Summary
Background
Different nutritional supplements may prevent respiratory tract infections (RTIs) in adults, but their comparative effectiveness remains unclear. We aimed to evaluate the effectiveness of oral nutritional supplements in preventing RTIs and reducing their symptom duration and severity.
Methods
In this systematic review and network meta-analysis, we searched for randomized controlled trials (RCTs) assessing oral micronutrients, flavonoids, probiotics, or synbiotics in adults for the prevention of RTIs in PubMed, Embase, Cochrane Central Register, and ClinicalTrials.gov from inception to February 25, 2025. Studies with participants under 18 years, immunodeficiencies, non-oral administration, or a therapeutic rather than preventive focus were excluded. We performed network meta-analyses (NMA) using frequentist random-effects models to compare interventions through direct and indirect evidence. We used the CINeMA framework to rate the overall certainty of evidence. The primary outcome was the incidence of RTIs. The secondary outcomes were upper respiratory tract infections (URTIs) or common cold, COVID-19 or influenza, RTI symptom duration, RTI symptom severity, and adverse events. The study is registered with PROSPERO, CRD420250653276.
Findings
We identified 120 eligible trials, among which 107 trials involving 101,751 adults were included in the network meta-analysis. Compared with placebo, catechin (RR = 0.79, 95% CI: 0.66, 0.95; n = 5; high certainty), Bifidobacterium animalis (RR = 0.79, 95% CI: 0.63, 0.99; n = 3; moderate certainty), and multi-strain probiotics (RR = 0.90, 95% CI: 0.82, 0.98; n = 16; moderate certainty) were the most effective interventions for reducing RTI incidence. For COVID-19 or influenza prevention, high-dose vitamin D was highly effective (RR = 0.66, 95% CI: 0.51, 0.86; n = 9; moderate certainty). Catechin (MD = −2.64 days/RTI, 95% CI: −4.92, −0.35; n = 2; moderate certainty) and multi-strain probiotics (MD = −0.97 days/RTI, 95% CI: −1.78, −0.16; n = 4; high certainty) most effectively shortened RTI symptom duration. Multi-strain probiotics (SMD = −0.33, 95% CI: −0.51, −0.14; n = 6; moderate certainty) also showed superior performance in alleviating RTI symptom severity. None of the interventions increased the risk of adverse events versus placebo.
Interpretation
Based on the current evidence, catechin, B. animalis, and multi-strain probiotics show relatively better effects in preventing adult RTI compared to other nutritional supplements. For COVID-19 or influenza prevention, high-dose vitamin D supplementation may be more effective. This study is limited by the lack of head-to-head comparisons between interventions (particularly across major categories) and heterogeneity from varied administration methods (e.g., capsules, beverages, mouthwashes), impacting the consistency and overall estimation of the NMA. Future high-quality studies are needed to provide direct evidence on the optimal intervention type, frequency, and form/dose of administration to guide clinical practice effectively.
Funding
This work was supported by the Ningbo Top Medical and Health Research Program and the Zhejiang Provincial Disease Prevention and Control Science and Technology Plan.
Keywords: Network meta-analysis, RCT, Respiratory tract infections, Nutritional supplements, Systematic review
Research in context.
Evidence before this study
We conducted a preliminary search of PubMed, Embase, Cochrane Library, and Web of Science databases, reviewing evidence from their inception to February 17, 2025 on the role of nutritional supplements in preventing respiratory tract infections (RTIs) among adults, with no language restrictions. Search terms included combinations of (flavonoid∗ OR flavonol OR probiotic∗ OR synbiotic∗ OR “multiple micronutrient” OR “micronutrient supplementation” OR “vitamin D” OR cholecalciferol OR “25-hydroxyvitamin D” OR “1,25-dihydroxyvitamin D” OR “vitamin C” OR “ascorbic acid” OR “vitamin A” OR retinol OR retinoids OR “beta-carotene” OR carotenoids OR “vitamin E” OR zinc) AND (“respiratory tract infection” OR “respiratory infection” OR “common cold” OR influenza OR pneumonia OR COVID-19 OR SARS-CoV-2) AND (adult∗) AND (“randomized controlled trial” OR RCT OR “clinical trial” OR “controlled trial” OR randomized). Eligible participants were adults aged 18 or older, taking oral nutritional supplements, and free of immune-related diseases. Approximately 20 systematic reviews and meta-analyses were identified, all using traditional pairwise meta-analysis with a narrow focus on specific supplement types. No network meta-analysis (NMA) was found that systematically compared the full range of nutritional interventions for RTI prevention.
Added value of this study
Our systematic review and NMA comprehensively assessed the efficacy and safety of oral nutritional supplements, including low-, moderate-, and high-dose vitamin C, standard- and high-dose vitamin D, vitamin E, zinc, flavonoids, probiotics, and synbiotics, for preventing RTIs in adults. Incorporating 107 randomized controlled trials (RCTs) with 101,751 participants, this study provides a substantially larger evidence base than previous reviews. We utilized the CINeMA framework to evaluate the confidence in NMA effect estimates and presented clear, intuitive rankings of intervention performance across outcomes. Additional analyses included subgroup comparisons by age (<65 vs ≥65 years) and meta-regression and sensitivity analyses considering factors such as participant age, sex, follow-up duration, outcome measurement methods, high-intensity training status, and risk of bias. Our results indicate that catechin, Bifidobacterium animalis, and multi-strain probiotics are among the most effective for reducing RTI incidence, while high-dose vitamin D shows potential for preventing COVID-19 and influenza.
Implications of all the available evidence
This review indicates that certain nutritional supplements are effective in preventing RTIs among adults. This study is limited by the absence of head-to-head comparisons between interventions, particularly across major categories, and by heterogeneity arising from diverse administration methods, which affects the consistency and overall estimation of the NMA. Future high-quality studies are needed to provide direct evidence on the optimal intervention type, frequency, and administration form or dose to effectively guide clinical practice.
Introduction
Respiratory tract infections (RTIs) are defined as diseases of the respiratory system causing infections of the sinuses, throat, airways, and lungs, categorized into upper respiratory tract infections (URTIs) and lower respiratory tract infections (LRTIs). The incidence of RTIs has been increasing annually, imposing a significant disease burden (URTI: 225,505.7 cases per 100,000 population per year [201,156.4–253,739.5]; LRTI: 4770 cases per 100,000 population per year [4510–5040]).1,2
Interest in the potential for nutrient supplementation to reduce the risk of RTIs has increased since the emergence of the COVID-19 pandemic3 and the resurgence of seasonal influenza globally.4 Nutrient supplementation has long been considered a potentially effective strategy for preventing RTIs.5 Supplementing with micronutrients (including vitamins A, C, D, E, zinc, selenium, iron, etc.) and plant-based antioxidants (such as flavonoids and polyphenols) can reduce the risk of RTI by activating the immune system, reducing oxidative stress, providing anti-inflammatory effects, and maintaining respiratory tract barrier function.3,6 Probiotics and synbiotics can mediate the balance and diversity of the gut microbiota, which plays a significant role in the host's response to many viral infections, either by promoting the attachment of viruses to host cells or by supporting antiviral immunity (such as interferons and innate immune activation).6,7
In recent years, many systematic reviews and meta-analyses of randomized controlled trials (RCTs) have explored the effectiveness of oral nutrient supplements in preventing RTIs,4,8, 9, 10, 11, 12, 13, 14, 15, 16 suggesting beneficial effects of flavonoids and probiotics, while the effects of vitamins A, C, D, E, and zinc remain controversial. No review has simultaneously examined the comparative effects of all these interventions. Although primarily affecting children under 5 years of age, RTIs continue to have high incidence and mortality rates in adults, particularly among middle-aged and elderly individuals over 50 years old.1,2 However, fewer systematic reviews have focused on the adult population.4,12 Additionally, there are still limited meta-analyses on the role of nutrient supplementation in preventing COVID-19 and influenza.17, 18, 19, 20 Therefore, we conducted a systematic review and network meta-analysis (NMA) of RCTs to investigate the effectiveness and safety of these interventions in reducing the incidence and severity of RTIs in adults.
Methods
Search strategy and selection criteria
We conducted a systematic review and NMA following the Preferred Reporting Items for Systematic Reviews-Network Meta-Analyses (PRISMA-NMA) guideline.21 The study protocol was registered on PROSPERO (CRD420250653276). The changes made in this study compared to the PROSPERO registration along with the reasons are detailed in Table S1.
Electronic databases, including PubMed, Embase, Cochrane Central Register, and ClinicalTrials.gov, were searched from their inception to February 25, 2025 without language restriction. Reference lists of included original studies, reviews, and meta-analyses were also examined to identify additional relevant studies. The detailed search strategy is provided in eMethods 1 in the Supplement. Following the initial search, five researchers (Z.Z., X.Z., Y.C., B.Z., and Y.C.) independently screened titles and abstracts in duplicate. Subsequently, three researchers (Z.Z., X.Z., and Y.C.) performed full-text screening, assessing eligibility based on the Population, Intervention, Comparison, Outcome, and Study Type (PICOT) criteria.
We included randomized clinical trials that investigated the preventive effects of oral micronutrients, flavonoids, probiotics, or synbiotics on the incidence, duration, or severity of respiratory tract infections in adults aged ≥18 years. Micronutrient supplements encompassed vitamin A (including alpha- and beta-carotenes), vitamin C (classified into low-dose VC: average daily dose ≤500 mg, moderate-dose VC: average daily dose 501–1000 mg and high-dose VC: average daily dose >1000 mg), vitamin D (classified into standard-dose VD: average daily dose <2000 IU, and high-dose VD: average daily dose ≥2000 IU), vitamin E (including alpha- and beta-tocopherols), and zinc. The grouping of VC and VD was primarily based on previous researches4,10 and data availability. Flavonoid supplements were defined as oral interventions with flavonoids including catechins and quercetins as the primary biologically active components. Probiotics were classified at the species level into single probiotics such as Lactobacillus casei (L. casei), Lactobacillus rhamnosus (L. rhamnosus), Lactobacillus helveticus (L. helveticus), and Bifidobacterium animalis (B. animalis), and multi-strain probiotics (combinations involving multiple strains of Lactobacillus ssp., Bifidobacterium ssp., Streptococcus ssp., Enterococcus ssp., and/or Saccharomyces boulardii).12,22 Synbiotics were defined as combinations of probiotics (single or multiple strains) and prebiotics (nondigestible carbohydrates fermented by gut microbiota).
