Abstract
Cerebral infarct (stroke) often causes devastating and irreversible losses of function, in part because of the brain's limited capacity for anatomical reorganization. The purine nucleoside inosine has previously been shown to induce neurons to express a set of growth-associated proteins and to extend axons in culture and in vivo. We show here that in adult rats with unilateral cortical infarcts, inosine stimulated neurons on the undamaged side of the brain to extend new projections to denervated areas of the midbrain and spinal cord. This growth was paralleled by improved performance on several behavioral measures.
Cerebral ischemic infarct, or stroke, affects approximately 750,000 people annually in the U.S. alone. Depending on the locus of damage and other complicating factors, stroke can result in devastating losses in sensory, motor, and cognitive functions. A limited amount of synaptic reorganization is thought to occur spontaneously in the brain after stroke (1–5) or focal injury (6, 7) and can be enhanced by training (8, 9). However, there is little evidence that undamaged neurons can extend lengthy projections to reinnervate brain regions that have lost their normal inputs, nor are any clinical methods available to improve functional outcome by stimulating axonal reorganization after stroke.
The purine nucleoside inosine enters cells via facilitated diffusion or can be synthesized readily from adenosine. In at least some neurons, inosine activates an intracellular signaling pathway that regulates the expression of multiple genes involved in axon outgrowth (10, 11). In vivo, inosine treatment can promote extensive sprouting of the intact corticospinal tract (CST) into areas denervated by transecting the contralateral CST (12). Here, we have investigated whether inosine treatment can stimulate axonal growth and help improve behavioral outcome after stroke. The rat's sensorimotor cortex is required for fine motor control of the contralateral limbs (5, 13, 14). After creating a stroke that damaged most of the sensorimotor cortex on one side of the brain, we tested whether inosine could induce neurons in the intact hemisphere to grow new connections to denervated target areas and restore cortical control to the hemiparetic side. Although a number of neural pathways may be important in this regard, we have focused on the corticorubral tract and CST, two pathways that help mediate fine motor coordination (15, 16). Our results demonstrate that inosine stimulates significant axonal reorganization after stroke and leads to improved performance on several sensorimotor tasks.
Methods
Surgery.
Male Sprague–Dawley rats (250–325 g, age range 8–10 weeks: Charles River Breeding Laboratories) were used throughout. In a pilot study done in collaboration with Cerebrotec (Boston), we investigated whether inosine treatment would improve behavioral outcome after stroke. Under halothane anesthesia, rats were placed on their sides, and a vertical incision was made midway between the right orbit and external auditory canal. The underlying temporalis muscle was incised, scraped from the skull, and retracted, with care to preserve the facial nerve. A large craniectomy was performed from the posterior zygoma and along the temporal ridge of the cranium extending ventrally to expose the middle cerebral artery (MCA) and olfactory tract. The dura was opened, and the base of the MCA and the anterior portion of the first branch were electrocoagulated ventral to the olfactory tract, resulting in infarction of the right dorsolateral cerebral cortex and underlying striatum (4, 17). Animals were randomly assigned to receive a continuous infusion of either inosine (50 mM in saline; n = 15) or saline (n = 12) into the cisterna magna using osmotic minipumps (0.25 μl/h, Alzet model 2004, Palo Alto, CA).
The second study, carried out at the University of Lethbridge, examined the behavioral consequences of inosine treatment in more detail and investigated whether changes in performance were related to anatomical reorganization. Rats were prescreened to select those that performed well on the battery of tests described below. Animals in the second study received more extensive right hemisphere strokes than those in the first to increase the amount of target denervation and hence increase the possible sprouting from the intact side. This was done by electrocoagulating the distal branches of the anterior cerebral artery along with the MCA. While still under anesthesia, animals received saline alone or inosine at concentrations of 2, 10, or 50 mM in saline (n = 12 per group) from an osmotic minipump (Model 2004, Alzet) at a flow rate of 0.25 μl/h. Catheters were stereotaxically implanted into the lateral ventricle on the undamaged side of the brain and were held in place with cyanoacrylate; reservoirs were buried under the dorsal fascia of the neck. Pumps were replaced after 28 days.
Behavioral Testing.
