Abstract
We present the syntheses of the hexafluoridouranates(V) MUF6 (M = Li–Cs, Ag, Tl, H3O) and of the dodecafluoridodiuranate(V) Ba[U2F12]·1.36HF. With the exception of AgUF6 and H3OUF6, all compounds were synthesized by reacting the respective metal fluorides with β-UF5 in anhydrous hydrogen fluoride (aHF). AgUF6 was obtained as a side product in the oxidation of Ag powder with UF6 under a CO atmosphere, while H3OUF6 was obtained from the controlled hydrolysis of β-UF5 with SiO2 in aHF. For this hydrolysis, silica glass wool proved to be the superior choice of SiO2. X-ray diffraction experiments on single crystals of the compounds as well as on polycrystalline samples allowed the unambiguous determination of their crystal structures, clarifying previously published structure models that were based on only powder X-ray diffraction. The structural chemistry of these compounds is discussed. In the case of LiUF6, NaUF6, and H3OUF6, molecular UF6 – anions are observed in the solid state, while one-dimensional infinite strands of ∞ {[UF4/1F4/2]−} anions are present in the crystal structures of KUF6, RbUF6, TlUF6, and AgUF6. The dodecafluoridodiuranate(V) Ba[U2F12]·1.36HF is the second example of a compound containing the peculiar [U2F12]2– anion. Its U atoms show a coordination number of seven in a capped trigonal prismatic coordination sphere, and these prisms share a common edge. In contrast to the Sr homologue, the Ba compound contains HF molecules of crystallization.


Introduction
Two allotropic forms of uranium(V) fluoride are known, a low-temperature form, β-UF5, and a high-temperature form, α-UF5. , β-Uranium(V) fluoride crystallizes in the tetragonal crystal system with a network structure. The uranium atoms are coordinated by eight fluorine atoms in the form of a distorted trigonal dodecahedron, and the crystal structure can be described by using the Niggli formula ∞ [UF2/1F6/2]. α-UF5 also crystallizes in the tetragonal crystal system but with a one-dimensional infinite chain motif in which the U atoms are coordinated by six F atoms in an octahedron-like manner. The structure can be described using the Niggli formula ∞ [UF4/1F2/2]. The chemical behavior of UF5 is related to that of Lewis acids PF5, AsF5, and SbF5. For example, hexa-, hepta- or octafluoridouranates(V) are formed with fluoride ion donors such as the alkali metal fluorides. ,
To the best of our knowledge, the first preparations of hexafluoridouranates(V) of the composition MUF6 (with M as a monovalent cation) took place in solid-state syntheses of uranium(V) fluoride and alkali metal fluorides, see reaction eq . Other methods are based on the reaction of UF5 with fluorides in various solvents; 48% hydrofluoric acid, anhydrous hydrogen fluoride (aHF), and acetonitrile have been described as suitable solvents. ,,, Both UF5 and the alkali metal fluorides are soluble in 48% hydrofluoric acid and acetonitrile. In contrast, only the alkali metal fluorides dissolve noticeably in aHF.
| 1 |
Another synthesis approach is the reduction of uranium(VI) fluoride with one-electron donors such as nitrogen monoxide or nitrogen dioxide. − The reaction of UF6 with gaseous ammonia should also lead to the formation of NH4UF6. , By reacting dry nitrates with nitrosyl hexafluoridouranate(V), NOUF6, hexafluoridouaranates(V) can also be obtained; see reaction eq . ,
| 2 |
We report here on the preparation of previously described alkali metal hexafluoridouranates(V), but here for the first time in single-crystalline form, and on a comprehensive structural-chemical study of these. We shed light on the structural peculiarities of previous crystal structure reports of some of these compounds.
Results and Discussion
Preparation of Alkali Metal Hexafluoridouranates(V)
The homologous series of alkali metal hexafluoridouranates(V) can be easily prepared in solution due to the high solubility of alkali metal fluorides in aHF, which has already been reported in the literature for these salts. ,− The formation can be described with reaction eq , and further details are given in the Experimental Section.
| 3 |
Photographs of the compounds as well as their powder X-ray diffraction patterns are given in the Supporting Information together with a comparison with diffraction patterns from the literature and those calculated from our structure models based on single-crystal X-ray diffraction.
Preparation of Silver(I) Hexafluoridouranate(V)
During attempts to synthesize a silver(I) carbonyl compound of the putative composition [Ag(CO)2][UF6] from the oxidation of Ag powder with UF6 in aHF under CO atmosphere, we obtained single crystals of AgUF6 as a side product. The reaction equation is shown in eq . Further details are given in the Experimental Section.
| 4 |
Preparation of Thallium(I) Hexafluoridouranate(V)
Thallium(I) hexafluoridouranate(V), TlUF6, was obtained in a solid-state reaction of TlF with UF5. Due to its good solubility in aHF, TlUF6 was prepared in analogy to the alkali metal hexafluoridouranates(V); see reaction eq . For further details, see the Experimental Section.
Preparation of Oxonium Hexafluoridouranate(V)
Previous studies have described the compounds HUF6·1.25H2O and HUF6·2.5H2O, which crystallize on cooling from hydrofluoric acid solution saturated with UF5 (48% HF). The reaction of equimolar amounts of water and UF5 in hydrogen fluoride leads to the formation of oxonium hexafluoridouranate(V), H3OUF6. It has been speculated that the former compounds may represent H3OUF6 hydrates.
