Abstract
The viral cap-snatching mechanism, facilitated by the endonuclease (EndoN) domain of viral polymerases, is critical for viral gene transcription and translation and is therefore an attractive target for antiviral development. The successful development of EndoN inhibitor XOFLUZA (Baloxavir marboxil) has opened the possibility that the EndoN domain of negative-sense RNA viruses (NSVs) can be targeted in a similar fashion. Rift Valley Fever Virus (RVFV) belongs to the Bunyaviricetes class, which includes other pathogens with pandemic potential, such as severe fever with thrombocytopenia syndrome virus (SFTSV) and Heartland virus (HRTV). Here, we identify MBXC-4522 as a RVFV EndoN inhibitor using a high-throughput FRET-based assay. We screened a library of >20,000 compounds and identified those that target the RVFV EndoN domain. MBXC-4522, a spiro piperidine pyrido pyridine, directly binds the RVFV EndoN domain, increases protein stability, and inhibits EndoN activity. MBXC-4522 acts in a metal binding-independent mechanism, while XOFLUZA’s mode of action is metal binding-dependent. Infectious assays also support the ability of MBXC-4522 to inhibit pathogenic RVFV (ZH501), suggesting that hit-to-lead optimization and future in vivo validation are warranted.
Keywords: antiviral, endonuclease inhibitor, Rift Valley Fever Virus, bunyavirus


In mammals, 5′ cap recognition is widely used by cytosolic innate immune proteins to determine whether an mRNA transcript is self or nonself. To avoid activating host innate responses and ensure translation of viral genes, some viruses steal 5′ caps from host transcripts in a process called cap-snatching. This replication-critical process not only facilitates the generation of viral mRNAs but also leads to the downregulation of host mRNAs, limiting host innate immune responses. Cap-snatching was first described and is best understood in the context of influenza A virus (IAV), a member of the family Orthomyxoviridae. The IAV PA protein contains an N-terminal endonuclease (EndoN) domain that requires divalent metal cation binding for activity. − Use of the snatched cap results in a host-capped viral transcript capable of evading recognition by host innate immune proteins. ,
In addition to IAV, several viruses use the cap-snatching mechanism, including some arenaviruses and most bunyaviruses. Rift Valley Fever Virus (RVFV), severe fever with thrombocytopenia syndrome virus (SFTSV), and Heartland virus (HRTV) are predominantly arthropod-borne viruses in the Bunyaviricete class that use their EndoN domain for RNA cap snatching. These viruses have tripartite RNA genomes, wide geographic distributions, and a history of severe infections in livestock and sporadic human disease. , Epizootic outbreaks of RVFV have occurred in livestock in Africa and the Middle East since the first reported outbreak in Kenya’s Rift Valley in 1931. During outbreaks, RVFV can cause spontaneous abortion rates of up to 100% in pregnant livestock and fatality rates greater than 90% in young animals. These livestock infections also cause significant socioeconomic impact in endemic regions of sub-Saharan Africa and the Arabian Peninsula. − Zoonotic RVFV outbreaks in humans occur through mosquito-vectored transmission or direct contact with the blood or tissue of infected animals, with severe cases progressing to ocular, hemorrhagic fever, or meningoencephalitis forms. Closely related to RVFV, SFTSV is an emerging tick-borne virus that causes severe fever and thrombocytopenia syndrome. SFTSV was identified as the causative agent of the syndrome two years after outbreaks began in the rural Chinese provinces of Hubei and Henan. , Like RVFV, SFTSV has spread from endemic regions to nonendemic regions, including other provinces in China as well as Japan, South Korea, Vietnam, Myanmar, Thailand, and Taiwan, with case fatality rates as high as 45.7%. , HRTV is another emerging tick-borne virus closely related to RVFV and SFTSV. Endemic to the United States, HRTV circulates in wildlife reservoirs such as deer and raccoons and is transmitted zoonotically to humans through the bites of infected ticks. , HRTV causes Heartland virus disease, and though fewer than 100 cases have been reported, two were fatal, resulting in a case fatality rate of 5–10%. , Because the disease is largely underreported, it is estimated that the actual impact of HRTV is far greater. Together, RVFV, SFTSV, and HRTV are emerging viruses with pandemic potential.
At present, there are no specific medical countermeasures for prophylactic or therapeutic treatment of RVFV. Availability of crystal structures of multiple endonuclease domains have set the stage to target a catalytic metal-binding pocket that appears to be highly conserved across cap-snatching NSVs. ,− These efforts are further supported by the approval of XOFLUZA, which reduces IAV replication by targeting this conserved interface upon metabolic conversion to the active form of XOFLUZA, baloxavir acid (BXA). , Here we describe efforts to use a high-throughput (HTS) assay to identify hit compounds that target the RVFV N-terminal EndoN domain (RVFV-EndoN) using a fluorescence resonance energy transfer (FRET)-based activity assay, modeled on IAV-PA activity studies. Initial screening of ∼ 20,000 compounds of a proprietary library (MBX) resulted in 55 distinct hit compounds, including MBXC-4522, a member of the spiro piperidine pyrido pyridine class of compounds. Biochemical characterization reveals that MBXC-4522 binds to and stabilizes RVFV-EndoN in a dose-dependent manner with a low-micromolar IC50. In further support, we show that MBXC-4522 inhibits the infection of both RVFV vaccine strain MP12 and pathogenic strain ZH501 in vitro. Our data also show modest inhibition of other bunyaviruses, including HRTV and SFTSV. Notably, MBXC-4522 inhibits EndoN activity independently of metal chelation, suggesting a mode of action distinct from BXA. These results support the identification of a new class of EndoN inhibitors that inhibit RVFV replication and warrant further optimization and functional validation.