Exclusion criteria included studies with incomplete data (lacking information on the age of the included population, specific types of interventions, number of cases, duration of symptoms, or symptom severity scores), reviews, commentaries, editorials, animal or in vitro studies, and observational studies; Studies with a mean or median participant age <18 years (due to differences in immune function, lifestyle, and the types and dosages of nutritional supplements applicable to children and adolescents compared to adults); Non-oral administrations such as nasal sprays, injections, and intestinal colonization; Studies investigating therapeutic effects rather than preventive effects; or cohorts of hospitalized patients, individuals with immunodeficiencies, autoimmune or other immune-related disorders, or those receiving immunomodulatory therapy.
Data analysis
Data extraction and risk-of-bias assessments were independently conducted by two reviewers (Z.Z. and X.Z.). Disagreements were resolved through group discussion. A standardized Excel template was used to extract the following: author, year, country, population characteristics, sample size, follow-up duration, sex, mean age, interventions, outcomes, and outcome ascertainment. In cases of overlapping data, the study with the most comprehensive dataset was selected.
Risk of bias was evaluated using the Cochrane Risk of Bias 2 tool for randomized trials.23 Small-study effects were assessed using comparison-adjusted funnel plots in the network, along with Egger's and Begg's tests for statistical significance.24 If publication bias is identified, we further correct pairwise comparisons with over 10 studies using the trim-and-fill method. The certainty of evidence was evaluated using the CINeMA (Confidence in Network Meta-Analysis) framework, which adapts the GRADE approach for network meta-analyses, addressing within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency (see eMethods 2 in the Supplement for details).25,26
The primary outcome was the overall incidence of RTIs, defined as the ratio of individuals reporting one or more RTIs to the total number of participants, including URTI, LRTI, common cold, or influenza. Data were collected regardless of whether the diagnosis was based on self-report, clinical assessment, or laboratory confirmation.
Secondary outcomes were categorized as follows: (1) URTI/common cold included cases specifically described in the original studies as “URTI” or “common cold.” Studies reported with a non-specific term such as “RTI” without further details were not included in this subgroup. (2) COVID-19/influenza included cases specifically identified as “COVID-19” (e.g., confirmed by PCR or antigen test), “influenza” (e.g., laboratory-confirmed or clinically diagnosed), or “influenza-like illness (ILI)”. ILI was defined according to the criteria provided in the original study, typically involving fever plus cough or sore throat. (3) Symptom duration was measured as the mean duration of symptoms per RTI episode. (4) Symptom severity was assessed by the mean severity score per RTI episode. (5) Adverse events were defined as any mild-to-severe intervention-related adverse reactions.
To obtain robust estimates, we included in the NMA only interventions supported by at least three studies or data points. We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for the incidence of RTI, and odds ratios (ORs) were used for adverse events. For symptom duration and severity, mean differences (MDs) and standardized mean differences (SMDs) with Hedges' g correction for small studies and corresponding 95% CIs were used, respectively. All pooled estimates were calculated based on intention to treat analysis. Primary and secondary outcomes were analyzed using frequentist NMAs based on a graph-theoretical approach, implemented with the netmeta package in R version 4.3.3 (R Project for Statistical Computing).
Ranking probabilities were estimated using the surface under the cumulative ranking curve (SUCRA) and rankograms.27 Heterogeneity was quantified with within-design and between-design Q statistics and I2. The transitivity assumption was evaluated by comparing key study characteristics and assessing local consistency (net splitting of direct evidence from head-to-head studies vs indirect evidence inferred through common comparators) and global consistency (design-by-treatment interaction test).28,29
Subgroup and sensitivity analyses were performed for the primary outcome to explore differences in RTI risk between adults aged <65 years and those ≥65 years, as well as changes in effect sizes after excluding high risk-of-bias studies, those with a “no treatment” control group, or high-intensity-trained population (including athletes and military recruits). Meta-regression analyses were conducted within a Bayesian framework, examining the influence of confounders such as participant age (<65 vs ≥65 years), proportion of females, follow-up duration (weeks), self-reported RTI (yes vs no), high-intensity-trained population (yes vs no) and risk of bias of studies. These models utilized 50,000 burn-in iterations and 100,000 additional simulations with four chains of different initial values via the Markov Chain Monte Carlo method, obtaining medians and 95% credible intervals (CrIs). The Deviance Information Criterion (DIC) was used to complement the assessment of the model differences. Additionally, to investigate the nonlinear dose-response relationship between vitamin D and C supplementation and RTI risk, a one-stage, nonlinear random-effects dose-response meta-analysis was performed using the dosresmet package, with knots placed at the 10th, 50th, and 90th percentiles of the exposure distribution.30
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data in the study and the corresponding author (Yi Chen) had final responsibility for the decision to submit for publication.
Results
An initial search yielded 8696 citations, which required abstract screening. Subsequently, 543 reports underwent full-text screening, and 120 clinical trials31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60,61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90,91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120,121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150 were included in the systematic review. After excluding 13 trials for reasons (Table S3), 107 involving 101,751 participants were included in the final NMA (Fig. 1). These trials included 36 on vitamin D, 22 on vitamin C, 4 on zinc, 10 on flavonoids (6 catechin and 4 quercetin), 40 on probiotics (24 single probiotic and 16 multi-strain probiotics), and 4 on synbiotics. Six studies had three arms, two studies had four arms, and the remaining studies were all two-arm RCTs. The median (IQR) of the duration of follow-up was 12 (8–25.7) weeks (Table S2).
Fig. 1.
Flowchart according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline.
In the 120 RCTs, 50 had a low risk of bias, 58 had some concerns, and 12 had a high risk of bias. Detailed information on the risk of bias assessment is provided in Table S4 of the Supplement. The certainty of the evidence for the relevant outcomes was classified as high, moderate, low, or very low, and the interventions were categorized as among the best (statistically significant difference vs placebo and >1 other treatment), among the intermediate (statistically significant difference vs placebo), and among the worst (no statistically significant difference vs placebo).
RTI incidence
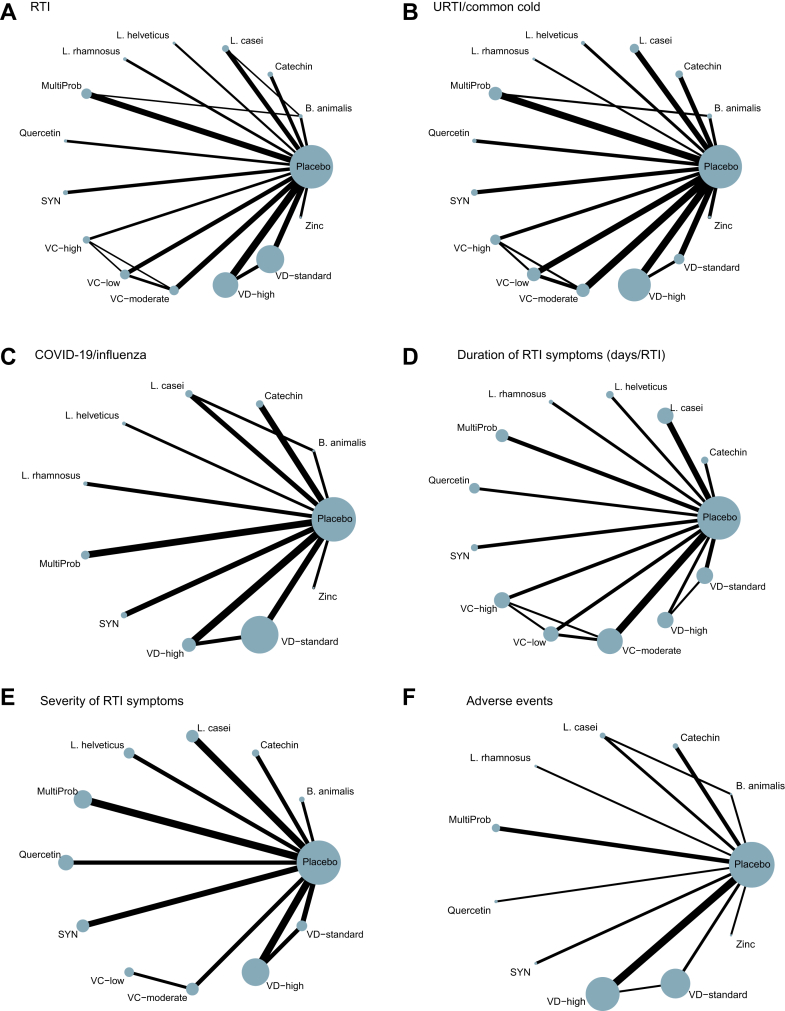
Of the 107 RCTs, 99 with 99,583 adults involving 15 preventive nutritional interventions addressed the primary outcome of RTI incidence (Fig. 2A). Of the 22 direct comparisons, 16 involved 2 studies or more. Comparisons of L. rhamnosus, multi-strain probiotics, moderate-dose VC, high-dose VD, and standard-dose VD, each versus Placebo, revealed significant heterogeneity (I2 > 50%). The three comparisons between high-dose VD, standard-dose VD, and placebo also exhibited local inconsistency (local P-inconsistency < 0.05). Global inconsistency was insignificant (P = 0.304) (Table S10 in Supplement).
Fig. 2.
Network of eligible comparisons. (A) RTI Incidence. (B) URTI/Common Cold. (C) COVID-19/Influenza. (D) Duration of RTI symptoms. (E) Severity of RTI symptoms. (F) Adverse events. The size of the nodes corresponds to the number of adults randomly assigned to that intervention. Interventions directly compared are connected by a line; the thickness of the line represents the number of trials evaluating that comparison. B. animalis: Bifidobacterium animalis; L. casei: Lactobacillus casei; L. rhamnosus: Lactobacillus rhamnosus; L. helveticus: Lactobacillus helveticus; RTI: respiratory tract infection; URTI: upper respiratory tract infection; PRO: probiotics; SYN: synbiotics; MultiProb: multiple-strain probiotics; VC-low: low-dose VC (average daily dose ≤ 500 mg); VC-moderate: moderate-dose VC (average daily dose of 501–1000 mg); VC-high: high-dose VC (average daily dose >1000 mg); VD-standard: standard-dose VD (average daily dose <2000 IU); VD-high: high-dose VD (average daily dose ≥2000 IU).