We therefore investigated whether the solvolysis of silicon dioxide in hydrogen fluoride in the presence of β-uranium(V) fluoride leads to the formation of H3OUF6. The rate-determining step should be the solvolysis in which solvated oxonium fluoride and silicon tetrafluoride are formed; see reaction eq .
| 5 |
The oxonium fluoride now acts as a fluoride ion donor for the Lewis acid UF5 and the solvated H3OUF6 is formed, as shown in reaction eq .
| 6 |
In three reaction batches, different forms of silicon dioxide (amorphous, annealed, and silica glass wool) were reacted with β-UF5 in a 1:2 ratio in hydrogen fluoride in FEP Schlenk tubes at room temperature. The reaction mixture with amorphous SiO2 showed a slight blue coloration of the solution and an inhomogeneous sediment after 4 days at room temperature. After a reaction time of 3 weeks, annealed SiO2 resulted in a weak blue solution with a gray, inhomogeneous sediment. The preparation with silica glass wool showed no sediment after 3 days of reaction, and the solution was blue in color. The slow removal of the hydrogen fluoride in a vacuum led to a blue-green homogeneous reaction product, which corresponds to the description of H3OUF6 in the literature. A photograph of the product received is shown in Figure S3. Among the reactants used, silica glass wool is, therefore, best suited for the synthesis of the oxonium salt.
Preparation of Barium Dodecafluoridodiuranate(V)–Hydrogen Fluoride(1/1.36)
The crystal structure of the compound Sr[U2F12] was already determined in the past. Similar to this, we synthesized barium dodecafluoridodiuranate(V)–hydrogen fluoride(1/1.36), Ba[U2F12]·1.36HF, by reacting BaF2 with UF5 in aHF according to eq . Further details are given in the Experimental Section.
| 7 |
The Single-Crystal Structure of Lithium Hexafluoridouranate(V), LiUF6
Obtaining suitable crystals for structure determination on the single-crystal diffractometer proved to be difficult for lithium hexafluoridouranate(V), as it has the lowest solubility reported among the alkali metal hexafluoridouranates(V) in aHF. Attempts to recrystallize microcrystalline LiUF6 from aHF, acetonitrile, as well as sulfur dioxide failed. In none of the solvents could a noticeable solubility, associated with a coloration of the solution or crystal growth, be observed. The resolvation thus seems to be strongly inhibited.
Single-crystalline LiUF6 of light blue color was finally obtained from a synthesis with a comparatively large amount of aHF (10 mL) as the solvent, in which it was distilled very slowly over several hours in a vacuum into a separate FEP Schlenk tube. Selected crystallographic data and details of the structure determination are given in Table S2, and atomic coordinates, Wyckoff positions, site symmetries, and isotropic and anisotropic displacement parameters are reported in Tables S3 and S4 in the Supporting Information.
LiUF6 crystallizes in the LiSbF6 structure type (hR24). Figure shows a section of the crystal structure; selected atom distances and angles are given in Table .
1.

Section of the crystal structure of LiUF6 on the left, crystal structure of LiUF6 with coordination polyhedra for the UF6 – ions in green. Displacement ellipsoids at 70% probability at 100 K. Symmetry transformations for the generation of equivalent atoms: #1 1 – y, x – y, z; #2 1 – x + y, 1 – x, z; #3 4/3 −x, 2/3 – y, 2/3 – z; #4 1/3 + y, 2/3 – x + y, 2/3 – z; #5 1/3 + x – y, −1/3 + x, 2/3 – z.
1. Selected Atom Distances d and Angles ∠XYZ of LiUF6 .
| atom distance d/Å | angle ∠XYZ/° | ||
|---|---|---|---|
| U(1)–F(1) | 2.0624(2) | F–U–F | 89.774(1)–90.218(1) |
| Li(1)–F(1) | 2.0061(2) | F–U–F | 179.964(1) |
| U(1)–U(1)#1 | 5.1902(7) | ||
The uranium atom U(1) on Wyckoff position 3a (3̅.) is coordinated in its first sphere by fluorine atom F(1) and its five symmetry-equivalents F(1)#1–F(1)#5 (Figure ). Overall, the molecular hexafluoridothiocyanate (V) ion UF6 – is formed. Figure shows the formed coordination polyhedron and crystal structure. Due to the position of the uranium atom, the UF6 – ion cannot have the ideal octahedral symmetry of O h , but only that of the subgroup S 6 (C 3i ). , This corresponds to a distortion along the C 3 axes of an octahedron. The deviation from the ideal values is also reflected in the angles formed between the fluorine and uranium atoms, which lie in the range of 89.774(1)–90.218(1)° and axially at 179.964(1)°. The tendency of distortion of the UF6 – ion has already been observed in single-crystal structure studies of other representatives of the compound class such as nitrosyl hexafluoridouranate(V), NOUF6, and cesium hexafluoridouranate(V), CsUF6. ,
The atomic distance U(1)–F(1) is 2.0624(2) Å, which is equal within the tripled standard uncertainty to the values of NOUF6, where a U–F distance of 2.0671(9) Å (T = 100 K) was reported. It also agrees with CsUF6 with a U–F distance of 2.057(6) Å (T not reported) and with the average U–F distances in bis(triphenylphosphonium)iminium hexafluoridouranate(V), (PPN)UF6, with 2.03(2) Å (T not reported). , In relation to binary uranium fluorides with U atoms in coordination number 6, α–UF5, ∞ [UF4/1F2/2], with a U–F distance of 2.020(5) and a μ-F–U distance of 2.236(1) Å (T not reported), and in UF6 with 2.023(6) Å (T = 77 K), the distances in the UF6 – salts are slightly increased due to the negative charge of the anion. ,
Around the lithium atom Li(1) (3b, 3̅.), also an octahedron-like coordination polyhedron with the fluorine atoms is formed. The atomic distance Li(1)–F(1) of 2.0061(2) Å is shorter than in LiF with 2.013 Å (T = 298 K).