Results and Discussion
We previously published a small-scale FRET-based assay which utilized a FITC-tagged, Iowa Black-quenched ssRNA probe as an effective reporter of endonuclease activity. To improve upon and adapt this assay to high-throughput screens of potential antiviral compounds, we designed a 21mer ssRNA probe, which uses a FAM fluorophore while maintaining the Iowa Black quencher. As depicted in the assay schematic in Figure A, the ssRNA linker is cleaved in a sequence-independent manner in the presence of active EndoN protein, resulting in separation of the quencher from the fluorescent tag and producing a fluorescent signal. Previous studies have documented that key catalytic residues of the EndoN chelation pocket are conserved across cap-snatching segmented NSVs. − , Published crystal structures also support this observation for SFTSV-EndoN and IAV-PA. , Sequence alignment reveals high homology among several EndoN domains from multiple viruses, including RVFV-EndoN (GenBank entry DQ375406.1) and SFTSV-EndoN (GenBank Accession AFK08658.1). We note that key catalytic residues D111A in RVFV-L and D112A in SFTSV-L were also conserved. , Based on the importance of the conserved catalytic pocket, we hypothesized that successful endonuclease inhibitors would target these conserved residues in a manner similar to BXA. We also expected to improve assay sensitivity and potentially identify hit compounds that may function distinctly from BXA.
1.
Hit compound MBXC-4522 inhibits RVFV-EndoN activity in biochemical assays. (A) A schematic of the FRET assay described in this study. A self-quenched ssRNA probe is cleaved in the presence of purified endonuclease protein, resulting in a quantifiable fluorescent signal with a real-time readout. F, FAM fluorophore; EndoN, endonuclease; Q, Iowa Black quencher. (B) Initial HTS FRET-based experiment shows that MBXC-4522 has a dose-dependent impact on RVFV-EndoN activity. Four-parameter nonlinear regression calculates that the concentration of compound required to inhibit activity by 50% (IC50) is 2.8 μM. Inset: Line drawing of the chemical structure of hit compound MBXC-4522. (C) Protein thermal shift assay (TSA) shows a dose-dependent increase in thermostability of EndoN in the presence of increasing MBXC-4522 concentrations. (D) Normalized protein TSA curves show the thermal shifts to RVFV-EndoN caused by the indicated additives. (E) A summary of the data from (D) shows that BXA (orange) only stabilizes RVFV-EndoN in the presence of MnCl2, indicating a dependence on metal binding. MBXC-4522 (blue) stabilizes RVFV-EndoN in the presence or absence of MnCl2, strongly suggesting that it is chelation-independent and has a different mode of action than BXA. Stabilization of RVFV-EndoN by MBXC-4522 is significantly larger than that by BXA even in the presence of cation. ns, not significant. ***, p < 0.001.
Following HTS assay optimization (see Supporting Information for details), small molecule libraries containing 20,480 compounds were screened at 1 μM in the HTS RVFV-EndoN FRET-based HTS assay, resulting in 55 hits with confirmed activity (≥70% inhibition). The 55 initial hits were also evaluated in secondary assays, such as cell cytotoxicity assays, expanded FRET-based activity assays, and protein thermal shift assays (TSA). Of the 55 initial hits, MBXC-4522 (Figure B inset), belonging to the spiro piperidine pyrido pyridine class of compounds, was prioritized based on its chemical structure and activity in the FRET assay. MBXC-4522 inhibits RVFV-EndoN activity in a dose-dependent manner, with an IC50 value of 2.8 μM in the dose-dependent FRET experiment (Figure B).
Protein TSA, or differential scanning fluorimetry or (DSF), can be used as an indirect measure of compound-protein binding. We found that addition of MBXC-4522 to RVFV-EndoN protein results in dose-dependent increases to RVFV-EndoN thermostability, suggesting binding (Figure C). Consistent with literature, addition of excess cation in the form of MnCl2 increases RVFV-EndoN activity. To determine the chelation-dependence of the inhibitory action of MBXC-4522 on RVFV-EndoN, we performed the protein TSA on RVFV-EndoN in the presence and absence of 1 mM MnCl2 with either MBXC-4522 or BXA. Increased stability of RVFV-EndoN with BXA was observed only in the presence of MnCl2, consistent with its chelation-dependent binding mechanism (Figure D). In contrast, MBXC-4522 increased the stability of RVFV-EndoN by the same amount with or without MnCl2 (Figure D). This data provide strong evidence that MBXC-4522 operates independently of chelation, providing a different mode of action compared with BXA. Comparison of BXA or MBXC-4522 binding to RVFV-EndoN was performed by subtracting the thermal stability of RVFV-EndoN with 5% DMSO from the values for matched conditions; e.g., the value for RVFV-EndoN + DMSO + MnCl2 was subtracted from the value for RVFV-EndoN + BXA + MnCl2. These results reveal that binding of BXA to RVFV-EndoN is dependent on the presence of MnCl2, while MBXC-4522 binds RVFV-EndoN independently of the presence or absence of MnCl2 as indicated by the thermostability results (Figure E). To ensure metal contaminants from purification were not confounding the interpretation of these results, RVFV-EndoN was treated overnight with EDTA, a common chelating agent, followed by desalting. The EDTA-treated protein was then compared to the untreated protein in both 2D HSQC NMR and in protein TSA (Figure S3). In the HSQC NMR, we found no differences between the peaks of treated and untreated protein (Figure S3A) as well as no differences in thermostability between treated and untreated protein (Figure S3B). These data indicate that EDTA treatment does not change the conformation or the compound-binding capabilities of RVFV-EndoN and show that there are no contaminating metals responsible for the results that we observe. Taken together, our data suggest that MBXC-4522 functions by a previously unreported mechanism for a small molecule inhibitor of RVFV EndoN.
To eliminate the question of MBXC-4522 causing background fluorescence, control experiments were conducted (Figure S4). First, a UV spectrum was obtained to assess the background fluorescence of MBXC-4522 in the FAM range. Relative to a sample containing DMSO and ssRNA digested by RVFV-EndoN (digested probe), the fluorescence of MBXC-4522 was negligible at all tested wavelengths (Figure S4A). Next, DMSO or MBXC-4522 was added to the ssRNA probe digested overnight by RVFV-EndoN. Following a 10 min incubation period, the fluorescent signal was read in the FAM range. We found that the fluorescent signals of these two samples were not significantly different at any wavelength tested (Figure S4B), suggesting the absence of signal quenching of FAM. Further, an excitation scan of MBXC-4522 with an emission of 535 nm showed two optimal excitation wavelengths, 270 and 370 nm, but not at 485 nm (FAM) or 488 nm (GFP) (Figure S4C). Finally, an absorbance spectrum of MBXC-4522 found that it does not absorb at 535 nm (Figure S4D). Taken together, these data show that MBXC-4522 does not interfere with the fluorescence signals used in our assays.