In the first study, sensorimotor integration was evaluated over a 19-day period by an investigator blind to the rats' treatment regimen. In the forelimb-placement test, animals were held gently by the torso and were moved slowly toward a table top until the dorsal forepaw surface barely touched the edge. Normal animals rapidly place their forelimb on the table top. Performance was scored between 0 (normal) and 10 (maximal impairment) (18, 19). Similarly, the hindlimb-placing test evaluated the animal's ability to place the hindpaw on a table in response to light stimulation and was scored on a 0–6 scale. In the “body-swing” test, extrapyramidal function was tested by holding the animal by its tail until a total of 30 swings were counted. Scores represent that percentage of times animals turned their head more than 10° to the side ipsilateral to the lesion (20).
In the second study, behavioral testing was done 3, 7, 14, 21, 28, 35, and 42 days after surgery by an experimenter blind to the animals' treatment. The animals that were not used to trace axonal projections (see below) were also tested at 56 days. Forepaw placing in the second study was evaluated by using a simplified three-point scale (3 = approximately normal speed and accuracy; 2 = significant delay but successful placement; 1 = successful placement after marked delay; 0 = no limb placement; see Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org) (21). In the reaching task, animals were trained to reach through bars to grasp food pellets (15). Rats with motor cortex lesions were severely impaired in this task, largely because of difficulty in pronating the wrist and digits over the food and in grasping and retrieving the pellets. Video recordings were used to score the number of times in 7 min that animals extended the impaired paw to the food tray and the percent of reaches that resulted in food consumption. Performance with the impaired paw was tested both with the unimpaired paw constrained and with the animal free to reach with either paw (Movie 2).
Normal animals use only their hindlimbs to swim in a straight line, holding the forepaws motionless under the chin. Unilateral sensorimotor cortex damage disinhibits movement by the contralateral limb, whereas the ipsilateral forelimb remains still (21). Cortical inhibition was evaluated by placing the animal in a glass tank and filming from the side as the rat swam toward a visible platform. The number of strokes made by each forelimb was counted in three trials (Movie 3). The tongue-protrusion test was carried out as described (22). Decreased length of tongue protrusion, measured in millimeters, reflects damage to the tongue representation area of the motor cortex (22). This test was given on days 28, 35, and 42.
Behavioral scores were evaluated by ANOVA to detect significant differences (P < 0.05) between and among treatment groups; Fisher's exact t test was used to determine where those differences occurred. In some cases, planned unpaired, t tests were carried out to compare the combined inosine groups and the untreated group (STATVIEW 5.0 software, SAS, Cary, NC).
Housing and behavioral testing facilities were routinely inspected by the Canadian Council on Animal Welfare. The animal protocol was approved by the Institutional Animal Care and Use Committees of the University of Lethbridge and of Children's Hospital, Boston.
Anatomical Studies.
Six weeks after the initial surgery, half of the animals in each group were anesthetized, the infusion needle and pump were removed, and, after craniotomy, biotinylated dextran amine (BDA: Molecular Probes: 10% wt/vol solution in sterile saline) was injected stereotaxically at depths of 0.5, 1.0, and 2.0 mm below the cortical surface at 18 points distributed over the sensorimotor cortex (70 nl per injection: Nanoject, Drummond Scientific, Broomall, PA). Animals were prepared for histology 2 weeks later. BDA-labeled axons were visualized as described (12, 23). In some cases, a light cellular counterstain was obtained by using neutral red dye to visualize anatomical boundaries. Some sections were double-stained to investigate whether BDA-labeled cortical efferents express growth-associated protein 43 (GAP-43). For this analysis, BDA-labeled axons were visualized with streptavidin conjugated to AlexaFluor-568 (1:1,000, Molecular Probes), whereas GAP-43 was visualized with a sheep anti-GAP-43 IgG (1:2,500) (24) followed by an anti-sheep IgG antibody conjugated to AlexaFluor-488 (1:1,000, Molecular Probes).
Axon crossings in the red nucleus were counted under ×200, with the treatment of the group being counted masked. The midline was identified by using the aqueduct and the paired red nuclei as landmarks. For each case, we averaged the number of BDA-labeled axons that crossed to the denervated side in four standard coronal sections that included the red nucleus; sections were spaced at 240 μm and were centered at the level of the oculomotor nuclei. Images were captured on a Nikon Eclipse Microscope with a Spot Digital Camera system (Universal Imaging System, Downington, PA), and the number of labeled axons on the denervated side was quantified. Results are from at least three randomly selected animals from each group. Within the cervical enlargement of the spinal cord, we used a different counting method, because it was difficult to identify exactly the same levels in each case. Using the central canal and dorsal median fissure as landmarks for the midline, we counted the number of axons crossing from the intact to the denervated side in 50–100 sections per case. The values reported represent the group means of the greatest number of crossed axons counted per section.