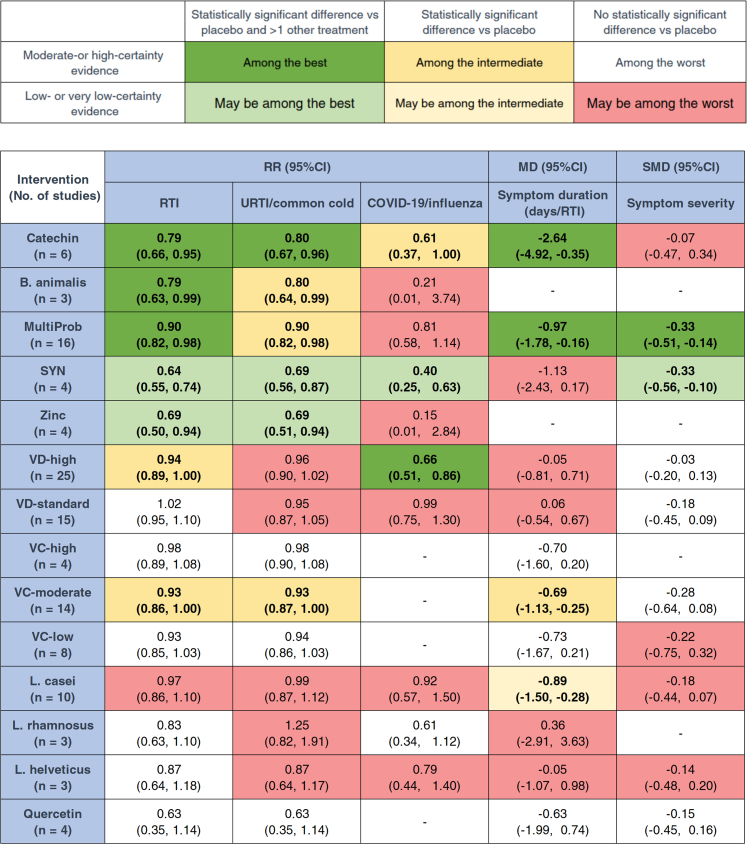
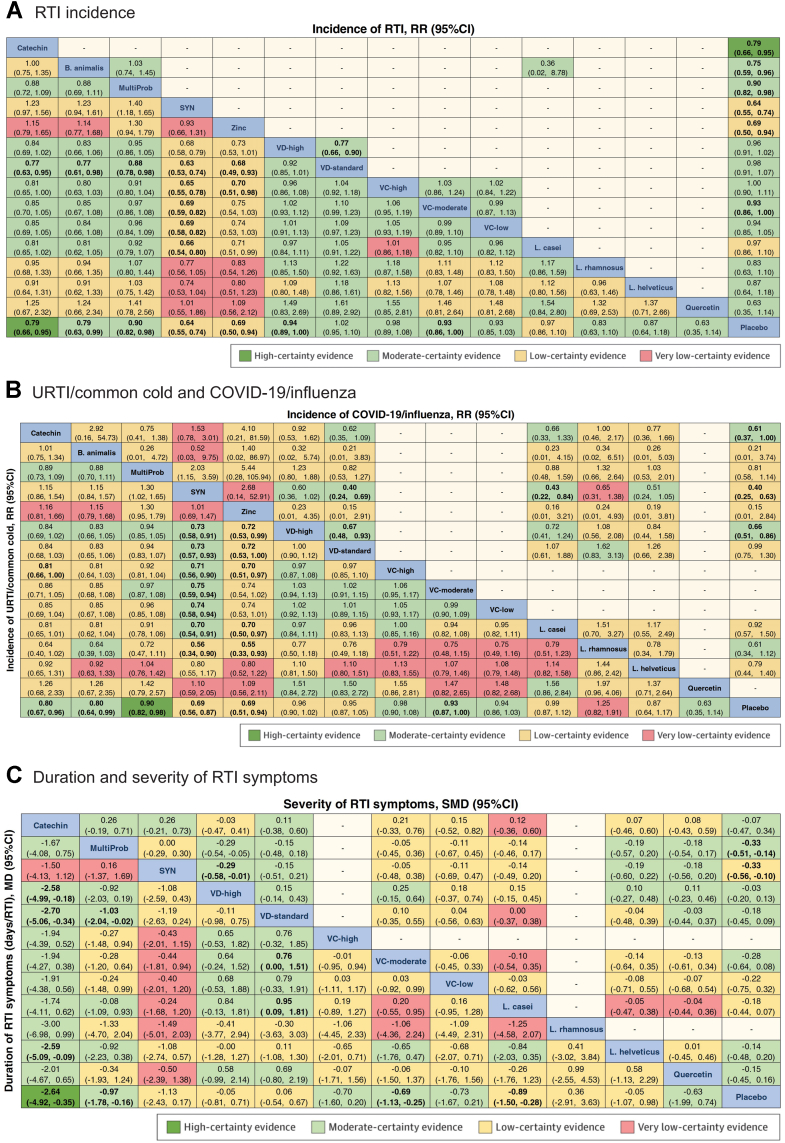
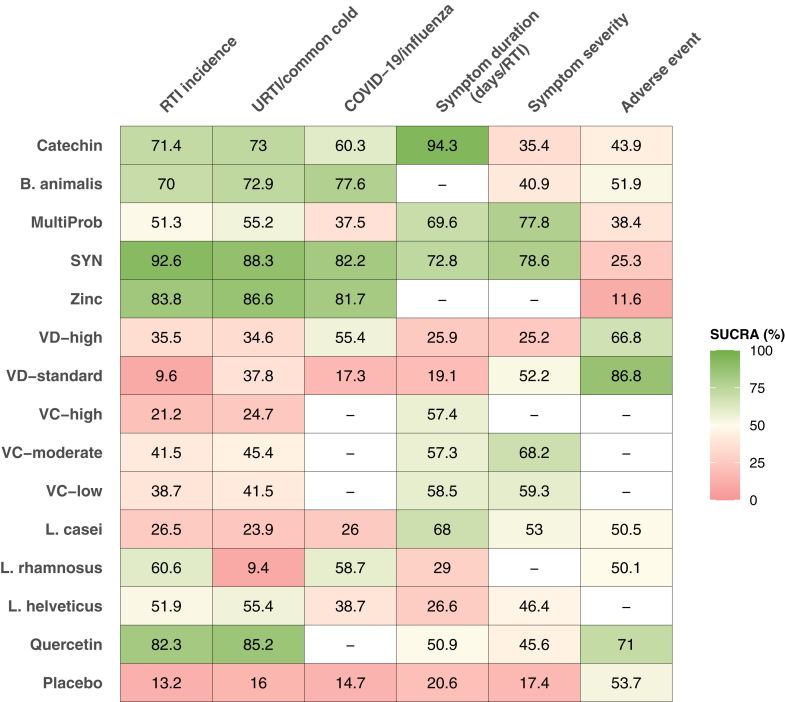
Fig. 3 summarizes the main findings for each outcome, presenting the network estimates for each intervention compared to placebo and their 95% confidence intervals. Compared with placebo, Catechin (RR = 0.79, 95% CI: 0.66, 0.95; SUCRA = 71.4; high certainty), B. animalis (RR = 0.79, 95% CI: 0.63, 0.99; SUCRA = 70.0; moderate certainty), and multi-strain probiotics (RR = 0.90, 95% CI: 0.82, 0.98; SUCRA = 51.3; moderate certainty) were among the most effective interventions in reducing RTI incidence. SYN (RR = 0.64, 95% CI: 0.55, 0.74; SUCRA = 92.6; low certainty) and zinc (RR = 0.69, 95% CI: 0.50, 0.94; SUCRA = 83.8; low certainty) may be among the most effective but lack certainty. High-dose VD (RR = 0.94, 95% CI: 0.89, 1.00; SUCRA = 35.5; moderate certainty) and Moderate-dose VC (RR = 0.93, 95% CI: 0.86, 1.00; SUCRA = 41.5; moderate certainty) were associated with intermediate effectiveness (Fig. 3, Fig. 4A, and Fig. 5). Fig. 4A shows the comparative effectiveness and certainty for all pairwise comparisons. Figure S1 shows the number of participants and effect size of each study. Table S5 provides specific details of the CINeMA certainty assessment for the RTI incidence outcome.
Fig. 3.
Network meta-analysis results sorted based on the CINeMA (Confidence in Network Meta-Analysis) certainty of evidence and effect estimates for the comparisons of active interventions vs placebo. Bold numbers represent statistically significant results. B. animalis: Bifidobacterium animalis; L. casei: Lactobacillus casei; L. rhamnosus: Lactobacillus rhamnosus; L. helveticus: Lactobacillus helveticus; RTI: respiratory tract infection; RR: risk ratio; URTI: upper respiratory tract infection; SMD: standardized mean difference; PRO: probiotics; SYN: synbiotics; MultiProb: multiple-strain probiotics; VC-low: low-dose VC (average daily dose ≤ 500 mg); VC-moderate: moderate-dose VC (average daily dose of 501–1000 mg); VC-high: high-dose VC (average daily dose >1000 mg); VD-standard: standard-dose VD (average daily dose <2000 IU); VD-high: high-dose VD (average daily dose ≥2000 IU).
Fig. 4.
Network meta-analysis results for the primary and secondary outcomes. (A) RTI Incidence: The effect size of the network meta-analysis is shown in the lower triangle, and direct comparisons are shown in the upper triangle. (B) URTI/Common Cold: Lower triangle; COVID-19/Influenza: Upper triangle. (C) Symptom Duration: Lower triangle; Symptom Severity: Upper triangle. For each row and column comparison, a risk ratio less than 0 or 1 indicates that the intervention in the blue cell in the upper-left corner is more favorable. Bold numbers represent statistically significant results. The color of each result cell represents the certainty of the evidence in the pairwise comparison. B. animalis: Bifidobacterium animalis; L. casei: Lactobacillus casei; L. rhamnosus: Lactobacillus rhamnosus; L. helveticus: Lactobacillus helveticus; RTI: respiratory tract infection; RR: risk ratio; URTI: upper respiratory tract infection; SMD: standardized mean difference; SYN: synbiotics; MultiProb: multiple-strain probiotics; VC-low: low-dose VC (average daily dose ≤ 500 mg); VC-moderate: moderate-dose VC (average daily dose of 501–1000 mg); VC-high: high-dose VC (average daily dose >1000 mg); VD-standard: standard-dose VD (average daily dose <2000 IU); VD-high: high-dose VD (average daily dose ≥2000 IU).
Fig. 5.