Next, we evaluated MBXC-4522 in cell cytotoxicity assays, which revealed MBXC-4522 has favorable CC50 values of 30.1 μM in HEK293 cells (Figure S5A), 73.8 μM in HepG2 cells (Figure S5B), 45.0 μM in Vero6 cells (Figure S5C), and 43.0 μM in BV2 cells (Figure S5D). To further explore the chemical scaffold, analogs of MBXC-4522 were synthesized, screened in the primary FRET assay and counterscreened for cytotoxicity (Figure ). The analog compounds, MBXC-4520, −4521, −4523, and −4524, maintain the core structure of MBXC-4522 with variations to the R1 group. R1 modifications include nonaromatic (MBXC-4520) and aromatic (MBXC-4521, −4523, and −4524) substituents with varied logP (Figure ; Table S1). Initial FRET-based activity data reveal that the analogs have differing effects on RVFV-EndoN (Figure C). Cell cytotoxicity assays also show that the analogs are less cytotoxic than the parent compound (Figure D; Figure S5; Table S1).
2.
Analog compounds MBXC-4520, −4521, −4523, and −4524 cause less cytotoxicity but showed lower potency relative to parent compound MBXC-4522. (A) Line drawings of the chemical scaffolds for compound synthesis. (B) A table excerpt from Table S1 showing the scaffolds, IC50 values, and CC50 values for the selected analog compounds. (C) FRET-based activity assay shows that the analog compounds have differing inhibitory impacts on RVFV-EndoN activity, with IC50 values calculated by four-parameter nonlinear regression and summarized in the table in (B). (D) Cytotoxicity of analogs in HEK293 cells was determined via CellTiter Glo 2.0 assay, plotted against compound concentration, and fitted with a nonlinear regression to calculate the CC50 values, which are summarized in (B). Error bars were calculated for each point but only displayed when the height of the error bar would exceed the height of the symbol.
Following these initial tests, the analogs were then evaluated against RVFV-EndoN in dose-dependent FRET-based activity assays as well as the protein TSA (Figure S6). MBXC-4520 and -4521 showed little to no activity in these assays, with negligible decreases to RVFV-EndoN activity and less than one degree of thermal shift (Figure S6A-D). Of the four analogs tested, the inhibition activity of MBXC-4523 is most similar to MBXC-4522 (Figure S6E). The protein TSA data reveal that MBXC-4523 is stabilizing to RVFV-EndoN, albeit to a lesser degree than MBXC-4522 (Figure S6F). MBXC-4524 modestly reduces the activity of RVFV-EndoN (Figure S6G) but does not appear to have a clear dose-dependent impact on the protein TSA results (Figure S6H). Due to the minimal inhibitory effects observed of MBXC-4520 and MBXC-4521, these two compounds were eliminated from subsequent evaluation.
Next, compounds were tested in a BSL2 infectivity assay to determine whether inhibition of EndoN activity resulted in defects in RVFV infection. Live-attenuated RVFV-MP12GFP virus was used to infect mouse BV2 cells which were pretreated with DMSO, MBXC-4522, −4523, or −4524. We found that MBXC-4522 significantly inhibits viral GFP signal at 50 μM (Figure A); further, this inhibition is dose-dependent and occurs with as little as 5 μM compound (Figure B). In contrast, MBXC-4523 and MBXC-4524 do not show significant visual or quantitative inhibition of the RVFV-MP12GFP infection in this assay. As a result, MBXC-4523 and −4524 were omitted from subsequent live virus experiments.
3.

Compound MBXC-4522 significantly inhibits RVFV-MP12 GFP infection of BV2 cells in a dose-dependent fashion. (A) Bright field and GFP fluorescence images of BV2 cells treated with 50 μM of each compound as well as untreated and DMSO-only control. White scale bar in the bottom right of each image indicates 100 μm. MBXC-4522 shows significant visual inhibition of infection. (B) Flow cytometry was used to quantify the percentage of BV2 cells infected at each tested concentration of the assayed compounds. Because DMSO treatment resulted in a statistically significant reduction in infectivity, the significance of each compound dose was calculated as compared with the DMSO condition. *, p < 0.05. ****, p < 0.0001.
To assess whether MBXC-4522 has activity against viruses closely related to RVFV, SFTSV, and HRTV were tested. Based on data obtained by viral antigen staining, viral plaque assay, and viral RNA quantification by qRT-PCR, we observed that MBXC-4522 significantly reduced viral antigen levels in cells infected with HRTV and SFTSV (Figure A). MBXC-4522 also significantly inhibited plaque formation of all three viruses by >1-log at the higher 25 and 50 μM doses (Figure B). For HRTV and SFTSV, inhibition is observed with compound concentrations as low as 5 μM; however, for RVFV (ZH501), 5 and 10 μM compound doses increase plaque formation (Figure B). Of note, RVFV (ZH501) viral RNA levels were not significantly elevated at these concentrations (Figure C) potentially due to the cytopathic effect of the virus, which likely led to a larger number of cells detaching from the plate. Further, RVFV viral RNA was significantly reduced at 25 and 50 μM MBXC-4522 (Figure C), suggesting that MBXC-4522 shows promising antiviral activity against multiple bunyaviruses in vitro.
4.