When BDA-labeled axons in the cervical enlargement were visualized with horseradish peroxidase histochemistry rather than with AlexaFluor-568-conjugated streptavidin, many more axons of variable intensity were visualized on the denervated side, as seen previously (12). However, because the latter were difficult to quantify accurately, the current counts are based on the fluorescently labeled material.
The remaining animals in each group were used to determine infarct volumes. After routine histological processing, aldehyde-fixed brains were sectioned frozen at 40 μm in the coronal plane. Images of mounted Cresyl violet-stained sections at standardized levels were captured digitally. The cross-sectional area of the neocortex and other structures was measured on both sides of the brain (NIH image software, Ver. 1.62) and was used to estimate volumetric loss. Infarct volumes were also determined in the first study.
Results
Inosine Improves Behavioral Outcome.
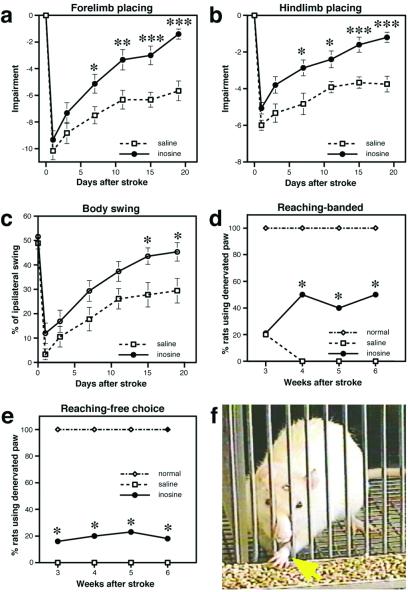
The first study investigated whether inosine would enhance functional recovery in rats with cortical strokes resulting from occlusion of the MCA (Fig. 1a). Tactile placing depends on the integrity of the sensorimotor cortex (19) and, as expected, animals had little use of their denervated forepaw in the first few days after surgery (Fig. 2a). Forepaw placement showed some improvement over time even without inosine, with saline-treated rats achieving approximately 50% of their baseline scores by 19 days. However, with inosine treatment, rats showed a much stronger recovery, returning to near-baseline levels of performance at 19 days; the superiority of the inosine-treated group was apparent by day 7 and increased in significance over the duration of the testing period (Fig. 2a). Inosine treatment similarly improved hindlimb placing, resulting in superior performance relative to controls from day 7 through the final day of testing (Fig. 2b). In the body-swing test, a measure of basal ganglia function (20), the performance of inosine-treated rats was superior to controls at the last two time points (Fig. 2c).
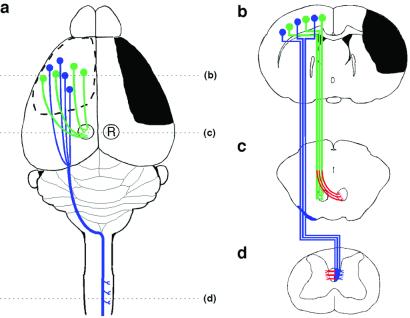
Figure 1.
Schematic illustration of the stroke and major cortical efferent pathways. (a) The sensorimotor cortex and other right hemisphere structures were damaged unilaterally by occluding the right MCA; in the second set of studies, parts of the anterior cerebral artery were occluded as well. The pathways originating from the intact left hemisphere that were traced with BDA include the corticorubral (green) and corticospinal (blue) tracts. (b) Coronal section through the forebrain showing the extent of injury (black) and the cells of origin of the intact corticospinal (blue) and corticorubral (green) tracts. (c) Projections from the sensorimotor cortex to the ipsilateral red nucleus (R). Compensatory growth to the denervated (Right) red nucleus, as investigated here, is shown in red. (d) CST projections from the intact hemisphere decussate in the caudal medulla, course in the contralateral dorsal funiculus, and synapse primarily on layer 4–6 interneurons in the cervical and lumbar enlargements of the spinal cord. Inosine-induced collateral projections to the denervated side are shown in red.