Heatmap of SUCRA for nutrient supplementation and RTI outcomes. SUCRA values rank nutrients on a continuous scale from 0 to 1. The higher the value, the better the preventive effect. B. animalis: Bifidobacterium animalis; L. casei: Lactobacillus casei; L. rhamnosus: Lactobacillus rhamnosus; L. helveticus: Lactobacillus helveticus; SYN: synbiotics; MultiProb: multiple-strain probiotics; VC-low: low-dose VC (average daily dose ≤ 500 mg); VC-moderate: moderate-dose VC (average daily dose of 501–1000 mg); VC-high: high-dose VC (average daily dose >1000 mg); VD-standard: standard-dose VD (average daily dose <2000 IU); VD-high: high-dose VD (average daily dose ≥2000 IU).
URTI/common cold incidence
75 studies reporting on the incidence of URTI or common cold enrolled 51,454 adults and evaluated 15 preventive interventions (Fig. 2B). Of the 19 available direct comparisons, the I2 of 4 comparisons was more than 50%. Inconsistency was only detected in comparisons between high-dose VD, standard-dose VD, and placebo (Table S11 in Supplement). Global inconsistency was not significant (P = 0.069).
Catechin (RR = 0.80, 95% CI: 0.67, 0.96; SUCRA = 73.0; moderate certainty) proved of best effectiveness in preventing URTI or common cold vs placebo. Low-certainty evidence suggests that SYN (RR = 0.69, 95% CI: 0.56, 0.87; SUCRA = 88.3) and zinc (RR = 0.69, 95% CI: 0.51, 0.94; SUCRA = 86.6) may be associated with a reduction in URTI or common cold incidence compared to placebo (Fig. 3, Fig. 4B, and Fig. 5). B. animalis (RR = 0.80, 95% CI: 0.64, 0.99; SUCRA = 72.9; moderate certainty), multi-strain probiotics (RR = 0.90, 95% CI: 0.82, 0.98; SUCRA = 55.2; high certainty), and Moderate-dose VC (RR = 0.93, 95% CI: 0.87, 1.00; SUCRA = 45.4; moderate certainty) demonstrated intermediate effectiveness (Fig. 3, Fig. 4B, and Fig. 5). Fig. 4B shows the comparative effectiveness and certainty for all pairwise comparisons. Figure S4 shows the number of participants and effect size of each study. Table S6 provides specific details of the CINeMA certainty assessment for the URTI/common cold incidence outcome.
COVID-19/influenza
The 32 studies (52,601 adults) reporting COVID-19 or influenza involved 11 interventions with 12 direct comparisons, among which High-dose VD vs placebo showed substantial heterogeneity (I2 > 50%) (Fig. 2C). No global or local inconsistency was found (Table S12 in Supplement). High-dose VD (vs placebo, RR = 0.66, 95% CI: 0.51, 0.86; SUCRA = 55.4; moderate certainty) proved of best effectiveness in reducing COVID-19 or influenza incidence. Synbiotics (vs placebo, RR = 0.40, 95% CI: 0.25, 0.63; SUCRA = 82.3; low certainty) may be among the best effectiveness. Catechin (vs placebo, RR = 0.61, 95% CI: 0.37, 1.00; SUCRA = 60.3; moderate certainty) was of intermediate effectiveness (Fig. 3, Fig. 4B, and Fig. 5). Figure S7 shows the number of participants and effect size of each study. Table S7 shows specific details of the CINeMA certainty assessment for the COVID-19/influenza incidence outcome.
Duration of RTI symptoms
We identified 41 studies (13,191 RTI episodes) that reported duration of RTI symptoms involving 13 interventions (Fig. 2D). Of the 16 direct comparisons, 3 had significant heterogeneity (I2 > 50%), and no global or local inconsistency was observed (Table S13 in Supplement).
Compared with placebo, Catechin (MD = −2.64 days/RTI, 95% CI: −4.92, −0.35; SUCRA = 94.3; high certainty) and multi-strain probiotics (MD = −0.97 days/RTI, 95% CI: −1.78, −0.16; SUCRA = 69.6; moderate certainty) were considered the best and most reliable interventions in shortening the duration of RTI symptoms. Moderate-dose VC (MD = −0.68 days/RTI, 95% CI: −1.13, −0.25; SUCRA = 57.3; moderate certainty) demonstrated intermediate effectiveness. Low-certainty evidence suggested L. casei (MD = −0.89 days/RTI, 95% CI: −1.50, −0.28; SUCRA = 68) may help reduce the duration of RTI symptoms (Fig. 3, Fig. 4C, and Fig. 5). Figure S10 shows the number of participants and effect size of each study. Table S8 provides specific details of the CINeMA certainty assessment for the duration of RTI symptoms outcome.
Severity of RTI symptoms
Based on 31 studies reporting on the severity of RTI symptoms, involving 5802 RTI episodes, 11 interventions were investigated (Fig. 2E). Of the 12 direct comparisons, two showing substantial heterogeneity (I2 > 50%). We found no statistical evidence of global or local inconsistency (Table S14 in Supplement).
Multi-strain probiotics (SMD = −0.33, 95% CI: −0.51, −0.14; SUCRA = 77.8; moderate certainty) were among the best effective intervention in alleviating the severity of RTI symptoms, compared with placebo. Synbiotics (SMD = −0.33, 95% CI: −0.56, −0.10; SUCRA = 78.6; low certainty) maybe effective (Fig. 3, Fig. 4C, and Fig. 5). Figure S13 shows the number of participants and effect size of each study. Table S9 shows specific details of the CINeMA certainty assessment for the duration of RTI symptoms outcome.
Adverse events
30 trials including 68,462 adults investigated adverse events, involving 11 interventions (Fig. 2F). None of the interventions increased the risk of adverse events versus placebo, indicating that all interventions had a good safety profile (Figure S12). However, safety evaluation data for VC was lacking.
Subgroup and sensitive analysis
We conducted a subgroup and sensitivity analysis for the primary outcome, RTI incidence. Figures S19–S21 presented the results for adults aged <65 years. The effect of high-dose VD improved further (RR = 0.69, 95% CI: 0.59, 0.80; SUCRA = 0.85), while the effects of B. animalis, zinc, and moderate-dose VC were non-significant. In adults aged ≥65, only multi-strain probiotics showed statistical significance, but only 7 interventions were available for comparison (Figures S22–S24).
When excluding studies with a high risk of bias, the effects of moderate-dose VC and zinc became statistically non-significant. When excluding studies involving high-intensity-trained population as the study population, the effect of moderate-dose VC also lost statistical significance. Other results did not change significantly (Figures S25–S33). Meta-regression analysis indicated that age, proportion of females, follow-up duration, self-reported RTI, high-intensity-trained population, and study risk of bias had no significant impact on the results (Table S15). Additionally, a nonlinear dose-response relationship between VC supplementation and RTI incidence was observed (Figures S34 and S35).
Publication bias
Comparison-adjusted funnel plots across outcomes showed significant evidence of small-study effect in RTI, URTI/common cold and COVID-19/influenza (Figures S36–S42).
Discussion
In this systematic review and NMA, the benefits of micronutrient supplementation, probiotics, synbiotics, and bioflavonoids in preventing the incidence of RTIs and alleviating RTI symptoms in adults were compared. We found moderate to high-certainty evidence suggesting that catechins were among the best measures for preventing RTIs and URTI/common cold as well as shortening symptom duration, high-dose vitamin D (average daily dose ≥2000 IU) may be the relatively optimal choice for reducing the incidence of COVID-19/influenza, and multi-strain probiotics were most effective in alleviating symptom severity. We did not observe any significant modification effect for age, proportion of females, follow-up duration, self-reported RTI, high-intensity-trained population, and risk of bias.
Flavonoids are diverse, and their specific effects cannot be generalized. A meta-analysis of 20 RCTs found flavonoid supplements reduced RTI incidence (RR = 0.81, 95% CI: 0.74, 0.89) and duration (Weighted mean difference = −0.56 days, 95% CI: −1.04, −0.08), but the purity and potency of the supplements were unclear, which may introduce heterogeneity and bias.14 Our NMA found that compared to other nutrient interventions, catechin supplementation was the best choice for preventing RTIs, while quercetin, another flavonoid, showed no effect. Catechins are a complex of flavanols, including epigallocatechin gallate (EGCg), which exhibits antiviral effects in vitro.151 Studies show that EGCg and EGC target viral RNA polymerase, inhibiting RNA synthesis and blocking viral proliferation.152 Recent docking simulation studies on SARS-CoV-2 also indicate that EGCg, EGC, and other catechins have significant binding affinity to the main protease of the virus.153 In mice, oral administration of an EGC/EGCg mixture increases IgA production in the intestinal mucosa, promoting mucosal immunity.154 A previous meta-analysis showed that the use of tea and catechins is effective in preventing influenza and URTIs (RR = 0.79, 95% CI: 0.66, 0.94), which is consistent with our study findings.8
Two meta-analyses found that VD supplementation reduced the incidence of COVID-19 and influenza,17,18 but neither explored the dose-dependency of VD effects. The relationship between VD dosage and RTIs remains controversial.48,64,141,155 A recent meta-analysis showed that ≥2000 IU/day VD (RR = 0.85, 95% CI: 0.75, 0.96) was more effective than <2000 IU/day (RR = 0.91, 95% CI: 0.94, 0.98) in preventing adult RTIs.4 In this NMA, high-dose VD was found to have a good effect in preventing COVID-19 or influenza. High-dose vitamin D exerts multiple immunomodulatory effects on innate and adaptive immune cells, enhances the antibacterial activity of macrophages, and improves immune health and defense against infectious diseases.156
We found that moderate-dose VC (501–1000 mg/day) were also effective in preventing RTIs. No dose-response relationship between VC supplementation and adult RTIs has been established. This study showed an inverted U-shaped relationship between VC dosage and RTI risk. Vitamin C plays a critical role in both innate and adaptive immunity, affecting epithelial barriers, supporting phagocytosis, and enhancing lymphocyte differentiation.157 However, studies indicate that daily supplementation exceeding the tolerable upper intake level (2000 mg) in adults may cause adverse reactions such as diarrhea, headaches, gastrointestinal discomfort, and an increased risk of other diseases, impacting patients' quality of life and potentially reducing compliance.158 Interestingly, our dose-response meta-analysis showed that the effect of vitamin C in preventing RTIs begins to weaken at doses greater than 1500 mg/day, which may be related to the above. A previous meta-analysis found that moderate-dose vitamin C significantly reduces adult RTIs (RR = 0.95, 95% CI: 0.92, 0.99),4 consistent with our findings. However, a Cochrane review suggested this effect is only significant in participants under severe physical stress (RR = 0.48, 95% CI: 0.35, 0.64), not the general population (RR = 0.97, 95% CI: 0.94, 1.00).15 Sensitivity analysis in our study showed that when excluding studies involving high-intensity-trained populations, the effect of moderate-dose vitamin C lost statistical significance. Therefore, further research is needed to establish the preventive effect of vitamin C on RTIs in the general adult population.