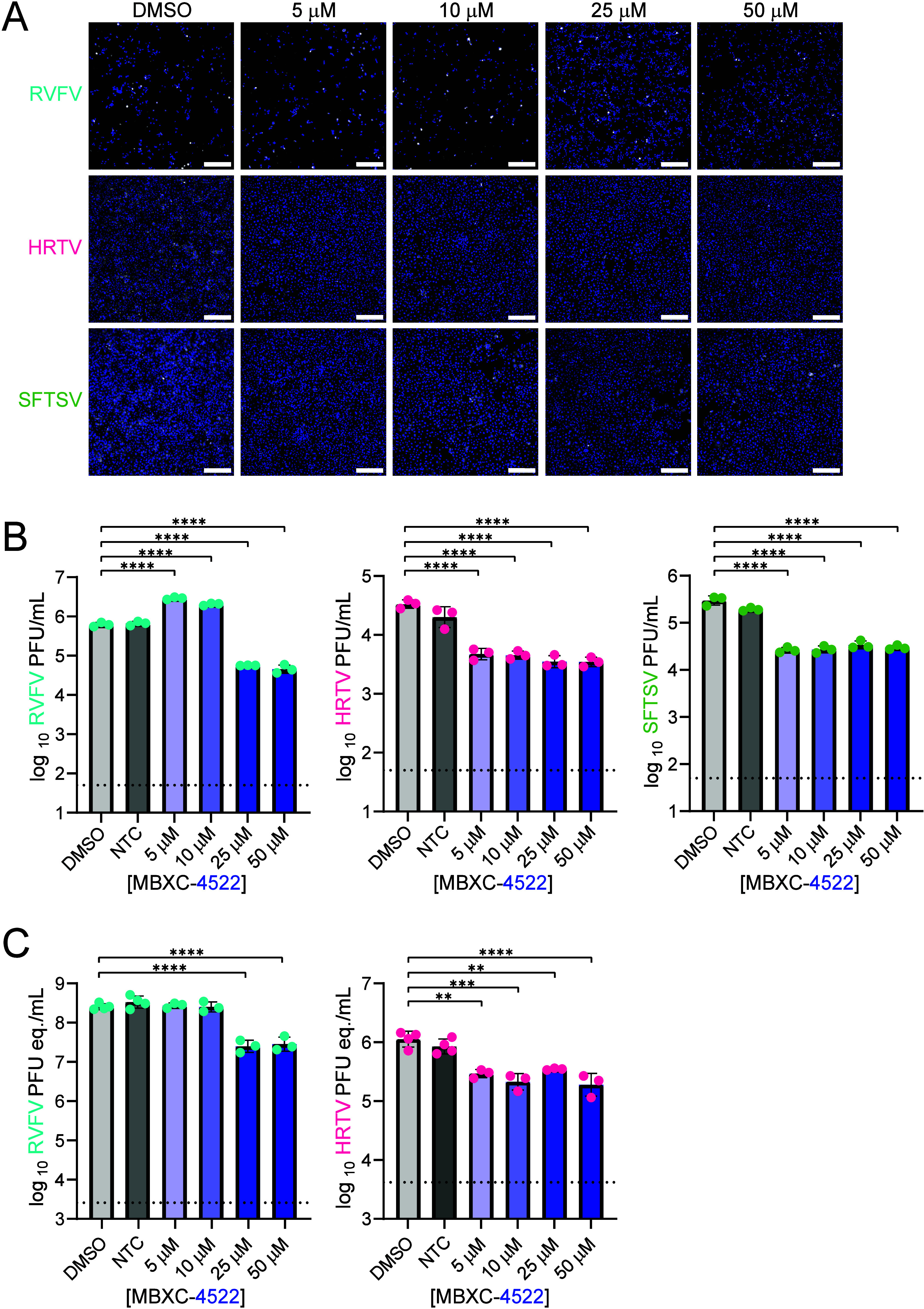
Compound MBXC-4522 exhibits inhibitory activity against multiple bunyaviruses. (A) Vero E6 cells were pretreated with compound and infected with the indicated WT viruses at an MOI of 1. At 18 hpi, cells were stained with DAPI (blue) and virus-specific anti-N antibody (white). The white scale bar in the bottom right of each image indicates 250 μm. (B and C) Infectious virus was measured by plaque assays (B) and viral RNA levels (C) were measured by qRT-PCR on samples obtained 18 hpi. NTC, nontreated control. Dotted horizontal lines indicate the limit of detection for the respective assay. *, p < 0.05. **, p < 0.01. ***, p < 0.001. ****, p < 0.0001.
Conclusions
Here, we describe a statistically robust HTS FRET-based activity assay for bunyaviral endonucleases, which can be used for identification of EndoN inhibitors. Using the assay, we identified a lead hit, MBXC-4522, as a potential RVFV-EndoN inhibitor. MBXC-4522 has favorable biochemical properties and shows activity against multiple bunyaviruses with a metal binding-independent mechanism. Thus, the MBXC-4522 inhibition MOA is distinct from that of BXA. Although additional structure-activity relationship studies and MOA work are warranted to improve the drug-like qualities of MBXC-4522 for in vivo studies and preclinical steps, the identification and characterization of MBXC-4522 described above represents an important first step toward developing a drug scaffold with a distinct MOA to combat RVFV and related viruses.
Methods
Cloning
The N-terminal region (aa 1–226) of RVFV-L protein (ZH501 strain, GenBank Accession DQ375406.1) was subcloned into the pET15b vector (Novagen) containing an N-terminal six-His tag and an ampicillin resistance gene. Sequences were verified by Sanger sequencing (Azenta, Burlington, MA, USA).
Protein Expression and Purification
RVFV-EndoN expression plasmid was transformed into BL21 cells (ATCC). Cells were grown at 37 °C in Miller LB Broth (Fisher Scientific, Hampton, NH, USA) containing ampicillin (Gold Biotechnology, Olivette, MO, USA) until cultures reached an optical density of 0.8 at 600 nm. For the 15N-labeled protein, cells were transferred to minimal media containing 15N-ammonium chloride (Millipore-Sigma, Burlington, MA, USA) prior to induction. Protein expression was induced with 0.5 mM IPTG (Gold Biotechnology) for 16 h in an 18 °C Innova44 shaking incubator (Eppendorf, Hamburg, Germany). Following induction, cells were pelleted at ∼ 8800 × g for 15 min (Beckman Coulter, Brea, CA, USA) and resuspended in lysis buffer (20 mM Tris [pH 8.0], 1 M NaCl, 20 mM imidazole [pH 7.0], 5 mM β-mercaptoethanol [β-Me], and protease inhibitors).