Figure 2.
Behavioral recovery after stroke. (a) In the first study, forelimb placing was evaluated by using a 10-point scale as described (4, 17). Animals treated with inosine performed better than saline-treated controls from day 7 onward. (b) Hindlimb placing of control and inosine-treated animals was scored on a six-point scale (4, 17). (c) Body swing, a measure of basal ganglion dysfunction, is scored by the percentage of times animals turn more than 10° to the side ipsilateral to the lesion when suspended from the tail (20). (d) Food retrieval: constrained. Score represents the percent of animals that used their impaired (left) paw when the right paw was constrained. (e) Food retrieval: free choice. Percent of animals using the impaired paw when free to use either paw. (f) Inosine-treated rat spontaneously using its impaired left paw (yellow arrow) to retrieve food pellets even when the intact right paw was unconstrained. *, P < 0 05; **, P <0.01; ***, P < 0.001.
Volumetric measures showed no differences in the extent of forebrain damage between inosine-treated animals (28.4 ± 1.0% volume loss) and controls (28.4 ± 1.5% loss). Thus, inosine does not appear to be neuroprotective.
To investigate whether the beneficial effects of inosine are related to a reorganization of neural pathways, we carried out a second study that included behavioral analysis and anatomical tracing of projections from the intact hemisphere. In addition to the MCA, we occluded several branches of the anterior cerebral artery to increase the denervation of the midbrain and spinal cord, thereby potentially increasing the area available for compensatory axon growth from the intact hemisphere (Fig. 1 b–d).
As in the first study, forepaw placing in inosine-treated animals was superior to that of controls at 3 weeks [mean ± SEM = 2.34 ± 0.17 for inosine-treated group, 1.40 ± 0.37 for controls; t(43) = 2.5, P < 0.02: Movie 1, which is published as supporting information on the PNAS web site]. In this and all other behavioral tests, no significant differences appeared among the groups receiving the three different inosine concentrations; we therefore pooled these groups to increase statistical reliability. On the basis of the simplified three-point scale that was used in the second study, the inosine- and vehicle-treated groups both appeared to attain normal forepaw placing by week 6. However, using a more sensitive measure of tactile performance, latency to place the paw, the superiority of inosine-treated animals remained evident at later time points [mean latency ± SEM = 185.1 ± 17.6 msec for inosine-treated groups at week 8, and 255.4 ± 25 msec for controls; t(21) = 2.6, P = 0.01].
Food retrieval is another task that requires the integrity of the sensorimotor cortex (14). When the normally innervated right paw was constrained, a few vehicle-treated controls attempted to use their denervated paw on week 3. However, none succeeded in gaining food, and this behavior became extinguished at later time points. In contrast, by week 4, 50% of the inosine-treated animals used their denervated left paw to retrieve food pellets when the right forelimb was constrained; these animals averaged from 37 ± 13 attempts per session at week 4 to 45 ± 9 attempts per session at week 6, the final time point, with a success rate of 18 ± 7% (difference between inosine-treated animals vs. controls significant at χ2 = 9.0, P < 0.02 for weeks 4–6: Fig. 2b). Remarkably, even when given a free choice of which limb to use, 20% of the inosine-treated rats preferentially used their denervated left forelimb, averaging 44 ± 16 reaches per session and an accuracy rate of 7% (Movie 2). During the entire test period, no vehicle-treated control ever spontaneously used its denervated paw to reach for food when the right limb was unconstrained nor did any animal with even less severe sensorimotor cortical damage in a previous study (B.K., unpublished data; difference in spontaneous use of denervated limb between inosine-treated rats and controls significant at χ2 = 7.3, P < 0.01). By week 8, animals treated with inosine showed significantly better forepaw inhibition during swimming than controls [mean number of strokes ± SEM = 6.7 ± 1.3 for inosine-treated group, 13.2 ± 1.1 for controls; t(21) = 6.5, P < 0.01: Movie 3] and by week 6 also performed better on the tongue-extension test [mean length ± SEM = 7.77 ± 0.24 mm for inosine, 6.35 ± 0.42 mm for controls; t(43) = 3.0, P < 0.01]. In sum, inosine treatment enhanced performance on four independent behavioral measures.
Anatomical Reorganization.