The coordination polyhedra of lithium and uranium atoms are oriented differently. One coordination polyhedron is surrounded by six polyhedra of the other cation type. The crystal structure can also be described as a sequence of alternating coordination polyhedron layers. When looking at such UF6 – layers, a stacking sequence of |:ABC:| is observed, corresponding to Jagodzinski symbol c. The stacking is therefore related to the cubic close packing.
The Single-Crystal Structure of Sodium Hexafluoridouranate(V), NaUF6Rhombohedral Modification
The sodium salt of alkali metal hexafluoridouranate(V), NaUF6, is the only one in the series to have been described as polymorphic to date. , Two modifications, one cubic and one rhombohedral, were reported. In the preparation of NaF and UF5 in hydrogen fluoride, the distillation rate of the solvent has an influence on the modification of the phase obtained. A high rate favors the formation of the higher-symmetry modification, while a low rate favors the formation of the lower-symmetry modification. The temperature also has an influence on the modification formed. Ostwald’s step rule, ,, the phenomenon that a metastable modification of a compound crystallizes first, which then transforms into a thermodynamically more favorable modification, seems to be also fulfilled here.
Blueish crystals of NaUF6 were obtained by using a low removal rate of aHF at room temperature from the reaction mixture. NaUF6 crystallizes, like LiUF6, in the LiSbF6 structure type, and no detailed structural description will be given. Selected crystallographic data and details of the structure determination are given in Tables S5–S7. Selected atom distances and angles are given in Table .
2. Selected Atom Distances d and Angles ∠XYZ of NaUF6 (Rhombohedral Polymorph).
| atom distance d/Å | angle ∠XYZ/° | ||
|---|---|---|---|
| U(1)–F(1) | 2.0661(7) | F–U–F | 89.563(2)–90.437(2) |
| Na(1)–F(1) | 2.2768(7) | F–U–F | 179.989(2); 179.985; 180.00 |
| U(1)–U(1) | 6.1078(9) | ||
The U–F distance is 2.0661(7) Å and is slightly larger than that in LiUF6. The formed octahedron-like coordination polyhedron, with angles in the range 89.563(2)–90.437(2)° and three different axial angles, shows a larger distortion than the UF6 – ion in LiUF6.
The sodium atoms are coordinated by six fluorine atoms at a distance of 2.2768(7) Å in the shape of an octahedron. The Na–F distance is comparable to that in NaF, which is 2.315 Å (T = 273 K). Compared to the lithium salt, the coordination polyhedra have a different orientation, which can be explained by the stronger distortion of the anions. This is probably due to the larger effective ionic radius of Na+ with 102 pm for the coordination number six in relation to Li+ with 76 pm.
The Single-Crystal Structure of Potassium Hexafluoridouranate(V), KUF6
Putative space groups and lattice parameters have already been reported for potassium hexafluoridouranate(V), KUF6, but single-crystal structure studies have not yet been carried out. A first study assigned the orthorhombic crystal system with lattice parameters a = 5.73, b = 5.6, and c = 3.98 Å, V = 127.7 Å3. Pccm (No. 49) was named as a possible space group. Another publication also proposed the orthorhombic crystal system with lattice parameters a = 5.61, b = 11.46, and c = 7.96 Å, V = 511.8 Å3, a quadrupled volume compared to the previous one, with the possible space groups Cmme (No. 67) or Aem2 (No. 39). In the course of a single-crystal structure investigation of rubidium hexafluoridoprotactinate(V), RbPaF6, space group Cmme, KUF6 was described as isotypic due to its similar lattice parameters. −
Blueish crystals of KUF6 were obtained at room temperature and subjected to single-crystal X-ray analysis. Initially, the structure solutions and refinements were attempted in the orthorhombic crystal system with the lattice parameters a = 11.442(2), b = 8.0345(1), and c = 5.5655(1) Å, V = 519.87 Å3 in space groups Cmme and Aem2. However, the refinements did not lead to any satisfactory structural model, as the displacement parameters showed unusual values. The successive descent into corresponding nonisomorphic orthorhombic subgroups and the application of twin laws showed no improvement of the structural models. , Finally, a proper structural model in the monoclinic subgroup C2/m (No. 12, mS32) of space group Cmme was obtained with the lattice parameters a = 11.442(2), b = 8.0345(1), c = 5.5655(1) Å, β = 90.138(9)°, V = 511.62(4) Å3, Z = 4 at T = 100 K. Selected crystallographic data and details of the structure determination are given in Tables S8–S10 in the Supporting Information. A section of the crystal structure of KUF6 is shown in Figure . Selected atom distances are listed in Table .
2.
Section of the crystal structure of KUF6 on the left; on the right, the crystal structure of KUF6, displacement ellipsoids at 70% probability at 100 K. The first coordination spheres of the U atoms are shown as green coordination polyhedra. Displacement ellipsoids at 70% probability at 100 K. Symmetry transformations for the generation of equivalent atoms: F(1)#1 1 – y, x – y, z; F(2)#1 1 – y, x – y, z; F(3)#1 1 – y, x – y, z; F(4)#1 1 – y, x – y, z.