For purification, cells were lysed using an EmulsiFlex-05 cell homogenizer (Avestin, Ottawa, ON, Canada) prior to centrifugation at ∼ 47500 × g for 40 min (Beckman Coulter). Supernatant was flowed through a Nickel Sepharose Fast Flow column (Cytiva) using buffer containing 20 mM Tris [pH 8.0], 1 M NaCl, 20 mM imidazole [pH 7.0], and 5 mM β-mercaptoethanol [β-Me]. Protein was eluted with buffer containing 20 mM Tris [pH 8.0], 50 mM NaCl, 500 mM imidazole [pH 7.0], and 5 mM β-Me. Protein was applied to an anion exchange column prior to size exchange chromatography. Final purified Protein was visualized by Coomassie staining of SDS-PAGE (Figure S1). The gel image was taken with a ChemiDoc MP imager (Bio-Rad, Hercules, CA, USA).
Hit Identification by FRET-Based High-Throughput Screen (HTS)
Purified RVFV-EndoN was diluted to a final concentration of 1 μM in reaction buffer containing 50 mM HEPES at pH 7.8, 150 mM KCl, and 1 mM MnCl2 and distributed to all wells of 384-well microplates by a Multidrop Combi microplate dispenser (Thermo-Fisher Scientific). 1 μL of screening compounds was added via robot, with BXA as a positive control and DMSO as a negative control. Following an incubation of 30 min at room temperature, the self-quenched ssRNA substrate (5′-6-FAM-21mer ssRNA-IowaBlack-3′, synthesized and HPLC purified by IDT) was added to all wells to a final concentration of 0.5 μM. Fluorescence signals of 6-FAM were measured with excitation at 485 nm and emission at 535 nm using an Envision 2102 Multilabel HTS Counter (PerkinElmer, Waltham, MA, USA). Z’-factor was calculated from the positive and negative controls for each plate as described in Data Analysis. Compounds with ≥ 70% inhibition were identified as initial hits.
Cell Cytotoxicity Assays
The cytotoxicities of selected compounds were tested to determine the selectivity of compounds in multiple mammalian cell lines, including HEK293 (ATCC), Vero (ATCC), BV2 cells (ATCC), and HepG2 (ATCC), using CellTiter Glo 2.0 (Promega) according to the manufacturer’s manual. Cells were seeded at 4,000 cells/well in 96-well plates. On the following day, cells were treated with initial hits in serially diluted doses from 100 to 0.1 μM. An internally acquired cytotoxic compound (Microbiotix, proprietary) was used as the positive control, and DMSO was used as the negative control. After 72 h at 37 °C, 5% CO2, cell viability was determined by adding CellTiter Glo 2.0 to cells, incubating for 15 min at room temperature, and measuring luminescence on SpectraMax (Molecular Devices, San Jose, CA, USA). The percentage of cell viability was normalized to the DMSO control and plotted against the compound concentration. Plots were fitted, and the concentration of compounds which reduces cell viability by 50% compared with the control (CC50) was calculated using GraphPad Prism.
Protein Thermal Shift Assays (protein TSAs)
Compounds were reconstituted in DMSO to a final concentration of 10 mM and stored at 4 °C. Purified RVFV-EndoN protein (in-house) was diluted on ice to a working concentration of 25 μM in reaction buffer containing 50 mM HEPES (pH 7.0), 150 mM KCl, and 1 mM MnCl2. 5000x SYPRO Orange Protein Gel Stain (Invitrogen, div. Thermo-Fisher Scientific) was diluted to 50x in the reaction buffer and kept on ice. Compounds were thawed briefly at room temperature, and condition-specific master mixes containing final concentrations of 5 μM protein, 5x SYPRO Orange, and hit compound in 5% DMSO were assembled on ice. For dose response experiments, compounds underwent 2-fold serial dilutions in DMSO (Acros Organics, div. Thermo-Fisher Scientific). The final amount of DMSO in the reactions was kept at 5% regardless of hit compound concentration except in DMSO-free controls. 25 μL of master mix per well was plated in triplicate in a MicroAmp EnduraPlate Optical 96-Well Clear 0.1 mL Reaction Plate (Applied Biosystems, div. Thermo-Fisher Scientific) and sealed with a MicroAmp Optical Adhesive Film (Applied Biosystems). The reaction plate was centrifuged 1 min at 90 × g, 4 °C (Beckman Coulter) before being loaded into a QuantStudio 5 qRT-PCR machine fitted with a fixed 0.1 mL block (Applied Biosystems). A QuantStudio 5 software protocol was generated such that the block cooled to 20 °C at 3.1 °C/s, held at 20 °C for 1 min, and then ramped up to 95 °C at a rate of 0.05 °C/s. To reduce background, the heated lid function was disabled, and the QuantStudio 5 was powered off until immediately before use.
FRET-Based Activity Assays
A HTS fluorescent ssRNA probe (5′-6-FAM-21mer ssRNA-IowaBlack-3′) was synthesized and HPLC-purified by IDT (Coralville, IA), reconstituted in DMSO to a stock concentration of 100 μM, and frozen at −20 °C. Purified RVFV-EndoN protein was diluted to a final concentration of 1 μM, 5 μM, or 20 μM in reaction buffer containing 50 mM HEPES (pH 7.0), 150 mM KCl, and 1 mM MnCl2. Triplicate reactions for each condition were loaded onto a black 384-well microplate (Corning #3575; Corning, Corning, NY, USA) and read at 25 °C with a Cytation 5 plate reader operating Gen5 software (BioTek, Winooski, VT, USA). Total volume per well was 25 μL. Fluorescent signal was read using excitation and emission wavelengths of 485 and 535 nm, respectively, a bandwidth of 20 nm each, a read height of 10.5 mm, 10 reads per time point, and extended gain. An automatic read protocol was developed in Gen5 such that measurements were taken every three or four min following a three s dual-orbital shake. For reproducibility characterization, fluorescent signals for 32 positive (probe-only) and 32 negative (probe plus protein) control reactions were measured using the read parameters described above. Descriptive statistics at 1 or 6 h were calculated in GraphPad Prism 10 (Dotmatics, Boston, MA, USA). All reproducibility reactions used a 100 nM probe and were conducted in buffer containing 50 mM HEPES (pH 7.0), 150 mM KCl, and 1 mM MnCl2 in a total volume of 25 μL per well. Z’ values were calculated using eq , while the signal-to-noise ratio (SNR) was calculated according to eq . SFTSV-EndoN, purified as described previously, was included as a control.