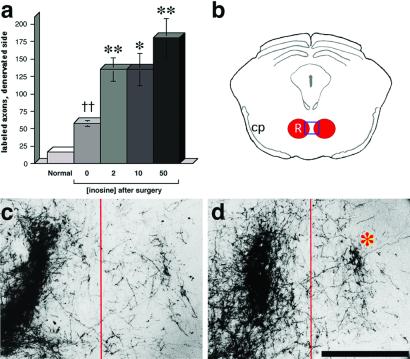
Axonal reorganization after brain injury is often difficult to prove convincingly because of the problem of distinguishing new connections from those that had not been damaged in the first place. However, because the corticospinal and corticorubral tracts are almost completely lateralized (25, 26), reorganization of these pathways would be apparent if the denervated side of the brain were to show a significant increase in the number of axons arising from the uninjured hemisphere (12, 27, 28) (Fig. 1). Axons originating in the intact hemisphere were labeled by injecting BDA into the left sensorimotor cortex at the end of the testing period (i.e., at 6 weeks). After allowing an additional 2 weeks for the BDA to be transported to distal axonal terminals, animals were prepared for histology. Midline crossings of the corticorubral tract were quantified by counting BDA-labeled fibers in the denervated red nucleus. The number of crossed corticorubral fibers was low in normal control animals and increased after stroke, even in animals treated with saline alone (Fig. 3 a and c). Inosine treatment had a greater effect, increasing the number of crossed corticorubral axons 8- to 10-fold above the number seen in normal animals, i.e., an additional 2- to 3-fold over the level seen in injured animals receiving saline (Fig. 3 a and d).
Figure 3.
Axon crossing at the level of the red nucleus. (a) Quantitation of axons crossing from the intact side of the brain to the denervated red nucleus (averaged from four sections per case). (b) Schematic coronal section through the midbrain at the level of the red nucleus. Box (red) indicates approximate area shown in c and d. Labeled axons in the vicinity of the red nucleus are shown on the normal side (Left) and on the denervated (Right) side in an animal treated with saline after stroke (c), and in an animal treated with inosine after stroke (d). *, P < 0.05; **, P < 0.01. (Bar in d = 500 μm.)
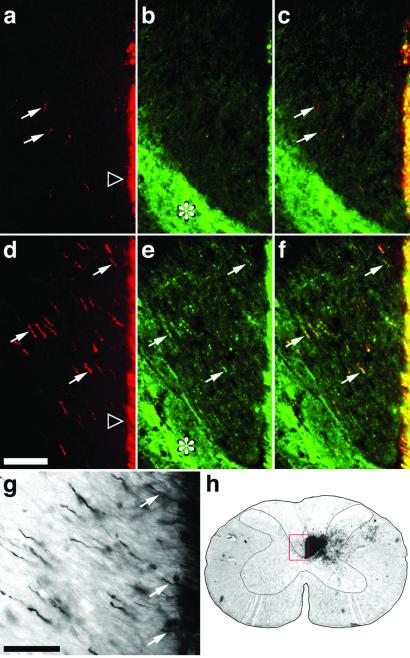
The effects of inosine on axon growth were also evident in the cervical enlargement, the region of the spinal cord that innervates the forelimbs and a principle target of the CST. In normal rats, BDA injections into the sensorimotor cortex labeled a maximum of 29 ± 2.5 (mean ± SEM) axons per section in the ipsilateral dorsal funiculus and adjacent neuropil. Eight weeks after stroke, saline-treated animals showed only a slight increase in this number (38.5 ± 2.5: Fig. 4a). In contrast, inosine had a profound effect (Fig. 4d), increasing the maximum number of crossed CST axons visualized per section to 121.6 ± 18.3 (difference from saline-treated controls significant at P < 0.005). Double labeling revealed that most of the crossed axons in inosine-treated animals were immunopositive for the growth-associated protein, GAP-43 (Fig. 4 e and f). This finding suggests that the crossed axons are still in a growth mode. In contrast, the few BDA-labeled CST axons seen on the denervated side of the spinal cord in saline-treated animals showed little or no GAP-43 staining (Fig. 4 b and c).
Figure 4.
