at first egg in geese. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E. Structural biology of laminins. Essays Biochem. 2019;63:285–295. doi: 10.1042/EBC20180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz G.G., Suarez S.S. Calcium/calmodulin and calmodulin kinase II stimulate hyperactivation in demembranated bovine sperm. Biol. Reprod. 2005;73:519–526. doi: 10.1095/biolreprod.105.040733. [DOI] [PubMed] [Google Scholar]
- Jaiswal B.S., Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. P Natl Acad Sci USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P., Calderwood D.A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat. Rev. Mol. Cell Biol. 2023;24:142–161. doi: 10.1038/s41580-022-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprara A., Huhtaniemi I.T. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2018;86:3–17. doi: 10.1016/j.metabol.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Kotlyarov S., Kotlyarova A. Participation of Kruppel-like factors in atherogenesis. Metabolites. 2023;13 doi: 10.3390/metabo13030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y., Russell S..J., Ussar S., Boucher J., Vernochet C., Mori M.A., Smyth G., Rourk M., Cederquist C., Rosen E.D., Kahn B.B., Kahn C.R. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang X., Yang G., Xu K., Yin Y., Brecchia G., Yin J. CD36 favours fat sensing and transport to govern lipid metabolism. Prog. Lipid Res. 2022;88 doi: 10.1016/j.plipres.2022.101193. [DOI] [PubMed] [Google Scholar]
- Lin B., Zhou X., Jiang D., Shen X., Ouyang H., Li W., Xu D., Fang L., Tian Y., Li X., Huang Y. Comparative transcriptomic analysis reveals candidate genes for seasonal breeding in the male Lion-Head goose. Br. Poult. Sci. 2023;64:157–163. doi: 10.1080/00071668.2022.2152651. [DOI] [PubMed] [Google Scholar]
- Liu Q., Song Y., Ma J., Mabrouk I., Zhou Y., Hu J., Sun Y. The combination of RNA-seq transcriptomics and data-independent acquisition proteomics reveal the mechanisms and function of different gooses testicular development at different stages of laying cycle. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.104007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Hu Y., Li L., He Y., Wang J., Guo N., Fang Y., Chen Q., Cai C., Tong J., Tang L., Wang Z. Targeting FABP4 in elderly mice rejuvenates liver metabolism and ameliorates aging-associated metabolic disorders. Metabolism. 2023;142 doi: 10.1016/j.metabol.2023.155528. [DOI] [PubMed] [Google Scholar]
- Ma Q., Gui Y., Ma X., Zhang B., Xiong W., Yang S., Cao C., Mo S., Shu G., Ye J., Liu K., Wang X., Gui Y., Wang F., Yuan S. N6-methyladenosine writer METTL16-mediated alternative splicing and translation control are essential for murine spermatogenesis. Genome Biol. 2024;25:193. doi: 10.1186/s13059-024-03332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher G.J., Goriely A.., Wilkie A.O. Cellular evidence for selfish spermatogonial selection in aged human testes. Andrology. 2014;2:304–314. doi: 10.1111/j.2047-2927.2013.00175.x. [DOI] [PubMed] [Google Scholar]
- Mfoundou J.D.L., Guo Y., Yan Z., Wang X. Morpho-histology and morphometry of chicken testes and seminiferous tubules among yellow-feathered broilers of different ages. Vet. Sci. 2022;9 doi: 10.3390/vetsci9090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian J., Pieri M., Ohanian V. Non-receptor tyrosine kinases and the actin cytoskeleton in contractile vascular smooth muscle. J. Physiol. 2015;593:3807–3814. doi: 10.1113/jphysiol.2014.284174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q., Bao D., Lu Y., Hu J., Hu B., Lan C., Hu S., He H., Liu H., Li L., Wang J. A comparative study of libido in drakes: from phenotypes to molecules. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Q., Hu S., Li L., Ran M., Zhu J., Zhao Y., Hu B., Hu J., He H., Li L., Wang J. Integrated mRNA and miRNA transcriptome analysis provides novel insights into the molecular mechanisms underlying goose pituitary development during the embryo-to-hatchling transition. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E. Apolipoprotein-L Functions in Membrane Remodeling. Cells. 2024;13 doi: 10.3390/cells13242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Frances M., van Gurp L., Abate M.V., Cigliola V., Furuyama K., Bru-Tari E., Oropeza D., Carreaux T., Fujitani Y., Thorel F., Herrera P.L. Author correction: pancreatic ppy-expressing gamma-cells display mixed phenotypic traits and the adaptive plasticity to engage insulin production. Nat. Commun. 2021;12:5783. doi: 10.1038/s41467-021-26062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthawala K., Hadjiangelis N., Jacoby S.C., Bayongan E., Zhao Z., Yang Z., Devitt M.L., Horan G.S., Weinreb P.H., Lukashev M.E., Violette S.M., Grant K.S., Colarossi C., Formenti S.C., Munger J.S. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Deng Z., Ye F., Gou J., Huang M., Xiang H., Li H. Analysis of the differentially expressed genes in the combs and testes of Qingyuan partridge roosters at different developmental stages. BMC. Genomics. 