Data Analysis
Averaging and normalization of protein TSA results as well as calculations of percent activity values for FRET-based assays were performed in Excel (Microsoft). Graphs, fit lines, and statistics for all experiments were visualized and calculated in GraphPad Prism 10 (Dotmatics), with data fitted and IC50 or CC50 values calculated according to four-parameter nonlinear regression unless otherwise specified. The Z’ factor was calculated using eq , where σ + and σ – are the standard deviation of the positive and negative controls, respectively, and μ + and μ – are the average fluorescence value of the positive and negative controls, respectively.
| 1 |
The SNR was calculated using eq , where μ + and μ – are the average fluorescence value of the positive and negative controls, respectively, and σ – is the standard deviation of the negative controls.
| 2 |
HSQC NMR
Purified 15N-labeled RVFV-EndoN was incubated overnight at 4 °C in either the presence or absence of EDTA to a final concentration of 0.33 M. EDTA-treated protein was desalted following incubation. Treated and untreated proteins were each diluted to a final concentration of 100 μM in buffer (20 mM HEPES [pH 7.0], 150 mM NaCl, 2 mM TCEP) with 5% D2O. Each sample was added to a clean 4 in. × 5 mm NMR tube and loaded into a 600 MHz magnet (Bruker, Billerica, MA, USA) for HSQC NMR analysis at 25 °C. A Linux computer running TopSpin software (Bruker) was used to analyze the resulting two-dimensional peaks.
Cells and Viruses
Wild-type (WT) BV2 cells (ATCC) were maintained by serial passage in a maintenance medium of DMEM + 10% FBS with 10 mM HEPES (pH 7) and 1% sodium pyruvate. HEK293. Vero6 cells were maintained in DMEM + 10% FBS with 1% l-Glutamine, 0.5% Nonessential Amino Acids, and 1% P/S. HepG2 cells were maintained in EMEM + 10% FBS with 1% P/S. All cells were grown at 37 °C, 5% CO2. RVFV-MP12GFP was passaged via serial infection of BV2 cells using an infection medium of DMEM + 2% FBS with 10 mM HEPES (pH 7) and 1% sodium pyruvate. HRTV (strain MO-4) was obtained from BEI Resources. SFTSV (strain CB1/2014) was a kind gift from Dr. Jae Jung (Cleveland Clinic, Cleveland, OH, USA). RVFV (ZH501) was generously provided by Dr. Barry Miller (CDC, Fort Collins, CO, USA) and Dr. Stuart Nichol (CDC, Atlanta, GA, USA). RVFV (ZH501), HRTV, and SFTSV were propagated in Vero E6 cells with standard culture conditions using D2 media composed of DMEM supplemented with 1% Pen/Strep, 1% l-Glut, and 2% FBS. A standard viral plaque assay was used to determine the infectious titers of these stocks.
RVFV-MP12GFP Infectivity Inhibition Assays
0.07 million BV2 WT cells were seeded in a 24-well plate (Corning) and allowed to reach adequate confluency at 37 °C with 5% CO2. Working concentrations of compounds at 5, 10, 25, and 50 μM were made in 1 mL of BV2 infection media (DMEM + 2% FBS with 10 mM HEPES and 1% sodium pyruvate) as well as media-only and DMSO controls. 10% of the cell maintenance media (DMEM + 10% FBS with 10 mM HEPES and 1% sodium pyruvate) was replaced with 250 μL of BV2 infection media containing DMSO or compound dilutions and incubated at 37 °C and 5% CO2 for 15 min. Following incubation, fluorescently attenuated RVFV-MP12GFP virus (in-house) was thawed and added to each well at an MOI of 1 under BSL2 conditions. The reaction plate was further incubated at 37 °C and 5% CO2 for 1 h with rocking every 15 min to prevent cell drying. Then, the remaining volumes of infection media dilutions were added to the respective wells and incubated at 37 °C in 5% CO2 for 16 h. The following day, cells were trypsinized with 0.05% Trypsin-EDTA (Life Technologies, div. Thermo-Fisher Scientific) and washed with PBS prior to fixation with 4% paraformaldehyde. Fixed cells were imaged on a light microscope as well as processed by flow cytometry.
RVFV ZH501 Inhibition studies
WT Vero E6 cells were cultured and pretreated with compounds or DMSO-only control as described above. Cells were then infected with RVFV (ZH501), SFTSV, or HRTV at an MOI of 1 under the BSL3 conditions. At 18 h post infection, media were harvested, and cells were fixed with 4% paraformaldehyde. The cells on coverslips were then stained with DAPI nuclear stain and virus-specific nucleoprotein antibody (Genscript) at 1:500 each and mounted with gelvatol onto glass slides. Coverslips were imaged on a Leica DMI8 to assess the reduction of viral antigen (white).
Viral RNA Isolation and qRT-PCR
Viral RNA isolation was performed on supernatant samples obtained from RVFV, SFTSV, or HRTV virus-infected cells at 18 h postinfection using an Invitrogen PureLink RNA kit (Invitrogen, div. Thermo-Fisher Scientific). 100 μL of supernatant from each sample was inactivated in 900 μL of Trizol. 200 μL of chloroform was then added to each sample and allowed to sit at room temperature for 3 min. Samples were then centrifuged for 15 min at 12,000 × g at 4 °C. Following centrifugation, the aqueous phase was removed and mixed with an equivalent volume of 70% ethanol. The remainder of the isolation was performed according to the PureLink RNA kit protocol. qRT-PCR was then performed by using the Invitrogen SuperScript III Platinum One-Step Quantitative Kit (Invitrogen). For RVFV (ZH501), the primers used to target the L segment were: RVFV-2912Fwd 5′-TGAAAATTCCTGAGACACATGG-3′ and RVFV-2981Rev 5′-ACTTCCTTGCATCATCTGATG-3′. The Taqman probe used was RVFV-2950-Probe 5′-56-FAM/CAATGTAAGGGGCCTGTGTGGACTTGTG/3BHQ_1–3′. For HRTV, primers targeting the S segment were used: HRTLDMO4–1186For 5′-CCTTTGGTCCACATTGATTG-3′ and HRTLDMO4–1273Rev 5′- CACTGATTCCACAGGCAGAT-3′. The Taqman probe used was HRTLDMO4–122Probe 5′ 6-FAM/TTG CCA AAG GGA ATA GGC ATC CA/BHQ_1 3′.
qRT-PCR was performed on a QuantStudio 5 instrument (Applied Biosystems).