Axon crossing in the cervical spinal cord. (a–g) Transverse sections through the cervical enlargement showing the area of the denervated CST. The midline and medial-most fibers of the intact CST are on the far right side of each frame. (h) Low-power photo through the cervical spinal cord showing the approximate area enlarged in a–g (red frame). (a–c) Double-labeled section from a control animal treated with saline after stroke. (d–f) Similarly stained sections from an animal treated with inosine after stroke. (a, d) BDA-labeled fibers originating in the intact hemisphere, stained red here, projecting to the denervated side of the spinal cord (arrows) in saline- (a) and inosine-treated (d) animals. (b, e) Fibers with high levels of the growth-associated protein GAP-43 (green fluorescence) in the same sections shown in a and d, respectively. The intense fluorescence in the neuropil lateral to the CST (asterisks) is unaffected by treatment. (c, f) Merged images showing that crossed, BDA-labeled fibers contain high levels of GAP-43 (yellow staining: arrows). (g) Section from an inosine-treated animal stained with horseradish peroxidase-conjugated avidin–biotin complex followed by diaminobenzidine. Arrows show axons crossing the midline. (Bar a–f, 100 μm; g, 50 μm.)
Postmortem measurements of stroke volume in the second series of animals again revealed no differences between inosine-treated animals (36.3 ± 1.1% volume loss) and controls (33.1 ± 4.1% loss). The cortical damage included most of the sensorimotor cortex, encompassing all of the parietal cortex, although the most dorsal cortex was spared direct damage. The motor cortex was nonetheless deafferented, as the anterior thalamus on the lesioned side showed severe gliosis and calcification of the ventral lateral complex, in addition to neuronal loss and gliosis in the anterior ventral, dorsal medial, and ventral medial nuclei in all brains. Most of the fibers forming the internal capsule were degenerated, as were much of the anterior striatum and globus pallidus. These morphological changes were highly consistent in all injured brains. Other infarcted areas included most of the insular, piriform, and perirhinal cortices and parts of the amygdaloid complex.
Discussion
These studies show that inosine induces significant axonal reorganization in the rat brain after stroke and helps restore cortical control of the denervated forelimb. Poststroke changes in brain organization have long been inferred from the partial recovery that often occurs clinically over time and, more recently, from changes in brain activity visualized by neuroimaging techniques and electrophysiology (1, 2, 8, 29, 30). In addition, animal experiments have shown changes in dendritic morphology after unilateral infarcts or focal injury, along with immunohistochemical changes for certain synaptic proteins, usually in the intact hemisphere (3–7, 13, 31, 32). A priori, however, most of these changes could reflect small local shifts in synaptic organization, rather than major alterations in brain wiring. The present study shows that some axonal reorganization does indeed occur spontaneously in the corticospinal and corticorubral tracts after stroke. However, these spontaneously occurring changes are small compared with those induced by inosine treatment. Although we have focused on two major pathways whose rewiring can be demonstrated readily, it is likely that inosine stimulated anatomical changes in other pathways as well, which may have contributed to the observed functional improvements. In other studies, spontaneous compensatory growth has been demonstrated in the hippocampus after lesioning the perforant pathway (33), in the spinal cord after transecting the CST (12, 34), and in the visual cortex after lesions (7).
Inosine, acting through a direct intracellular mechanism, induces neurons in culture to express a constellation of genes associated with axon growth, including GAP-43, L1, and α-1 tubulin (10, 11). The increased GAP-43 immunoreactivity seen in the crossed CST fibers of the present study suggests that inosine may act similarly in vivo, inducing a program of gene expression that enables cortical pyramidal cells to extend axonal branches. The growth of these branches through the heavily myelinated dorsal funiculus further suggests that inosine enables growing axons to overcome at least some of the molecular signals that normally inhibit growth. In vitro, 6-thioguanine, a purine analog that is structurally related to inosine, blocks the effects of trophic factors on neurite outgrowth and on the induction of growth-associated proteins (10, 11, 35–37). In low micromolar concentrations, 6-thioguanine acts selectively as an inhibitor of N-kinase, a partially characterized, growth factor-sensitive, serine-threonine kinase (36). Inosine competitively reverses the inhibitory effects of 6-thioguanine, and we have therefore suggested that inosine stimulates axon growth by acting as an N-kinase agonist (11).