3. Selected Atom Distances d of KUF6 .
| Atom distances d/Å | |||
|---|---|---|---|
| U(1)–F(1) | 2.0633(3) | K(1)–F(1) | 2.6995(3) |
| U(1)–F(2) | 2.0635(3) | K(1)–F(1) | 3.0950(3) |
| U(1)–F(3) | 2.3400(2) | K(1)–F(2) | 2.6897(3) |
| U(1)–F(4) | 2.3403(2) | K(1)–F(2) | 3.0902(3) |
| U(1)–U(1)#1 | 4.0102(5) | K(1)–F(3) | 2.6762(4) |
| U(1)–U(1)#2 | 4.0243(6) | K(1)–F(4) | 2.6886(4) |
The asymmetric unit contains a uranium atom U(1), which is located at the Wyckoff position 4h with site symmetry 2. , In the first sphere, the U atom is coordinated by eight fluorine atoms. Of these, the fluorine atoms F(1) and F(2) occupy the Wyckoff position 8j and the fluorine atoms F(3) and F(4) the position 4i. The isopointal fluorine atoms each lie on different crystallographic orbits. The equivalent fluorine atoms F(1)#1–F(4)#1 are generated by symmetry transformations; for these, see the caption of Figure . The uranium atom is thus coordinated by the fluorine atoms in the form of a distorted trigonal dodecahedron. The ideal polyhedron is also known as the Johnson solid J84 and has the symmetry D 2d . The edges formed by the atoms F(3) and F(3)#1 and by F(4) and F(4)#1 are joined by further coordination polyhedra, so that a one-dimensional infinite chain is formed, which can be described using the Niggli notation ∞ {[UF4/1F4/2]−}. The chain is aligned parallel to the crystallographic b axis, see Figure . Such a structural motif of the anions can also be observed in other fluorides. For example, in RbPaF6, previously assumed to be isotypic, in tetramethylammonium hexafluoridoprotactinate(V), (N(CH3)4)PaF6, and in potassium hexafluoridozirconate(IV) and -hafnate(IV), K2ZrF6 and K2HfF6. ,
Looking at the coordination sphere around the uranium atom at a distance of 4.10 to 4.25 Å, the fluorine atoms form a distorted gyrobifastigium with corner and edge linkages. The ideal polyhedron is also known as a twisted double wedge, has the symmetry D 2d and also belongs to the Johnson solids with the number J26. Gyrobifastigium is the only Johnson solid that can completely fill the three-dimensional (3D) space.
In KUF6, two different distances between the uranium atom and the fluorine atoms can be observed: a short distance with 2.0633(3) Å for the terminally bound fluorine atoms and a larger one with 2.3400(2) Å for the bridging fluorine atoms. The former is comparable to those in simple UF6 – salts with molecular anions. The distances for the bridging fluorine atoms in the ∞ {[UF4/1F4/2]−} anion are slightly larger, as expected, compared to those in the electrically neutral α-UF5 at 2.236(1) Å (T not reported), β-UF5 (∞ [UF2/1F6/2]) at 2.27(2) Å (T = r.t.), and the hydrogen cyanide adduct ∞ [UF4/1F2/2(HCN)2/1] with 2.227(6)–2.330(5) Å (T = 100 K). ,,
The distances of the uranium atoms within the chains of 4.0102(5) and 4.0243(6) Å are quite small compared to those of hexafluoridouranates(V) investigated so far. In NOUF6, U–U distances of 5.1732(2) Å (T = 100 K) are observed, in CsUF6 of 5.417(3) Å (T not reported). , Magnetochemical investigations were carried out on the latter two, but no coupling of the magnetic centers was observed there. , However, an interaction could occur in KUF6 due to the smaller distance.
The potassium atom K(1) is located at Wyckoff position 4i with site symmetry m. It is coordinated by six fluorine atoms with a smaller distance of approximately 2.68 Å and four with a larger distance of ca. 3.09 Å. Of these, two are terminally bound with distances of 2.6762(4) Å for F(3) and 2.6886(4) Å for F(4), which are comparable to those in KF with 2.664 Å (T not reported). The remaining fluorine atoms are bridging, so that one-dimensional infinite chains are obtained in which two opposite quadrilateral faces are linked. A distorted pentagonal prism is thus obtained as a coordination polyhedron in which, compared with the ideal body, one fluorine atom is displaced out of the plane on each of the two pentagonal faces.
The Single-Crystal Structure of Rubidium Hexafluoridouranate(V), RbUF6
For rubidium hexafluoridouranate(V), RbUF6, similar speculations were made for the possible space group as for KUF6, see above. ,, Blueish-green crystals were obtained and analyzed by X-ray diffraction. Due to the similar lattice parameters and comparable effective ionic radii of the Rb+ and K+ ions, the compound crystallizes in the monoclinic crystal system with Z = 4 in space group C2/m (No. 12). Selected crystallographic data and details of the structure determination are given in Tables S11–S13 in the Supporting Information.
RbUF6 is isotypic to KUF6 so that no detailed structural discussion will be made. Selected atom distances are listed in Table .