Previous meta-analyses suggested probiotics reduce RTI risk by 9%–47%12,159, 160, 161 and shorten RTI duration by 0.8–2.7 days.159,160,162 In this NMA, B. animalis and multi-strain probiotics reduced adult RTI risk by 20% and 10% respectively, and multi-strain probiotics and L. casei shortened RTI duration by 0.97 and 0.89 days respectively, similar to the recent research findings.12 In single-strain studies, B. animalis demonstrated better efficacy compared to strains within the Lactobacillus genus. Research indicates that B. animalis subsp. lactis (e.g., BB-12 strain) significantly enhances natural killer (NK) cell activity, reduces inflammatory cytokines (e.g., IL-6, TNF-α), and boosts antiviral responses (e.g., production of IFN-γ and IL-12).163 Additionally, B. animalis has high cell wall hydrophobicity, which facilitates better adhesion to the intestinal epithelium, resulting in superior gut colonization compared to L. casei.164 The ability of B. animalis subsp. lactis to produce short-chain fatty acids is significantly greater than that of Lactobacillus salivarius, Lactiplantibacillus plantarum, and others, benefiting respiratory defense by enhancing gut barrier integrity, reducing systemic inflammation, and supporting immune cell function.165
For multi-strain probiotics, studies indicate that different strains can enhance bioactivity through synergistic effects.166 However, more strains are not necessarily better. A systematic review evaluated the preventive effects of single-strain probiotics versus multi-strain mixtures on various diseases (including URTI), and in most cases, single strains were comparable to mixtures.167 Therefore, selecting appropriate probiotics should be based on evidence from efficacy trials to identify specific single or multi-strain probiotic combinations. The multi-strain probiotics in our NMA primarily involve the genera Lactobacillus ssp., Bifidobacterium ssp., Streptococcus ssp., providing some guidance. In fact, the results of this NMA show that B. animalis (RR = 0.79, 95% CI: 0.63, 0.99; SUCRA = 70.0) outperforms multi-strain probiotics (RR = 0.90, 95% CI: 0.82, 0.98; SUCRA = 51.3) in efficacy ranking.
Although synbiotics and zinc showed significant beneficial effects in our study, their evidence level was downgraded due to the limited number of studies and low evidence quality. Recent two meta-analyses found that zinc was ineffective for RTIs, but they both included experimental challenge studies, which may differ from infections under natural conditions.4,16 Additionally, the use of VA, VE, and other types of single-strain probiotics could not be pooled due to insufficient data points. More high-quality related studies are necessary in the future.
To elucidate our key findings, catechins exhibit superior efficacy in preventing respiratory infections due to their multifaceted mechanisms: potent antioxidant and anti-inflammatory effects, direct inhibition of viral replication, and enhancement of immune responses and mucosal barriers.168 Probiotics rank second, exerting broader systemic effects via the gut-lung axis,169 surpassing the localized or single-target actions of VC and VD, though their effects are slower and indirect. VC supports antioxidant and immune functions but is limited by its singular target and weak antiviral activity.157 VD modulates immune balance via the vitamin D receptor pathway, with restricted antiviral and anti-inflammatory efficacy.156
Considering cost-benefit is necessary. Studies show probiotics reduce RTI burden, saving Can$1.3–8.9 million in healthcare and Can$61.2–99.7 million in productivity in Canada by averting 0.57–2.3 million RTI days.170 In France, savings are €14.6–37.7 million, cutting 2.4–6.6 million RTI days.171 In the US, savings are $4.6–373 million for healthcare and up to $1.4 billion with productivity, reducing 19–54.5 million RTI days.172 This NMA and current evidence suggest therapeutic supplementation with VD,173 VC,4,174 and zinc16 may outperform preventive supplementation in reducing RTI duration or symptom severity, indicating a potentially more cost-effective approach for symptom relief. Evidence for catechins and probiotics in adult RTI treatment is limited, requiring further research.
Investigating optimal supplementation dosage and intervention duration is crucial for effective practical implementation. A dose-response relationship may exist for catechins,8 suggesting that supplementation can be appropriately increased within safe limits (<704 mg EGCG/day).175 Results from one meta-analyses failed to show a relationship between probiotic efficacy and dose administered176; selecting appropriate strains (e.g., B. animalis) is more critical. Moderate-dose vitamin C (average daily dose of 501–1000 mg) and high-dose vitamin D (average daily dose ≥2000 IU) may represent optimal choices. Additionally, the median preventive intervention duration of trials included in this network meta-analysis was 12 weeks, with winter being the peak season for respiratory infectious diseases, indicating that supplementation during winter may be the most effective timing.177
Our review has the following strengths: the first comprehensive comparative assessment of all relevant nutritional interventions for reducing the incidence of adult RTIs; the analysis of different dosage groups for VC and VD, as well as different types of flavonoids and probiotics, addressed the current controversies; and the use of the CINeMA method to semi-objectively assess the certainty of NMA effect estimates, as well as providing a simple and user-friendly representation of the relative performance of each intervention for each outcome (Fig. 3).
The limitations of this review: First, apart from direct comparisons with the control group, there is a lack of head-to-head direct comparisons between other interventions (especially those involving different major categories of interventions), making it difficult to assess inconsistency in many comparisons. Second, differences in administration methods (such as capsules, beverages, and mouthwashes) may lead to heterogeneity, which to some extent affects the overall estimation of the NMA. Finally, for many interventions, only low or very low-quality evidence was available (Fig. 3), and potential publication bias may further reduce the quality of the evidence.
In conclusion, this systematic review and NMA find that moderate to high-quality evidence suggest catechin, B. animalis, and multi-strain probiotics are among the best measures for preventing RTIs. High-dose vitamin D is optimal in reducing the incidence of COVID-19 or influenza. Catechin and multi-strain probiotics are associated with a shorter symptom duration. Multi-strain probiotics are most effective in alleviating symptom severity. Future high-quality studies are needed to provide direct evidence on the optimal intervention type, frequency, and form/dose of administration to guide clinical practice effectively.
Contributors
YC and ZXZ designed the study. ZXZ, XXZ, YRC, BZ, and YC conducted the literature search and literature screening. ZXZ and XXZ extracted the data. ZXZ performed the data analysis and statistical interpretation. YC supervised the statistical methodology. ZXZ and XXZ wrote the first draft of the manuscript. All authors contributed to data interpretation and critical revision of the manuscript. All authors read and approved the final version of the manuscript. ZXZ, XXZ, and YC had access to and verified the underlying data.
Data sharing statement
The data generated in this study are available from the corresponding authors upon reasonable request, for research purposes only. The data will be available starting from the date of publication.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This work was supported by the Ningbo Top Medical and Health Research Program (No. 2023020713) and the Zhejiang Provincial Disease Prevention and Control Science and Technology Plan (2025JK075).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103479.
Appendix A. Supplementary data
References
- 1.Jin X., Ren J., Li R., et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender R.G., Sirota S.B., Swetschinski L.R., et al. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect Dis. 2024;24(9):974–1002. doi: 10.1016/S1473-3099(24)00176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James P.T., Ali Z., Armitage A.E., et al. The role of nutrition in COVID-19 susceptibility and severity of disease: a systematic review. J Nutr. 2021;151(7):1854–1878. doi: 10.1093/jn/nxab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abioye A.I., Bromage S., Fawzi W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: a systematic review and meta-analysis. BMJ Glob Health. 2021;6(1) doi: 10.1136/bmjgh-2020-003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi Z., Askari M., Jafari A., et al. Dietary patterns and micronutrients in respiratory infections including COVID-19: a narrative review. BMC Public Health. 2024;24(1):1661. doi: 10.1186/s12889-024-18760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasmi A., Tippairote T., Mujawdiya P.K., et al. The microbiota-mediated dietary and nutritional interventions for COVID-19. Clin Immunol. 2021;226 doi: 10.1016/j.clim.2021.108725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehtoranta L., Latvala S., Lehtinen M.J. Role of probiotics in stimulating the immune system in viral respiratory tract infections: a narrative review. Nutrients. 2020;12(10) doi: 10.3390/nu12103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umeda M., Tominaga T., Kozuma K., et al. Preventive effects of tea and tea catechins against influenza and acute upper respiratory tract infections: a systematic review and meta-analysis. Eur J Nutr. 2021;60(8):4189–4202. doi: 10.1007/s00394-021-02681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahmina Afrose K., Anthony L., Kevin F., Nasrin H., Mumunur R. Effect of vitamin C supplements on respiratory tract infections: a systematic review and meta-analysis. Curr Rev Clin Exp Pharmacol. 2022;17(3):205–215. doi: 10.2174/2772432817666211230100723. [DOI] [PubMed] [Google Scholar]
- 10.Jolliffe D.A., Camargo C.A., Sluyter J.D., et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 11.Vlieg-Boerstra B., de Jong N., Meyer R., et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: a systematic review and meta-analysis. Allergy. 2022;77(5):1373–1388. doi: 10.1111/all.15136. [DOI] [PubMed] [Google Scholar]
- 12.Coleman J.L., Hatch-McChesney A., Small S.D., et al. Orally ingested probiotics, prebiotics, and synbiotics as countermeasures for respiratory tract infections in nonelderly adults: a systematic review and meta-analysis. Adv Nutr. 2022;13(6):2277–2295. doi: 10.1093/advances/nmac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan C.K.Y., Tao J., Chan O.S., Li H.B., Pang H. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(4):979–988. doi: 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao J., Zhao J., Wen J.-R., et al. Flavonoid-containing supplements for preventing acute respiratory tract infections: a systematic review and meta-analysis of 20 randomized controlled trials. Complement Ther Med. 2022;70 doi: 10.1016/j.ctim.2022.102865. [DOI] [PubMed] [Google Scholar]