Trauma elevates inosine levels in the brain (38, 39); this, together with other trophic factors that are released from neurons, glia, or monocytes (40–43), may contribute to the limited reorganization that occurs spontaneously after stroke. Among the polypeptide growth factors that are released after injury, fibroblast growth factor (FGF) -1 and neurotrophin-3 have been shown to promote some regeneration of the injured CST (44, 45). Osteogenic protein-1, nerve growth factor, and FGF-2 help improve functional outcome after stroke and are associated with either enhanced dendritic growth or increased levels of particular synaptic proteins, e.g., synaptophysin and/or GAP-43 (4, 32, 46). It will be important to establish the temporal window of opportunity in which inosine can help stimulate axon growth after stroke. In a previous study, inosine strongly stimulated CST sprouting when delivery was begun 24 h after transecting the contralateral CST (12). Also of clinical importance is the spectrum of neurons that can be targeted by inosine. Finally, another question is whether the effects of inosine can be potentiated by training, because training can enhance functional and anatomical changes even without pharmacological interventions (8, 9).
In animal models, antioxidants, caspase inhibitors, glutamate receptor blockers, and a number of other agents improve functional outcome after stroke by inhibiting cell death (47). Inosine does not appear to exert neuroprotective effects, as evidenced by its failure to diminish stroke volume. Because its effects on stimulating axonal rewiring are complementary to those of neuroprotective agents, and because it is a naturally occurring metabolite with few adverse effects, inosine treatment may represent a novel approach to improving function after stroke or CNS trauma.
Supplementary Material
Acknowledgments
We thank Drs. Zhigang He, Sonal Jhaveri, Nina Irwin, and Paul Rosenberg for critically reading the manuscript; Lijie Wang and Mingyan Hu (Children's Hospital) for carrying out the histology; Dr. Seth Finklestein and other investigators at Cerebrotec for assistance with the first set of behavioral studies; Dic Charge and Claudia Gonzalez (NeuroDetective, Inc., Lethbridge, Alberta, Canada) for assistance with the behavioral analysis in the second set of studies; and Jen Lata (Boston Life Sciences, Incorporated) for coordinating parts of this project. We are grateful for the support of the Christopher Reeve Paralysis Foundation (to L.B.), the National Institutes of Health (R21 NS41996 to L.B.), and Boston Life Sciences, Incorporated.
Abbreviations
- BDA
biotinylated dextran amine
- CST
corticospinal tract
- MCA
middle cerebral artery
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nelles G, Spiekramann G, Jueptner M, Leonhardt G, Muller S, Gerhard H, Diener H C. Ann Neurol. 1999;46:901–909. doi: 10.1002/1531-8249(199912)46:6<901::aid-ana13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Weiller C, Chollet F, Friston K J, Wise R J, Frackowiak R S. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 3.Jones T A, Schallert T. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 4.Kawamata T, Dietrich W D, Schallert T, Gotts J E, Cocke R R, Benowitz L I, Finklestein S P. Proc Natl Acad Sci USA. 1997;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroemer R P, Kent T A, Hulsebosch C E. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 6.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 7.Darian-Smith C, Gilbert C D. Nature (London) 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 8.Nudo R J, Wise B M, SiFuentes F, Milliken G W. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 9.Jones T A, Schallert T. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benowitz L I, Jing Y, Tabibiazar R, Jo S A, Petrausch B, Stuermer C A, Rosenberg P A, Irwin N. J Biol Chem. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- 11.Petrausch B, Tabibiazar R, Roser T, Jing Y, Goldman D, Stuermer C A, Irwin N, Benowitz L I. J Neurosci. 2000;20:8031–8041. doi: 10.1523/JNEUROSCI.20-21-08031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benowitz L I, Goldberg D E, Madsen J R, Soni D, Irwin N. Proc Natl Acad Sci USA. 1999;96:13486–13490. doi: 10.1073/pnas.96.23.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schallert T, Fleming S M, Leasure J L, Tillerson J L, Bland S T. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 14.Whishaw I Q, Pellis S M, Gorny B P, Pellis V C. Behav Brain Res. 1991;42:77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 15.Whishaw I Q, Kolb B. Brain Res. 1988;451:97–114. doi: 10.1016/0006-8993(88)90753-6. [DOI] [PubMed] [Google Scholar]