4. Selected Atom Distances d of RbUF6 .
| atom distances d/Å | |||
|---|---|---|---|
| U(1)–F(1) | 2.0600(4) | Rb(1)–F(1) | 2.833(4) |
| U(1)–F(2) | 2.0573(4) | Rb(1)–F(1) | 3.1123(4) |
| U(1)–F(3) | 2.3286(2) | Rb(1)–F(2) | 2.8308(4) |
| U(1)–F(4) | 2.3286(2) | Rb(1)–F(2) | 3.1168(4) |
| U(1)–U(1)#1 | 4.0019(9) | Rb(1)–F(3) | 2.7691(5) |
| U(1)–U(1)#2 | 4.0148(9) | Rb(1)–F(4) | 2.7694(5) |
The uranium atom U(1) is coordinated by fluorine atoms at a distance of approximately 2.057(4) and 2.3286(2) Å, forming a distorted trigonal dodecahedron as a coordination polyhedron. The measured distances show a slight shortening compared to KUF6, which is probably due to the larger Rb+ ion and a shortening of the U–F bond. The U–U distances within the chains are also slightly smaller at 4.0019(9) and 4.0148(9) Å.
The rubidium atoms are coordinated by 10 fluorine atoms in the first coordination sphere, resulting in a distorted pentagonal prism as the coordination polyhedron. Smaller Rb–F distances of about 2.8 Å and larger distances of about 3.1 Å are observed. The two terminally bound fluorine atoms, F(3) with 2.7691(5) Å and F(4) with 2.7694(5) Å, have a comparable Rb–F distance as in RbF, 2.827 Å (T not reported).
The Single-Crystal Structure of Silver Hexafluoridouranate(V), AgUF6
During studies of the formation of silver carbonyl compounds, we obtained yellowish needle-shaped crystals of AgUF6. Silver hexafluoridouranate(V) was previously reported to crystallize in space group P42/mcm, which was established based on powder X-ray diffraction patterns. , However, our single-crystal X-ray diffraction study showed that AgUF6 crystallizes in the CaTbF6 structure type in the tetragonal space group P42/m (No. 84, tP16) with the lattice parameters a = 5.4188(2), c = 7.9458(4) Å, V = 233.32(2) Å3, Z = 2 at T = 100 K. Selected crystallographic data and details of the structure determination are given in Tables S14–S16 in the Supporting Information. A section of the crystal structure of AgUF6 is shown in Figure . Selected atom distances are given in Table .
3.
Section of the crystal structure of AgUF6 on the left; on the right, the crystal structure of AgUF6. The first coordination spheres of the U atoms are shown as green coordination polyhedra. Displacement ellipsoids at 70% probability at 100 K. Symmetry transformations for the generation of equivalent atoms: Ag(1)#1 1 + x, y, z; U(1)#1 1 – y, x, −1/2 + z; F(1)#1 y, 1 – x, −1/2 + z; F(1)#2 1 – y, x, −1/2 + z; F(1)#3 x, y, −1 + z; F(1)#4 1 – x, 1 – y, −1 + z; F(2)#1 – x, 1 – y, z; F(2)#2 1 + x, y, z; F(2)#3 1 – y, 1 + x, 1/2 – z; F(2)#4 y, −x, 1/2 – z.
5. Selected Atom Distances d of AgUF6 .
| atom distances d/Å | |||
|---|---|---|---|
| U(1)–F(1) | 2.3292(5) | Ag(1)–F(1) | 2.6499(5) |
| U(1)–F(2) | 2.0641(7) | Ag(1)–F(2) | 2.4345(8) |
| U(1)–U(1) | 3.9729(2) | ||
The asymmetric unit contains a uranium atom U(1), which is located at the Wyckoff position 2f (4̅.). , In the first sphere, the U atom is coordinated by eight fluorine atoms. Of these, the fluorine atom F(1) occupies Wyckoff position 4j (m..) and fluorine atom F(2) the position 8k (1). The equivalent fluorine atoms F(1)#1 to F(2)#4 are generated by symmetry transformations; for these, see the caption of Figure . Since the coordination sphere of the uranium atom is similar to those in KUF6 and RbUF6, we refrain from a detailed discussion here.
The silver atom Ag(1) is located at Wyckoff position 2a with site symmetry 2/m... It is coordinated by four fluorine atoms with a smaller distance of 2.4345(8) Å and two with a larger distance of 2.6499(5) Å in the shape of a distorted octahedron. The coordination polyhedra of the silver atoms are connected via shared corners to the coordination polyhedra of the uranium atoms. The packing of the octahedra along the crystallographic a and c axes is shown in Figure . If Ag–F distances of up to 3.1 Å are considered, after which a larger gap comes, then a distorted pentagonal antiprism results.
4.
Sections of the crystal structure of AgUF6. The packing of the coordination polyhedra of Ag is shown in gray, and those of U in green along the crystallographic a and c axes. Displacement ellipsoids at 70% probability at 100 K.
In the previously proposed structure model in space group P42/mcm, the coordination numbers of the U atoms were six, and those of the Ag atoms were eight with octahedral and square-antiprismatic coordination spheres. As said above, our structure model shows a coordination number of eight for the U atoms in the shape of a distorted trigonal dodecahedron as in the respective K and Rb compounds. The coordination number of the Ag+ cation is six in the shape of a distorted octahedron or ten in the shape of a distorted pentagonal antiprism, considering Ag– distances up to 3.1 Å. The coordination numbers of the K+ and the Rb+ cations of the UF6 – salts are also 10; however, a distorted pentagonal prism is observed. So, the difference in coordination spheres is where the different crystal structures of AgUF6 compared to KUF6 and RbUF6 come from.