- 15.Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;2013(1) doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nault D., Machingo T.A., Shipper A.G., et al. Zinc for prevention and treatment of the common cold. Cochrane Database Syst Rev. 2024;5(5) doi: 10.1002/14651858.CD014914.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartini M., Del Puente F., Oliva M., et al. Preventive vitamin D supplementation and risk for COVID-19 infection: a systematic review and meta-analysis. Nutrients. 2024;16(5) doi: 10.3390/nu16050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z.X., Zhu X.X., Gu L.F., Zhan Y.C., Chen L., Li X.Y. Association between vitamin D and influenza: meta-analysis and systematic review of randomized controlled trials. Front Nutr. 2022;8 doi: 10.3389/fnut.2021.799709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Y., Wang R.T., Xia J., Cao H.J. Interventions for preventing influenza: an overview of Cochrane systematic reviews and a Bayesian network meta-analysis. J Integr Med. 2021;19(6):503–514. doi: 10.1016/j.joim.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Sinopoli A., Sciurti A., Isonne C., Santoro M.M., Baccolini V. The efficacy of multivitamin, vitamin A, vitamin B, vitamin C, and vitamin D supplements in the prevention and management of COVID-19 and Long-COVID: an updated systematic review and meta-analysis of randomized clinical trials. Nutrients. 2024;16(9) doi: 10.3390/nu16091345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Florez I.D., Morgan R.L., et al. Probiotics, prebiotics, lactoferrin, and combination products for prevention of mortality and morbidity in preterm infants: a systematic review and network meta-analysis. JAMA Pediatr. 2023;177(11):1158–1167. doi: 10.1001/jamapediatrics.2023.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Harbord R.M., Egger M., Sterne J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 25.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papakonstantinou T., Nikolakopoulou A., Higgins J.P.T., Egger M., Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16(1) doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaimani A., Higgins J.P.T., Mavridis D., Spyridonos P., Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu G., Ades A.E. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101(474):447–459. [Google Scholar]
- 29.Higgins J.P.T., Jackson D., Barrett J.K., Lu G., Ades A.E., White I.R. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim S.R., Lee J. Dose-response meta-analysis: application and practice using the R software. Epidemiol Health. 2019;41 doi: 10.4178/epih.e2019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahanchian H., Ranjbar A., Reihani H., et al. Synbiotic for prevention of SARS-Cov2 infection in high risk hospital staffs: a randomized controlled trial. Open J Nurs. 2021;11(5):281–290. [Google Scholar]
- 32.Ahrén I.L., Hillman M., Nordström E.A., Larsson N., Niskanen T.M. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomized, placebo-controlled clinical trial. J Nutr. 2021;151(1):214–222. doi: 10.1093/jn/nxaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Nakib W., Higgins P.G., Barrow I., et al. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother. 1987;20:893–901. doi: 10.1093/jac/20.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aloia J.F., Islam S., Mikhail M. Vitamin D and acute respiratory infections-the PODA trial. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aloia J.F., Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–1096. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altadill T., Espadaler-Mazo J., Liong M.T. Effects of a lactobacilli probiotic on reducing duration of URTI and fever, and use of URTI-associated medicine: a re-analysis of a randomized, placebo-controlled study. Microorganisms. 2021;9(3):528. doi: 10.3390/microorganisms9030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson T.W., Reid D.B., Beaton G.H. Vitamin C and the common cold: a double-blind trial. Can Med Assoc J. 1972;107:503–508. [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson T.W., Suranyi G., Beaton G.H. The effect on winter illness of large doses of vitamin C. Can Med Assoc J. 1974;111:31–36. [PMC free article] [PubMed] [Google Scholar]
- 39.Arihiro S., Nakashima A., Matsuoka M., et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(6):1088–1095. doi: 10.1093/ibd/izy346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auinger A., Riede L., Bothe G., Busch R., Gruenwald J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body's defence against pathogens: a double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur J Nutr. 2013;52:1913–1918. doi: 10.1007/s00394-013-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berggren A., Lazou Ahrén I., Larsson N., Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011;50(3):203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 42.Bergman P., Norlin A.C., Hansen S., Björkhem-Bergman L. Vitamin D supplementation improves well-being in patients with frequent respiratory tract infections: a post hoc analysis of a randomized, placebo-controlled trial. BMC Res Notes. 2015;8:498. doi: 10.1186/s13104-015-1504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bischoff-Ferrari H.A., Vellas B., Rizzoli R., et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briggs M.H. CRC Press; Boca Raton, FL: 1984. Vitamin C and Infectious Disease: A Review of the Literature and the Results of a Randomised Double Blind Prospective Study Over Eight Years. [Google Scholar]
- 45.Brunvoll S.H., Nygaard A.B., Ellingjord-Dale M., et al. Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial. BMJ. 2022;378 doi: 10.1136/bmj-2022-071245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler C.C., Lau M., Gillespie D., et al. Effect of probiotic use on antibiotic administration among care home residents: a randomized clinical trial. JAMA. 2020;324(1):47–56. doi: 10.1001/jama.2020.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camargo C.A., Schaumberg D.A., Friedenberg G., et al. Effect of daily vitamin D supplementation on risk of upper respiratory infection in older adults: a randomized controlled trial. Clin Infect Dis. 2024;78(5):1162–1169. doi: 10.1093/cid/ciad770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo C.A., Sluyter J., Stewart A.W., et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin Infect Dis. 2020;71(2):311–317. doi: 10.1093/cid/ciz801. [DOI] [PubMed] [Google Scholar]
- 49.Carr A.B., Einstein R., Lai L.Y., Martin N.G., Starmer G.A. Vitamin C and the common cold: a second MZ Cotwin control study. Acta Genet Med Gemellol. 1981;30(4):249–255. doi: 10.1017/s0001566000006450. [DOI] [PubMed] [Google Scholar]
- 50.Carson M., Cox H., Corbett M., Pollitt N. Vitamin C and the common cold. J Soc Occup Med. 1975;25(3):99–102. doi: 10.1093/occmed/25.3.99. [DOI] [PubMed] [Google Scholar]
- 51.Charleston S., Clegg K.M. Ascorbic acid and the common cold. Lancet. 1972;299:1401–1402. doi: 10.1016/s0140-6736(72)91143-9. [DOI] [PubMed] [Google Scholar]
- 52.Clegg K.M., Macdonald J.M. L-Ascorbic acid and D-isoascorbic acid in a common cold survey. Am J Clin Nutr. 1975;28:973–976. doi: 10.1093/ajcn/28.9.973. [DOI] [PubMed] [Google Scholar]
- 53.Cowan D.W., Diehl H.S., Baker A.B. Vitamins for the prevention of colds. J Am Med Assoc. 1942;120:1268–1271. [Google Scholar]
- 54.Dahlberg G., Engel A., Rydin H. The value of ascorbic acid as a prophylactic against »common colds».1. Acta Med Scand. 1944;119:540–561. [Google Scholar]
- 55.de Gruijl F.R., Pavel S. The effects of a mid-winter 8-week course of sub-sunburn sunbed exposures on tanning, vitamin D status and colds. Photochem Photobiol Sci. 2012;11:1848–1854. doi: 10.1039/c2pp25179e. [DOI] [PubMed] [Google Scholar]
- 56.de Vrese M., Winkler P., Rautenberg P., et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24(4):481–491. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Denlinger L.C., King T.S., Cardet J.C., et al. Vitamin D supplementation and the risk of colds in patients with asthma. Am J Respir Crit Care Med. 2016;193(6):634–641. doi: 10.1164/rccm.201506-1169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elwood P.C., Lee H.P., St Leger A.S., Baird M., Howard A.N. A randomized controlled trial of vitamin C in the prevention and amelioration of the common cold. Br J Prev Soc Med. 1976;30(3):193–196. doi: 10.1136/jech.30.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farr B.M., Conner E.M., Betts R.F., et al. Two randomized controlled trials of zinc gluconate lozenge therapy of experimentally induced rhinovirus colds. Antimicrob Agents Chemother. 1987;31:1183–1187. doi: 10.1128/aac.31.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernández-Ferreiro A., Formigo-Couceiro F.J., Veiga-Gutierrez R., et al. Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the immune response of elderly subjects to COVID-19 vaccination: a randomized controlled trial. Nutrients. 2022;14(1):228. doi: 10.3390/nu14010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franz W.L., Heyl H.L., Sands G.W. Blood ascorbic acid level in bioflavonoid and ascorbic acid therapy of common cold. J Am Med Assoc. 1956;162:1224–1226. doi: 10.1001/jama.1956.02970300024009. [DOI] [PubMed] [Google Scholar]
- 62.Fujita R., Iimuro S., Shinozaki T., et al. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am J Infect Control. 2013;41(12):1231–1235. doi: 10.1016/j.ajic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Furushima D., Nishimura T., Takuma N., et al. Prevention of acute upper respiratory infections by consumption of catechins in healthcare workers: a randomized, placebo-controlled trial. Nutrients. 2019;12(1):4. doi: 10.3390/nu12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ginde A.A., Blatchford P., Breese K., et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gleeson M., Bishop N.C., Oliveira M., McCauley T., Tauler P., Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exerc Metab. 2012;22(4):235–242. doi: 10.1123/ijsnem.22.4.235. [DOI] [PubMed] [Google Scholar]
- 66.Gleeson M., Bishop N.C., Oliveira M., Tauler P. Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exerc Metab. 2011;21(1):55–64. doi: 10.1123/ijsnem.21.1.55. [DOI] [PubMed] [Google Scholar]
- 67.Gleeson M., Bishop N.C., Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. Eur J Appl Physiol. 2016;116(8):1555–1563. doi: 10.1007/s00421-016-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Godan Hauptman A., Lukić-Grlić A., Vraneš J., Milošević M., Gagro A. The effect of standard-dose wintertime vitamin D supplementation on influenza infection in immunized nursing home elderly residents. Croat Med J. 2021;62(5):495–503. doi: 10.3325/cmj.2021.62.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodall E.C., Granados A.C., Luinstra K., et al. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: a randomized controlled trial. BMC Infect Dis. 2014;14:273. doi: 10.1186/1471-2334-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guillemard E., Tanguy J., Flavigny A., de la Motte S., Schrezenmeir J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J Am Coll Nutr. 2010;29(5):455–468. doi: 10.1080/07315724.2010.10719882. [DOI] [PubMed] [Google Scholar]
- 71.Harrison S.E., Oliver S.J., Kashi D.S., et al. Influence of vitamin D supplementation by simulated sunlight or oral D3 on respiratory infection during military training. Med Sci Sports Exerc. 2021;53(7):1505–1516. doi: 10.1249/MSS.0000000000002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinz S.A., Henson D.A., Austin M.D., Jin F., Nieman D.C. Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial. Pharmacol Res. 2010;62(3):237–242. doi: 10.1016/j.phrs.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemilä H., Virtamo J., Albanes D., Kaprio J. The effect of vitamin E on common cold incidence is modified by age, smoking and residential neighborhood. J Am Coll Nutr. 2006;25(4):332–339. doi: 10.1080/07315724.2006.10719543. [DOI] [PubMed] [Google Scholar]