Viral Plaque Assays
Vero E6 cells were seeded in 12-well plates and allowed to reach near confluency overnight at 37 °C and 5% CO2. Supernatant samples obtained 18 hpi were serially diluted 10-fold in D2 media. Cells were inoculated and incubated for 1 h at 37 °C with 5% CO2 prior to removing the inoculum. Agar overlay composed of 1x MEM, 2% FBS, 1% Pen/Strep, 1% HEPES buffer, and 0.8% SeaKem agarose (Fisher Scientific, div. Thermo-Fisher Scientific) was added to each well. Plates were incubated at 37 °C with 5% CO2 for 3 (RVFV) or 8 (HRTV, SFTSV) days to allow plaque formation. Plates were then fixed with 37% formaldehyde for at least 3 h, then stained with 0.1% crystal violet for visualization and plaque counting.
Quantification and Statistical Analysis
To perform the statistical analysis, Prism Version 8.0 (GraphPad) was used. Statistical significance was determined by either the student t test for two groups or 1-way ANOVA analysis, followed by Dunnett’s test for comparing more than two groups. Unless otherwise specified, error bars represent the mean and standard deviations (mean ± SD). The dose–response curves were fitted and IC50 calculated using Sigmoidal dose–response (variable slope) in GraphPad.
Supplementary Material
Acknowledgments
We thank Dr. Kevin Blake, Scientific Editor, Division of Laboratory & Genomic Medicine in the Pathology and Immunology Department at Washington University in St. Louis for his input. We also thank the members of Hartman, Krezel, Leung, and Amarasinghe laboratories and members of Microbiotix, Inc. for their input. This work was partially supported by NIH grants R41AI165102 (G.L. and G.K.A.); R01AI178378, R01AI161765, and R01AI169850 (A.L.H., D.W.L., and G.K.A.); and T32 AI049820-20 (R.E.R.). BSL3 work at University of Pittsburgh was supported by UC7 AI180311.
Glossary
Abbreviations
- sNSV
segmented negative-sense virus.
- EndoN
endonuclease.
- IAV
Influenza A virus.
- SFTSV
severe fever with thrombocytopenia syndrome virus.
- RVFV
Rift Valley fever virus.
- HRTV
Heartland virus.
- BXA
baloxavir acid.
- FRET
fluorescence resonance energy transfer.
- HTS
high-throughput screen.
- MOA
mode of action.
- DMSO
dimethyl sulfoxide.
- β-Me
β-Mercaptoethanol.
- SNR
signal-to-noise ratio.
- SAR
structure–activity relationship.
- Protein TSA
protein thermal shift assay.
- HSQC
heteronuclear single quantum coherence.
- NMR
nuclear magnetic resonance
All reagents and raw data are available upon request following, including those subjected to institutional material transfer agreements (MTAs).
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.5c00088.
Table S1: In vitro activity of the hit MBX series. Figure S1: Recombinant RVFV-EndoN protein used in this study. Figure S2: The FRET-based EndoN assay is sensitive, reproducible, and has a broad dynamic range. Figure S3: Purified RVFV-EndoN does not contain contaminating metals. Figure S4: MBXC-4522 does not interfere with fluorescent signal of FAM. Figure S5: Cytotoxicity of hit compound MBXC-4522. Figure S6: Structural differences in analogs render them less effective than MBXC-4522 (PDF)
∇.
(C.D.K., G.L.) These authors contributed equally.
The authors declare the following competing financial interest(s): Amarasinghe, G.K., Leung, D.W., Kirby, C.D, Liu, G. and G-Dayanandan, N. are inventors on a provisional patent application related to the work presented. Other authors declare that they have no conflict of interest.
References
- Leung D. W., Amarasinghe G. K.. When your cap matters: structural insights into self vs non-self recognition of 5′ RNA by immunomodulatory host proteins. Curr. Opin Struct Biol. 2016;36:133–141. doi: 10.1016/j.sbi.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. S. Y., Angel M., Ma Y., Sloan E., Wang G., Martinez-Romero C., Alenquer M., Roudko V., Chung L., Zheng S., Chang M., Fstkchyan Y., Clohisey S., Dinan A. M., Gibbs J., Gifford R., Shen R., Gu Q., Irigoyen N., Campisi L., Huang C., Zhao N., Jones J. D., van Knippenberg I., Zhu Z., Moshkina N., Meyer L., Noel J., Peralta Z., Rezelj V., Kaake R., Rosenberg B., Wang B., Wei J., Paessler S., Wise H. M., Johnson J., Vannini A., Amorim M. J., Baillie J. K., Miraldi E. R., Benner C., Brierley I., Digard P., Łuksza M., Firth A. E., Krogan N., Greenbaum B. D., MacLeod M. K., van Bakel H., Garcìa-Sastre A., Yewdell J. W., Hutchinson E., Marazzi I.. Hybrid Gene Origination Creates Human-Virus Chimeric Proteins during Infection. Cell. 2020;181(7):1502–1517. doi: 10.1016/j.cell.2020.05.035. e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling E., Murphy V., Mai M. C., Stone E. T., Casals A. G., Hassert M., O’Dea A. T., Cao F., Donlin M. J., Elagawany M., Elgendy B., Pardali V., Giannakopoulou E., Zoidis G., Schiavone D. V., Berkowitz A. J., Agyemang N. B., Murelli R. P., Tavis J. E., Pinto A. K., Brien J. D.. Metal coordinating inhibitors of Rift Valley fever virus replication. PLoS One. 2022;17(9):e0274266. doi: 10.1371/journal.pone.0274266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M.. Selected host cell capped RNA fragments prime influenza viral RNA transcription in vivo. Nucleic Acids Res. 1981;9(17):4423–4436. doi: 10.1093/nar/9.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M.. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983;34(2):611–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- De Vlugt C., Sikora D., Pelchat M.. Insight into Influenza: A Virus Cap-Snatching. Viruses. 2018;10(11):641. doi: 10.3390/v10110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A., Bouvier D., Crepin T., McCarthy A. A., Hart D. J., Baudin F., Cusack S., Ruigrok R. W.. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458(7240):914–918. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- Lutz, M. M. ; Dunagan, M. M. ; Kurebayashi, Y. ; Takimoto, T. . Key role of the influenza A virus PA gene segment in the emergence of pandemic viruses. In Viruses; MDPI AG: 2020; Vol. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J. H., Brown K., Adkins S., de la Torre J. C., Digiaro M., Ergunay K., Firth A. E., Hughes H. R., Junglen S., Lambert A. J., Maes P., Marklewitz M., Palacios G., Sasaya T., Shi M., Zhang Y. Z., Wolf Y. I., Turina M.. Promotion of order Bunyavirales to class Bunyaviricetes to accommodate a rapidly increasing number of related polyploviricotine viruses. J. Virol. 2024;98(10):e0106924. doi: 10.1128/jvi.01069-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron F., Weber F., de la Torre J. C., Reguera J.. Transcription and replication mechanisms of Bunyaviridae and Arenaviridae L proteins. Virus Research. 2017;234:118–134. doi: 10.1016/j.virusres.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A.. Rift Valley Fever. Clin Lab Med. 2017;37(2):285–301. doi: 10.1016/j.cll.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health, O. Rift Valley fever. In WHO Fact Sheet, 2018.