2024;25:33. doi: 10.1186/s12864-024-09960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Huang T., Wang Z., Zhang X., Wang J., Dang Q., Cui D., Wang X., Zhai Y., Zhao L., Lu G., Shao C., Li S., Liu H., Liu Z. Bud31-mediated alternative splicing is required for spermatogonial stem cell self-renewal and differentiation. Cell Death. Differ. 2023;30:184–194. doi: 10.1038/s41418-022-01057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran L., Chen Q., Lu X., Gao Z., Cui F., Liu X., Xue B. Novel treatment and insight for irradiation-induced injuries: dibucaine ameliorates irradiation-induced testicular injury by inhibiting fatty acid oxidation in primary Leydig cells. Biomed. PharmacOther. 2023;164 doi: 10.1016/j.biopha.2023.114903. [DOI] [PubMed] [Google Scholar]
- Roy Choudhury D., Small C., Wang Y., Mueller P.R., Rebel V.I., Griswold M.D., McCarrey J.R. Microarray-based analysis of cell-cycle gene expression during spermatogenesis in the mouse. Biol. Reprod. 2010;83:663–675. doi: 10.1095/biolreprod.110.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Jayasena C.N., Dhillo W.S. Regulation of the hypothalamic-pituitary-testicular axis: pathophysiology of hypogonadism. Endocrinol. Metab. Clin. North Am. 2022;51:29–45. doi: 10.1016/j.ecl.2021.11.010. [DOI] [PubMed] [Google Scholar]
- Shi L., Li Y., Yuan J., Ma H., Wang P., Ni A., Ge P., Chen C., Li D., Sun Y., Chen J. Effects of age at photostimulation on sexual maturity and reproductive performance in rooster breeders. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E., Kuypers F.A. Mammalian long-chain acyl-CoA synthetases. Exp. Biol. Med. (Maywood) 2008;233:507–521. doi: 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Niu R., Su K., Wang B., Wang J., Zhang J., Wang J. Effects of sodium fluoride on hyperactivation and Ca2+ signaling pathway in sperm from mice: an in vivo study. Arch. Toxicol. 2010;84:353–361. doi: 10.1007/s00204-009-0508-x. [DOI] [PubMed] [Google Scholar]
- Tang B., Hu S., Ouyang Q., Wu T., Lu Y., Hu J., Hu B., Li L., Wang J. Comparative transcriptome analysis identifies crucial candidate genes and pathways in the hypothalamic-pituitary-gonadal axis during external genitalia development of male geese. BMC. Genomics. 2022;23:136. doi: 10.1186/s12864-022-08374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Li X., Wang Y., Xie F., Li R., Ren M., Hu Q., Li S. Comprehensive analysis of mRNA and miRNA differential expression profiles in the hypothalamus-pituitary-gonadal axis in laying and broodiness period of Wanxi white geese. Poult. Sci. 2025;104 doi: 10.1016/j.psj.2024.104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoumoh A.F., Igwebuike U..M., Okoye C.N., Ugwu U.M., Oguejiofor C.F. Assessment of age-related morphological changes in the testes of post-hatch light ecotype Nigerian indigenous chicken. Anat. Histol. Embryol. 2021;50:459–466. doi: 10.1111/ahe.12649. [DOI] [PubMed] [Google Scholar]
- Wang J., Li J., Xu W., Xia Q., Gu Y., Song W., Zhang X., Yang Y., Wang W., Li H., Zou K. Androgen promotes differentiation of PLZF(+) spermatogonia pool via indirect regulatory pattern. Cell Commun. Signal. 2019;17:57. doi: 10.1186/s12964-019-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Tian W., Wang D., Guo Y., Cheng Z., Zhang Y., Li X., Zhi Y., Li D., Li Z., Jiang R., Li G., Tian Y., Kang X., Li H., Dunn I.C., Liu X. Comparative analyses of dynamic transcriptome profiles highlight key response genes and dominant isoforms for muscle development and growth in chicken. Genet. Sel. Evol. 2023;55:73. doi: 10.1186/s12711-023-00849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellard S.R., Skinner M..W., Zhao X., Shults C., Jordan P.W. PLK1 depletion alters homologous recombination and synaptonemal complex disassembly events during mammalian spermatogenesis. Mol. Biol. Cell. 2022;33:ar37. doi: 10.1091/mbc.E21-03-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk J., Mukhina I., Kaczmarek L., Dityatev A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev. Neurobiol. 2011;71:1040–1053. doi: 10.1002/dneu.20958. [DOI] [PubMed] [Google Scholar]
- Xin Q., Jiao H., Wang X., Zhao J., Liu M., Li H., Zhou Y., Lin H. Effect of energy level of pullet diet and age on laying performance and expression of hypothalamus-pituitary-gonadal related genes in laying hens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Chen J., Qing E., Li X., Wang W., Ling Z., Chen Z., Jiang S., Yan Y., Deng S., Hu J., Li L., Wang J., Hu S. Developmental variations of the reproductive organs of ganders from different goose breeds and the underlying mechanisms. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.104233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shen Z., Yang Z., Jiang H., Chu S., Mao Y., Li M., Chen Z., Aboragah A., Loor J.J., Yang Z. Abundance of solute carrier family 27 member 6 (SLC27A6) in the bovine mammary gland alters fatty acid metabolism. Food Funct. 2021;12:4909–4920. doi: 10.1039/d0fo03289a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.