The Single-Crystal Structure of Thallium(I) Hexafluoridouranate(V), TlUF6
In the literature, thallium hexafluoridouranate(V), TlUF6, has been assigned several possible space groups, similar to the cases of KUF6 and RbUF6 described above. However, a single-crystal structure investigation has not yet been reported. − Blue-green crystals were obtained and investigated by X-ray diffraction. Due to the cell parameters being similar to KUF6 and RbUF6, the structure model was refined in space group C2/m (No. 12). Selected crystallographic data and details of the structure determination are given in Tables S14–S16 in the Supporting Information.
TlUF6 is isotypic to KUF6 so that no detailed structural discussion will be made. Selected atom distances are given in Table . The uranium atom U(1) is coordinated at distances of 2.05 to 2.32 Å by a total of eight fluorine atoms in the shape of a distorted trigonal dodecahedron. In relation to KUF6, the terminal and bridging fluorine atoms, like in RbUF6, have smaller distances to the uranium atom, which can be explained by the larger effective ionic radius of the Tl+ ion and the resulting shortening of the U–F bond. This influence can also be observed in the U–U distances within the chain, which are the smallest in the series at 3.9937(9) and 4.0046(9) Å.
6. Selected Atom Distances d of TlUF6 .
| atom distances d/Å | |||
|---|---|---|---|
| U(1)–F(1) | 2.0538(4) | Tl(1)–F(1) | 2.8394(4) |
| U(1)–F(2) | 2.0611(4) | Tl(1)–F(1) | 3.1510(3) |
| U(1)–F(3) | 2.3241(3) | Tl(1)–F(2) | 2.8311(4) |
| U(1)–F(4) | 2.3264(3) | Tl(1)–F(2) | 3.1485(4) |
| U(1)–U(1)#1 | 3.9937(9) | Tl(1)–F(3) | 2.7962(5) |
| U(1)–U(1)#2 | 4.0046(9) | Tl(1)–F(4) | 2.8052(6) |
As in KUF6 and RbUF6, the Tl atoms are coordinated by ten F atoms in the first coordination sphere, resulting in a distorted pentagonal prism as a polyhedron. Smaller Tl–F distances of approximately 2.82 Å and larger ones of ca. 3.15 Å are observed. The two terminally bound fluorine atoms, F(3) with 2.7962(5) Å and F(4) with 2.8052(6) Å, have a comparable Tl–F distance as in TlF, 2.590–3.040 Å (T not reported).
The Single-Crystal Structure of Oxonium Hexafluoridouranate(V), H3OUF6
So far, only the crystal system of the oxonium salt H3OUF6 had been determined. It was described as cubic with the lattice parameter a = 5.2229(5) Å with the volume V = 142.47 Å3 (T = r.t.). On the basis of the powder X-ray diffractograms, however, it was not possible to distinguish whether the compound crystallizes cubic primitive, such as NOUF7, or body-centered cubic, such as NOUF6. ,
Blueish crystals of H3OUF6 were obtained and investigated by X-ray diffraction, indicating the space group Ia3̅ (No. 206). Selected crystallographic data and details of the structure determination are given in Tables S17–S19 in the Supporting Information. The hydrogen atoms could not be located due to the site symmetry. A section of the crystal structure is shown in Figure , and selected atom distances and angles are given in Table .
5.
Section of the crystal structure of H3OUF6 on the left; crystal structure of H3OUF6 with green coordination polyhedra for the UF6 – ions on the right. Displacement ellipsoids with 70% probability at 100 K. Symmetry transformations for the generation of equivalent atoms: #1 1 – y, −1/2 + z, 3/2 – x; #2 3/2 – z, 1 – x, 1/2 + y; #3 3/2 – x, 1/2 – y, 3/2 – z; #4 1/2 + y, 1 – z, x; #5 z, −1/2 + x, 1 – y.
7. Selected Atom Distances d and Angles ∠XYZ of H3OUF6 .
| atom distances d/Å | angles ∠XYZ/° | ||
|---|---|---|---|
| U(1)–F(1) | 2.0545(4) | F–U–F | 89.183(2); 90.817(2) |
| O(1)–F(1) | 2.6378(4) | F–U–F | 179.982(2); 180.00; 180.00 |
| U(1)–U(1) | 5.1752(6) | ||
The uranium atom U(1) resides on Wyckoff position 8b (.3̅.). At a distance of 2.0545(4) Å from this, the fluorine atom F(1) is located at general position 48e. Symmetry transformations generate the equivalent fluorine atoms F(1)#1 to F(1)#5, whereby the uranium atom is coordinated by six fluorine atoms in an octahedron-like manner; see Figure . Consistent with the site symmetry of the U(1) atom, the coordination polyhedron exhibits distortions compared to the ideal octahedron, as indicated by the F–U–F angles of 89.183(2) and 90.817(2)° and the axial angle of 179.982(2)°, in addition to the 180° angles. The distances and angles in the UF6 – ion are comparable to those in LiUF6 and NaUF6, see above.