- McMillen, C. M. ; Hartman, A. L. . Rift Valley Fever: a Threat to Pregnant Women Hiding in Plain Sight? Journal of Virology 2021, 95 (9). 10.1128/JVI.01394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li Q., Hu W., Wu J., Wang Y., Mei L., Walker D. H., Ren J., Wang Y., Yu X.-J.. Person-to-Person Transmission of Severe Fever with Thrombocytopenia Syndrome Virus. Vector-Borne and Zoonotic Diseases. 2012;12(2):156. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- Yu X.-J., Liang M.-F., Zhang S.-Y., Liu Y., Li J.-D., Sun Y.-L., Zhang L., Zhang Q.-F., Popov V. L., Li C., Qu J., Li Q., Zhang Y.-P., Hai R., Wu W., Wang Q., Zhan F.-X., Wang X.-J., Kan B., Wang S.-W., Wan K.-L., Jing H.-Q., Lu J.-X., Yin W.-W., Zhou H., Guan X.-H., Liu J.-F., Bi Z.-Q., Liu G.-H., Ren J., Wang H., Zhao Z., Song J.-D., He J.-R., Wan T., Zhang J.-S., Fu X.-P., Sun L.-N., Dong X.-P., Feng Z.-J., Yang W.-Z., Hong T., Zhang Y., Walker D. H., Wang Y., Li D.-X.. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J. Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. C., Zhao J., Li H., Fang L. Q., Liu W.. Epidemiology, clinical characteristics, and treatment of severe fever with thrombocytopenia syndrome. Infectious Medicine. 2022;1(1):40–49. doi: 10.1016/j.imj.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A. L., Myler P. J.. Bunyavirales: Scientific Gaps and Prototype Pathogens for a Large and Diverse Group of Zoonotic Viruses. J. Infect Dis. 2023;228(Supp6):S376–S389. doi: 10.1093/infdis/jiac338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembek Z. F., Mothershead J. L., Cirimotich C. M., Wu A.. Heartland Virus Disease-An Underreported Emerging Infection. Microorganisms. 2024;12(2):286. doi: 10.3390/microorganisms12020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm T., Kopicki J. D., Busch C., Olschewski S., Rosenthal M., Uetrecht C., Günther S., Reindl S.. Biochemical and structural studies reveal differences and commonalities among cap-snatching endonucleases from segmented negative-strand RNA viruses. J. Biol. Chem. 2018;293(51):19686–19698. doi: 10.1074/jbc.RA118.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin B., Coutard B., Lelke M., Ferron F., Kerber R., Jamal S., Frangeul A., Baronti C., Charrel R., de Lamballerie X., Vonrhein C., Lescar J., Bricogne G., Günther S., Canard B.. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathogens. 2010;6(9):e1001038. doi: 10.1371/journal.ppat.1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera J., Weber F., Cusack S.. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathogens. 2010;6(9):e1001101. doi: 10.1371/journal.ppat.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Shin W. J., Zhang B., Choi Y., Yoo J. S., Zimmerman M. I., Frederick T. E., Bowman G. R., Gross M. L., Leung D. W., Jung J. U., Amarasinghe G. K.. The Cap-Snatching SFTSV Endonuclease Domain Is an Antiviral Target. Cell Reports. 2020;30(1):153–163. doi: 10.1016/j.celrep.2019.12.020. e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B., Tchesnokov E. P., Götte M.. The active form of the influenza cap-snatching endonuclease inhibitor baloxavir marboxil is a tight binding inhibitor. J. Biol. Chem. 2021;296:100486. doi: 10.1016/j.jbc.2021.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto, S. ; Speranzini, V. ; Hashimoto, T. ; Noshi, T. ; Yamaguchi, H. ; Kawai, M. ; Kawaguchi, K. ; Uehara, T. ; Shishido, T. ; Naito, A. ; Cusack, S. . Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018, 8 (1). 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu C., Ye W., Wang J., Dong X., Xu J., Li X., Zhang M., Lu H., Zhang F., Wu W., Dai S., Wang H.-W., Chen Z.. Structure of Rift Valley Fever Virus RNA-Dependent RNA Polymerase. Journal of Virology jvi.asm.org. 2022;96:1713–1734. doi: 10.1128/jvi.01713-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-H., Chung T. D. Y., Oldenburg K. R.. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang, X. D. Optimal High-Throughput Screening: Practical Experimental Design and Data Analysis for Genome-Scale RNAi Research; Cambridge University Press, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All reagents and raw data are available upon request following, including those subjected to institutional material transfer agreements (MTAs).