- 16.Raineteau O, Fouad K, Noth P, Thallmair M, Schwab M E. Proc Natl Acad Sci USA. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura A, Graham D I, McCulloch J, Teasdale G M. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- 18.Kawamata T, Alexis N E, Dietrich W D, Finklestein S P. J Cereb Blood Flow Metab. 1996;16:542–547. doi: 10.1097/00004647-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 19.De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- 20.Borlongan C V, Sanberg P R. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb B, Tomie J A. Behav Brain Res. 1988;28:259–274. doi: 10.1016/0166-4328(88)90129-5. [DOI] [PubMed] [Google Scholar]
- 22.Whishaw I Q, Haun F, Kolb B. In: Modern Techniques in Neuroscience Research. Widhorst U, editor. Heidelberg: Springer; 1999. pp. 1243–1275. [Google Scholar]
- 23.Grill R, Murai K, Blesch A, Gage F H, Tuszynski M H. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benowitz L I, Apostolides P J, Perrone-Bizzozero N, Finklestein S P, Zwiers H. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown L T. J Comp Neurol. 1974;154:149–167. doi: 10.1002/cne.901540204. [DOI] [PubMed] [Google Scholar]
- 26.Brown L T., Jr Exp Brain Res. 1971;13:432–450. doi: 10.1007/BF00234340. [DOI] [PubMed] [Google Scholar]
- 27.Z'Graggen W J, Metz G A, Kartje G L, Thallmair M, Schwab M E. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thallmair M, Metz G A, Z'Graggen W J, Raineteau O, Kartje G L, Schwab M E. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 29.Cramer S C, Nelles G, Benson R R, Kaplan J D, Parker R A, Kwong K K, Kennedy D N, Finklestein S P, Rosen B R. Stroke (Dallas) 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz B E, Dietrich W D, McCabe P M, Watson B D, Ginsberg M D, Schneiderman N. Brain Res. 1990;512:210–220. doi: 10.1016/0006-8993(90)90628-o. [DOI] [PubMed] [Google Scholar]
- 31.Jones T A. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- 32.Kolb B, Cote S, Ribeiro-da-Silva A, Cuello A C. Neuroscience. 1997;76:1139–1151. doi: 10.1016/s0306-4522(96)00448-4. [DOI] [PubMed] [Google Scholar]
- 33.Cotman C W, Nieto-Sampedro M. Annu Rev Psychol. 1982;33:371–401. doi: 10.1146/annurev.ps.33.020182.002103. [DOI] [PubMed] [Google Scholar]
- 34.Weidner N, Ner A, Salimi N, Tuszynski M H. Proc Natl Acad Sci USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volonte C, Rukenstein A, Loeb D M, Greene L A. J Cell Biol. 1989;109:2395–2403. doi: 10.1083/jcb.109.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batistatou A, Volonte C, Greene L A. Mol Biol Cell. 1992;3:363–371. doi: 10.1091/mbc.3.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene L A, Volonte C, Chalazonitis A. J Neurosci. 1990;10:1479–1485. doi: 10.1523/JNEUROSCI.10-05-01479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson P, Hillered L, Ponten U, Ungerstedt U. J Cereb Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 39.Bell M J, Kochanek P M, Carcillo J A, Mi Z, Schiding J K, Wisniewski S R, Clark R S, Dixon C E, Marion D W, Jackson E. J Neurotrauma. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- 40.Charytoniuk D A, Traiffort E, Pinard E, Issertial O, Seylaz J, Ruat M. Neuroscience. 2000;100:33–43. doi: 10.1016/s0306-4522(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 41.Finklestein S P, Caday C G, Kano M, Berlove D J, Hsu C Y, Moskowitz M, Klagsbrun M. Stroke (Dallas) 1990;21:III122–III124. [PubMed] [Google Scholar]
- 42.Lindvall O, Ernfors P, Bengzon J, Kokaia Z, Smith M L, Siesjo B K, Persson H. Proc Natl Acad Sci USA. 1992;89:648–652. doi: 10.1073/pnas.89.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batchelor P E, Liberatore G T, Wong J Y, Porritt M J, Frerichs F, Donnan G A, Howells D W. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng H, Cao Y, Olson L. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 45.Schnell L, Schneider R, Kolbeck R, Barde Y A, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 46.Kawamata T, Ren J, Chan T C, Charette M, Finklestein S P. NeuroReport. 1998;9:1441–1445. doi: 10.1097/00001756-199805110-00035. [DOI] [PubMed] [Google Scholar]
- 47.Lee J M, Zipfel G J, Choi D W. Nature (London) 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.