The oxygen atom O(1) resides on Wyckoff position 8a (.3̅.). The hydrogen atom positions could not be determined due to the special position of the oxygen atom. However, the presence of the oxonium ion could be confirmed by IR spectroscopy; see Figure , where the O–H band is broadened and shifted due to hydrogen bonding (3268, 2808 cm–1). The bands at 1617 and 957 cm–1 can also be assigned to the H3O+ cation. ,, The band at 485 cm–1 is due to the UF6 – anion. From the crystal structure, no precise statements can be made about hydrogen bonds of the type O–H···F. Comparing the donor···acceptor distances, O···F, of 2.6378(4) Å with those of other oxonium salts in which hydrogen bonding has been observed by X-ray diffraction, such as H3OAsF6, 2.6691(3) Å (T = 238 K), H3OSb2F11, 2.553(8) to 2.903(9) Å (T = 294 K), or H3OBF4, 2.5771(4) to 2.6090(4) Å (T = 247 K), the presence of these in H3OUF6 is nevertheless quite probable and proven by the IR spectrum. −
6.
ATR-IR spectrum of H3OUF6 recorded at room temperature with 32 scans and a resolution of 4 cm–1.
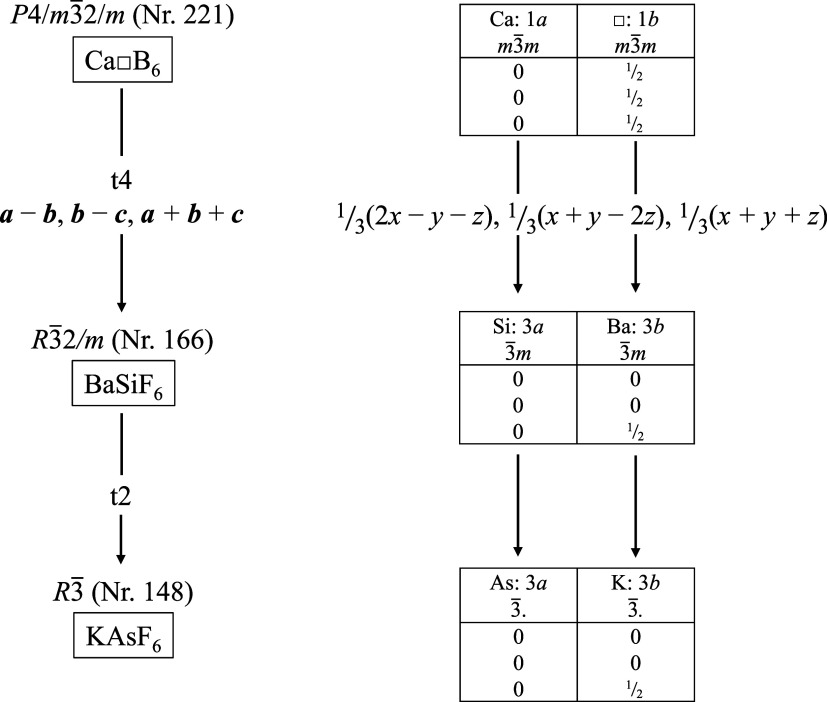
The lattice parameters indicate a structural relationship to O2PtF6, or NOUF6, in which, however, the 8a position is occupied by uranium atoms and the 8b position by the center of gravity of the cations. , Both crystal structures are superstructures of the CsCl structure type. H3OUF6 can therefore be considered more as an anti-O2PtF6 type due to the positional parameters, related to the example of the fluorite and “antifluorite” structure type. In comparison, H3OSbF6 and potassium hexafluoridoantimonate(V), KSbF6, crystallize in the same space group, similar lattice parameters and with the same occupation of the Wyckoff positions, making H3OUF6 isotypic to KSbF6. ,, In the following, the structural-chemical relationship between the CsCl type and the KSbF6 type will be analyzed by a group–subgroup relationship with the help of a Bärnighausen tree, see Figure . ,
7.
Bärnighausen tree with a group–subgroup relationship of CsCl to KSbF6, to which H3OUF6 is isotypic.
Starting from the aristotype CsCl, space group P4/m3̅2/m (No. 221), a translationengleiche transition of index 2 leads to subgroup P2/m3̅ (No. 200). , This lowers the site symmetry of the 1a and 1b position from m3̅m (Oh symmetry) to m3̅. (Th symmetry). , Based on this subgroup, a klassengleiche transition of index 4 leads to space group I21/a3̅ (No. 206) of hettotype KSbF6. In this case, the unit cell must be multiplied by a factor of 8; the Wyckoff positions develop as follows due to the origin shift of −1/2, −1/2, −1/2: 1a → 8b, 1b → 8a, which corresponds to swapping the centers of gravity of cation and anion sites. This transition reduces the site symmetries according to 3̅. (S 6 symmetry).
Powder X-ray Diffraction on Powders of the Above Compounds
The powder X-ray diffractogram of LiUF6 recorded at room temperature agrees well with the calculated diffractogram from the single-crystal structure analysis (T = 100 K), see Figure S4. A shift in the reflex positions can be attributed to the different measurement temperatures. The reflections could be indexed in the trigonal crystal system with the lattice parameters a = 5.27726(2) Å and c = 14.3676(4) Å, V = 346.52(2) Å3. The parameters are comparable to those previously reported for LiUF6 (ICDD entry [22–0411]) of a = 5.262(4), b = 14.295(5) Å and V = 342.78 Å3 (T not reported).
The NaUF6 obtained from hydrogen fluoride solution shows reflections in the powder X-ray diffractogram recorded at room temperature that can be assigned both to the cubic modification, ICDD entry [16–0720] (T not reported), and to the rhombohedral modification, ICDD entry [18 1220] (T not reported), see Figure S5. , A phase-pure synthesis from HF is therefore difficult, as the concentration, temperature, and distillation rate of the hydrogen fluoride have an influence on the supersaturation and polymorph formation.