- 74.Henson D., Nieman D., Davis J.M., et al. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int J Sports Med. 2008;29(10):856–863. doi: 10.1055/s-2007-989424. [DOI] [PubMed] [Google Scholar]
- 75.Himmelstein S.A., Robergs R.A., Koehler K.M. Vitamin C supplementation and upper respiratory tract infections in marathon runners. J Exerc Physiol. 1998;1(2):1–27. [Google Scholar]
- 76.Jespersen L., Tarnow I., Eskesen D., et al. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Am J Clin Nutr. 2015;101(6):1188–1196. doi: 10.3945/ajcn.114.103531. [DOI] [PubMed] [Google Scholar]
- 77.Johnston C.S., Barkyoumb G.M., Schumacher S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: a randomized controlled trial. Nutrients. 2014;6:2572–2583. doi: 10.3390/nu6072572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jolliffe D.A., Holt H., Greenig M., et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT) BMJ. 2022;378 doi: 10.1136/bmj-2022-071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jorde R., Witham M., Janssens W., et al. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44(2):126–132. doi: 10.3109/00365548.2011.621446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalima K., Lehtoranta L., He L., et al. Probiotics and respiratory and gastrointestinal tract infections in Finnish military conscripts—a randomised placebo-controlled double-blinded study. Benef Microbes. 2016;7(4):463–471. doi: 10.3920/BM2015.0172. [DOI] [PubMed] [Google Scholar]
- 81.Karonova T.L., Chernikova A.T., Golovatyuk K.A., et al. Vitamin D intake May reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients. 2022;14(3):505. doi: 10.3390/nu14030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kekkonen R.A., Vasankari T.J., Vuorimaa T., Haahtela T., Julkunen I., Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exerc Metab. 2007;17(4):352–363. doi: 10.1123/ijsnem.17.4.352. [DOI] [PubMed] [Google Scholar]
- 83.Kim T.K., Lim H.R., Byun J.S. Vitamin C supplementation reduces the odds of developing a common cold in Republic of Korea Army recruits: randomised controlled trial. BMJ Mil Health. 2020;168(2):117–123. doi: 10.1136/bmjmilitary-2019-001384. [DOI] [PubMed] [Google Scholar]
- 84.Kinoshita T., Maruyama K., Suyama K., et al. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: a randomized controlled trial. Food Funct. 2019;10(12):8129–8136. doi: 10.1039/c9fo02128k. [DOI] [PubMed] [Google Scholar]
- 85.Koesnoe S., Masjkuri N., Adisasmita A., et al. A randomized controlled trial to evaluate the effect of influenza vaccination and probiotic supplementation on immune response and incidence of influenza-like illness in an elderly population in Indonesia. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0250234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumpu M., Kekkonen R.A., Korpela R., et al. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef Microbes. 2015;6(5):631–639. doi: 10.3920/BM2014.0164. [DOI] [PubMed] [Google Scholar]
- 87.Laaksi I., Ruohola J.P., Mattila V., Auvinen A., Ylikomi T., Pihlajamäki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;202(5):809–814. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 88.Langkamp-Henken B., Rowe C.C., Ford A.L., et al. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;113(3):426–434. doi: 10.1017/S0007114514003997. [DOI] [PubMed] [Google Scholar]
- 89.Lehouck A., Mathieu C., Carremans C., et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 90.Lewis T.L., Karlowski T.R., Kapikian A.Z., Lynch J.M., Shaffer G.W., George D.A. A controlled clinical trial of ascorbic acid for the common cold. Ann N Y Acad Sci. 1975;258:505–512. doi: 10.1111/j.1749-6632.1975.tb29309.x. [DOI] [PubMed] [Google Scholar]
- 91.Li-Ng M., Aloia J.F., Pollack S., et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137(10):1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 92.Makino S., Ikegami S., Kume A., Horiuchi H., Sasaki H., Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br J Nutr. 2010;104(7):998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- 93.Martineau A.R., Hanifa Y., Witt K.D., et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu) Thorax. 2015;70(10):953–960. doi: 10.1136/thoraxjnl-2015-206996. [DOI] [PubMed] [Google Scholar]
- 94.Martineau A.R., James W.Y., Hooper R.L., et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- 95.Martineau A.R., MacLaughlin B.D., Hooper R.L., et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs) Thorax. 2015;70(5):451–457. doi: 10.1136/thoraxjnl-2014-206449. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto K., Yamada H., Takuma N., Niino H., Sagesaka Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: a randomized controlled trial. BMC Complement Altern Med. 2011;11:15. doi: 10.1186/1472-6882-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meydani S.N., Han S.N., Hamer D.H. Vitamin E and respiratory infection in the elderly. Ann N Y Acad Sci. 2004;1031:214–222. doi: 10.1196/annals.1331.021. [DOI] [PubMed] [Google Scholar]
- 98.Michalickova D., Minic R., Dikic N., et al. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2016;41(7):782–789. doi: 10.1139/apnm-2015-0541. [DOI] [PubMed] [Google Scholar]
- 99.Moolla M.E. The Role of Anti-oxidants in the Prevention of Post-race Upper Respiratory Tract Infection. University of Cape Town; Cape Town, South Africa: 1997. [Google Scholar]
- 100.Mullish B.H., Marchesi J.R., McDonald J.A.K., et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: should we be considering probiotics during viral pandemics? Gut Microbes. 2021;13(1):1–9. doi: 10.1080/19490976.2021.1900997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murdoch D.R., Slow S., Chambers S.T., et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 102.Nieman D.C., Henson D.A., Gross S.J., et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007;39(9):1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 103.Nishihira J., Moriya T., Sakai F., Kabuki T., Kawasaki Y., Nishimura M. Lactobacillus gasseri SBT2055 stimulates immunoglobulin production and innate immunity after influenza vaccination in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Funct Foods Health Dis. 2016;6(9):544. [Google Scholar]
- 104.Ozato N., Yamaguchi T., Kusaura T., et al. Effect of catechins on upper respiratory tract infections in winter: a randomized, placebo-controlled, double-blinded trial. Nutrients. 2022;14(9):1856. doi: 10.3390/nu14091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters E., Goetzsche J., Joseph L. Vitamin C as effective as combinations of anti-oxidant nutrients in reducing symptoms of upper respiratory tract infection in ultramarathon runners. S Afr J Sports Med. 1996;11:23–27. [Google Scholar]
- 106.Peters E.M., Goetzsche J.M., Grobbelaar B., Noakes T.D. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr. 1993;57(2):170–174. doi: 10.1093/ajcn/57.2.170. [DOI] [PubMed] [Google Scholar]
- 107.Pham H., Waterhouse M., Baxter C., et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial. Lancet Diabetes Endocrinol. 2021;9(2):69–81. doi: 10.1016/S2213-8587(20)30380-6. [DOI] [PubMed] [Google Scholar]
- 108.Pitt H.A., Costrini A.M. Vitamin C prophylaxis in marine recruits. JAMA. 1979;241:908–911. [PubMed] [Google Scholar]
- 109.Pooya A., Mahmoudian A., Hazavei M.M., Farajzadegan Z., Ramezani M.A. The effect of zinc and “Health Belief Model” based education on common cold prevention in soldiers. Am J Infect Dis. 2006;2(4):193–196. [Google Scholar]
- 110.Prasad A.S., Beck F.W.J., Bao B., et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85(3):837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 111.Pregliasco F., Anselmi G., Fonte L., Giussani F., Schieppati S., Soletti L. A new chance of preventing winter diseases by the administration of synbiotic formulations. J Clin Gastroenterol. 2008;42(Suppl 3):S224–S233. doi: 10.1097/MCG.0b013e31817e1c91. [DOI] [PubMed] [Google Scholar]
- 112.Pu F., Guo Y., Li M., et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clin Interv Aging. 2017;12:1223–1231. doi: 10.2147/CIA.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pumpa K.L., McKune A.J., Harnett J. A novel role of probiotics in improving host defence of elite rugby union athlete: a double blind randomised controlled trial. J Sci Med Sport. 2019;22(8):876–881. doi: 10.1016/j.jsams.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Rake C., Gilham C., Bukasa L., et al. High-dose oral vitamin D supplementation and mortality in people aged 65-84 years: the VIDAL cluster feasibility RCT of open versus double-blind individual randomisation. Health Technol Assess. 2020;24(10):1–54. doi: 10.3310/hta24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rees J.R., Hendricks K., Barry E.L., et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57(10):1384–1392. doi: 10.1093/cid/cit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rizzardini G., Eskesen D., Calder P.C., Capetti A., Jespersen L., Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107(6):876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez J.A.M., Bifano M., Roca Goma E., et al. Effect and tolerability of a nutritional supplement based on a synergistic combination of β-glucans and selenium- and zinc-enriched Saccharomyces cerevisiae (ABB C1®) in volunteers receiving the influenza or the COVID-19 vaccine: a randomized, double-blind, placebo-controlled study. Nutrients. 2021;13(12) doi: 10.3390/nu13124347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez-Blanque R., Sánchez-García J.C., Cobos-Vargas Á., et al. Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.962566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romero-Ibarguengoitia M.E., Gutiérrez-González D., Cantú-López C., Sanz-Sánchez M.Á., González-Cantú A. Effect of vitamin D3 supplementation vs. dietary-hygienic measures on SARS-CoV-2 infection rates in hospital workers with 25-hydroxyvitamin D3 [25(OH)D3] levels ≥20 ng/mL. Microorganisms. 2023;11(2):282. doi: 10.3390/microorganisms11020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rowe C.A., Nantz M.P., Bukowski J.F., Percival S.S. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma,delta T cell function: a randomized, double-blind, placebo-controlled study. J Am Coll Nutr. 2007;26(5):445–452. doi: 10.1080/07315724.2007.10719634. [DOI] [PubMed] [Google Scholar]
- 121.Sabiston B., Radomski M. Health Problems and Vitamin C in Canadian Northern Military Operations. Defence and Civil Institute of Environmental Medicine; Toronto, ON, Canada: 1974. Report No 74-R-M2. [Google Scholar]
- 122.Sasazuki S., Sasaki S., Tsubono Y., Okubo S., Hayashi M., Tsugane S. Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr. 2006;60(1):9–17. doi: 10.1038/sj.ejcn.1602261. [DOI] [PubMed] [Google Scholar]
- 123.Schröder C., Schmidt S., Garbe E., Röhmel J., Giersiepen K. Effects of the regular intake of the probiotic Lactobacillus reuteri (DSM 17938) on respiratory and gastrointestinal infections in a workplace setting: a double-blind randomized placebo-controlled trial. BMC Nutr. 2015;1(1):3. [Google Scholar]
- 124.Shida K., Sato T., Iizuka R., et al. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. 2017;56(1):45–53. doi: 10.1007/s00394-015-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shimizu Y., Ito Y., Yui K., Egawa K., Orimo H. Intake of 25-hydroxyvitamin D3 reduces duration and severity of upper respiratory tract infection: a randomized, double-blind, placebo-controlled, parallel group comparison study. J Nutr Health Aging. 2018;22(4):491–500. doi: 10.1007/s12603-017-0952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simpson S., van der Mei I., Stewart N., Blizzard L., Tettey P., Taylor B. Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: results from the CIPRIS pilot RCT. BMC Nutr. 2015;1(1):7. [Google Scholar]
- 127.Slow S., Epton M., Storer M., et al. Effect of adjunctive single high-dose vitamin D(3) on outcome of community-acquired pneumonia in hospitalised adults: the VIDCAPS randomised controlled trial. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-32162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith T.J., Rigassio-Radler D., Denmark R., Haley T., Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109(11):1999–2007. doi: 10.1017/S0007114512004138. [DOI] [PubMed] [Google Scholar]
- 129.Strasser B., Geiger D., Schauer M., et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8(11):752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sugimura T., Takahashi H., Jounai K., et al. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br J Nutr. 2015;114(5):727–733. doi: 10.1017/S0007114515002408. [DOI] [PubMed] [Google Scholar]
- 131.Tiollier E., Chennaoui M., Gomez-Merino D., Drogou C., Filaire E., Guezennec C.Y. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med. 2007;172(9):1006–1011. doi: 10.7205/milmed.172.9.1006. [DOI] [PubMed] [Google Scholar]
- 132.Tiralongo E., Wee S.S., Lea R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: a randomized, double-blind placebo-controlled clinical trial. Nutrients. 2016;8(4):182. doi: 10.3390/nu8040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tominaga T., Ikukawa T., Furushima D., Nakamura T.J., Yamada H. An exploratory randomized controlled study to investigate concentration-dependence of green tea catechin gargling on acute upper respiratory tract infections. Biol Pharm Bull. 2024;47(7):1331–1337. doi: 10.1248/bpb.b24-00113. [DOI] [PubMed] [Google Scholar]
- 134.Tran B., Armstrong B.K., Ebeling P.R., et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2014;99(1):156–161. doi: 10.3945/ajcn.113.063271. [DOI] [PubMed] [Google Scholar]
- 135.Turner R.B., Woodfolk J.A., Borish L., et al. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—a randomised controlled trial. Benef Microbes. 2017;8(2):207–215. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaisberg M., Paixão V., Almeida E.B., et al. Daily intake of fermented milk containing Lactobacillus casei Shirota (Lcs) modulates systemic and upper airways immune/inflammatory responses in marathon runners. Nutrients. 2019;11(7):1678. doi: 10.3390/nu11071678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Helmond N., Brobyn T.L., LaRiccia P.J., et al. Vitamin D3 supplementation at 5000 IU daily for the prevention of influenza-like illness in healthcare workers: a pragmatic randomized clinical trial. Nutrients. 2022;15(1):180. doi: 10.3390/nu15010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Straten M., Josling P. Preventing the common cold with a vitamin C supplement: a double-blind, placebo-controlled survey. Adv Ther. 2002;19(3):151–159. doi: 10.1007/BF02850271. [DOI] [PubMed] [Google Scholar]
- 139.Veverka D.V., Wilson C., Martinez M.A., Wenger R., Tamosuinas A. Use of zinc supplements to reduce upper respiratory infections in United States Air Force Academy cadets. Complement Ther Clin Pract. 2009;15(2):91–95. doi: 10.1016/j.ctcp.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 140.Villasis-Keever M.A., López-Alarcón M.G., Miranda-Novales G., et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch Med Res. 2022;53(4):423–430. doi: 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wall-Gremstrup G., Holt R., Yahyavi S.K., et al. High-dose vitamin D(3) supplementation shows no beneficial effects on white blood cell counts, acute phase reactants, or frequency of respiratory infections. Respir Res. 2024;25(1):11. doi: 10.1186/s12931-023-02642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang B., Hylwka T., Smieja M., Surrette M., Bowdish D.M.E., Loeb M. Probiotics to prevent respiratory infections in nursing homes: a pilot randomized controlled trial. J Am Geriatr Soc. 2018;66(7):1346–1352. doi: 10.1111/jgs.15396. [DOI] [PubMed] [Google Scholar]
- 143.Wang H., Tao L., Cui L., et al. Randomized trial of influence of vitamin D on the prevention and improvement of symptomatic COVID-19. Sci Rep. 2024;14(1) doi: 10.1038/s41598-024-66267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weismann K., Jakobsen J.P., Weismann J.E., et al. Zinc gluconate lozenges for common cold. A double-blind clinical trial. Dan Med Bull. 1990;37(3):279–281. [PubMed] [Google Scholar]
- 145.West N.P., Horn P.L., Pyne D.B., et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014;33(4):581–587. doi: 10.1016/j.clnu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 146.West N.P., Pyne D.B., Cripps A.W., et al. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10(1):30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wischmeyer P.E., Tang H., Ren Y., et al. Efficacy of probiotic treatment as post-exposure prophylaxis for COVID-19: a double-blind, Placebo-Controlled Randomized trial. Clin Nutr. 2024;43(1):259–267. doi: 10.1016/j.clnu.2023.11.043. [DOI] [PubMed] [Google Scholar]
- 148.Yamada H., Daimon T., Matsuda K., Yoshida M., Takuma N., Hara Y. A randomized controlled study on the effects of gargling with tea catechin extracts on the prevention of influenza infection in healthy adults. Jpn J Clin Pharmacol Therapeut. 2007;38(5):323–330. [Google Scholar]
- 149.Zhang H., Miao J., Su M., Liu B.Y., Liu Z. Effect of fermented milk on upper respiratory tract infection in adults who lived in the haze area of Northern China: a randomized clinical trial. Pharm Biol. 2021;59(1):647–652. doi: 10.1080/13880209.2021.1929344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang H., Yeh C., Jin Z., et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3(2):113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steinmann J., Buer J., Pietschmann T., Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weber J.M., Ruzindana-Umunyana A., Imbeault L., Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003;58(2):167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 153.Ghosh R., Chakraborty A., Biswas A., Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. 2021;39(12):4362–4374. doi: 10.1080/07391102.2020.1779818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monobe M., Ema K., Tokuda Y., Maeda-Yamamoto M. Effect on the epigallocatechin gallate/epigallocatechin ratio in a green tea (Camellia sinensis L.) extract of different extraction temperatures and its effect on IgA production in mice. Biosci Biotechnol Biochem. 2010;74(12):2501–2503. doi: 10.1271/bbb.100498. [DOI] [PubMed] [Google Scholar]
- 155.Wang C.-H., Porta L., Yang T.-K., et al. Optimal methods of vitamin D supplementation to prevent acute respiratory infections: a systematic review, dose–response and pairwise meta-analysis of randomized controlled trials. Nutr J. 2024;23(1):92. doi: 10.1186/s12937-024-00990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vanherwegen A.S., Gysemans C., Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46(4):1061–1094. doi: 10.1016/j.ecl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11) doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alberts A., Moldoveanu E.T., Niculescu A.G., Grumezescu A.M. Vitamin C: a comprehensive review of its role in health, disease prevention, and therapeutic potential. Molecules. 2025;30(3):748. doi: 10.3390/molecules30030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hao Q., Dong B.R., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2) doi: 10.1002/14651858.CD006895. [DOI] [PubMed] [Google Scholar]
- 160.Li L., Hong K., Sun Q., et al. Probiotics for preventing upper respiratory tract infections in adults: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/8734140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y., Li X., Ge T., et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95(31) doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.King S., Glanville J., Sanders M.E., Fitzgerald A., Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jungersen M., Wind A., Johansen E., Christensen J.E., Stuer-Lauridsen B., Eskesen D. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12(®) Microorganisms. 2014;2(2):92–110. doi: 10.3390/microorganisms2020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martins F.S., Silva A.A., Vieira A.T., et al. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191(8):623–630. doi: 10.1007/s00203-009-0491-x. [DOI] [PubMed] [Google Scholar]
- 165.Kang C.H., Kim J.S., Park H.M., Kim S., Paek N.S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech. 2021;11(5):217. doi: 10.1007/s13205-021-02767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kwoji I.D., Aiyegoro O.A., Okpeku M., Adeleke M.A. Multi-strain probiotics: synergy among isolates enhances biological activities. Biology (Basel) 2021;10(4):322. doi: 10.3390/biology10040322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McFarland L.V. Efficacy of single-strain probiotics versus multi-strain mixtures: systematic review of strain and disease specificity. Dig Dis Sci. 2021;66(3):694–704. doi: 10.1007/s10620-020-06244-z. [DOI] [PubMed] [Google Scholar]
- 168.Lotito S.B., Zhang W.J., Yang C.S., Crozier A., Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51(2):454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen C., Lin J., Wang X., et al. Novel insights into immune mechanisms in acute lung injury: focusing on gut microbiota and its metabolites. Microbiol Res. 2025;300 doi: 10.1016/j.micres.2025.128279. [DOI] [PubMed] [Google Scholar]
- 170.Lenoir-Wijnkoop I., Gerlier L., Roy D., Reid G. The clinical and economic impact of probiotics consumption on respiratory tract infections: projections for Canada. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lenoir-Wijnkoop I., Gerlier L., Bresson J.L., Le Pen C., Berdeaux G. Public health and budget impact of probiotics on common respiratory tract infections: a modelling study. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lenoir-Wijnkoop I., Merenstein D., Korchagina D., Broholm C., Sanders M.E., Tancredi D. Probiotics reduce health care cost and societal impact of flu-like respiratory tract infections in the USA: an economic modeling study. Front Pharmacol. 2019;10:980. doi: 10.3389/fphar.2019.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ghoreshi Z.A., Charostad J., Arefinia N., Nakhaie M., Rezaei Zadeh Rukerd M., Salajegheh F. Effect of vitamin D supplementation on clinical outcomes in adult patients with COVID-19: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Pharmacol Res Perspect. 2024;12(5) doi: 10.1002/prp2.70013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hemilä H., Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23(1):2468. doi: 10.1186/s12889-023-17229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults – results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 176.Zhao Y., Dong B.R., Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2022;8(8) doi: 10.1002/14651858.CD006895.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hawkes M.T., Lee B.E., Kanji J.N., et al. Seasonality of respiratory viruses at Northern Latitudes. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.