KUF6 was previously described in the literature as orthorhombic. In the above single-crystal structure analysis, however, the monoclinic crystal system proved to be superior for a proper structure model; see above. The powder X-ray diffractogram (Figure S6), which was recorded at room temperature, is underlaid with the data of the ICDD entry [14–0661] for KUF6 (previous single-crystal structure) in addition to the calculated reflex positions from our single-crystal data (T = 100 K). The reflections could be indexed with both an orthorhombic and monoclinic cell with comparable quality factors. The orthorhombic cell has the lattice parameters a = 11.4785(2), b = 8.0181(1), and c = 5.6082(1) Å and V = 516.16(1) Å3, those for the monoclinic cell are a = 11.4856(2), b = 8.0187(1), c = 5.6118(9) Å, β = 90.08(2)°, and V = 516.84(2) Å3. Based on the single-crystal and powder X-ray data, the crystal structure of KUF6 can also be described as pseudo-orthorhombic. This pseudosymmetry is also evident in the diffractogram compared to the literature data set. The monoclinic unit cell differs only minimally from the orthorhombic unit cell and leads to a splitting of some reflections of the orthorhombic crystal system.
Similar to KUF6, an orthorhombic unit cell has been described in the literature for RbUF6. , The lattice parameters were determined to be a = 5.82 Å, b = 11.89 Å, c = 8.03 Å, and V = 555.7 Å3 (T not reported). The powder X-ray diffractogram recorded at room temperature with underlaid reflex positions and intensities calculated from our single-crystal structure analysis (T = 100 K) and the ICDD entry [16–0748] for RbUF6 are shown in Figure S7. Indexing of the reflections was possible with comparable quality factors using orthorhombic and monoclinic cells, as stated above. The lattice parameters of the monoclinic cell are a = 11.859(3), b = 8.0561(1), c = 5.7945(3) Å, β = 90.09(3)°, and V = 553.6(3) Å3 (T = 298 K).
The powder X-ray diffractogram of CsUF6 is shown in Figure S8. The calculated reflection intensities from the crystal structure of CsUF6 are underlaid on the diffractogram. The lattice parameters for the single crystal were determined to be a = 8.0189, c = 8.4373 Å, and V = 469.85 Å3 (T not reported). An indexing of the reflections of the powder pattern also revealed a trigonal crystal system with the lattice parameters a = 8.0284(9), c = 8.4388(1) Å, and V = 471.05(1) Å3.
For thallium(I) hexafluoridouranate(V), similar to KUF6 and RbUF6, an orthorhombic cell has been described in the literature so far. − The powder X-ray diffractogram recorded at room temperature is shown in Figure S9 and underlaid with the calculated reflection positions from our single-crystal structure analysis (T = 100 K) and the ICDD entry [16–0748] of the isotypic RbUF6, see above. The lattice parameters were determined as a = 5.956, b = 5.774, c = 4.001 Å, and V = 137.59 Å3 (T not reported). However, an indexing of the reflections of our powder pattern indicates a quadrupling of the unit cell, in agreement with the results of the single-crystal data. The description with orthorhombic and monoclinic cells provides comparable quality factors. The lattice parameters of the monoclinic unit cell are a = 11.951(3), b = 8.0435(2), c = 5.7818(2) Å, β = 89.99(3)°, and V = 556.0(3) Å3.
The powder X-ray diffractogram of H3OUF6 measured at room temperature, which is shown in Figure S10, was underlaid with the calculated reflection layers from our single-crystal structure analysis and for comparison with the ICDD entry [11–0246] of NOUF6 (T not reported). Certain similarities with H3OUF6 were attributed to the latter, but the single-crystal structure analysis has shown that the oxonium salt also crystallizes in space group Ia3̅ (No. 206), but NOUF6 is isotypic to O2PtF6 and H3OUF6 is isotypic to H3OSbF6, which corresponds to a swapping of the cation and anion sites. ,, The extraneous reflection at approximately 28° 2θ is probably due to the reaction of H3OUF6 with the borosilicate glass. The capillary was dissolved by the oxonium salt approximately 2 days after the measurement. Ignoring the reflection, the others can be indexed in the cubic crystal system with the lattice parameters a = 5.2256(6) Å and V = 142.69(3) Å3. However, the single-crystal structure analysis shows an 8-fold increase in the primitive unit cell to a = 10.4496(1) Å and V = 1141.04(2) Å3, as was also shown for NOUF6.
The Single-Crystal Structure of Barium Dodecafluoridodiuranate(V)–Hydrogen Fluoride(1/1.36), Ba[U2F12]·1.36HF
Greenish plate-like crystals of Ba[U2F12]·1.36HF were obtained at −40 °C, isolated at room temperature, and subjected to single-crystal X-ray analysis. Ba[U2F12]·1.36HF crystallizes in the orthorhombic space group Pnma (No. 62, oP68) with the lattice parameters a = 8.9989(8), b = 12.6822(9), c = 9.3514(6) Å, V = 1067.24(14) Å3, and Z = 4 at T = 100 K. The structure solution was also carried out in space group Pna21, but this structure model resulted in negative displacement parameters of the fluorine atoms. Selected crystallographic data and details of the structure determination are given in Tables S20–S22 in the Supporting Information. A section of the crystal structure of Ba[U2F12]·1.36HF is shown in Figure . Selected atom distances are given in Table .
8.
Section of the crystal structure of Ba[U2F12]·1.36HF on the left with displacement ellipsoids at 70% probability at 100 K, and on the right its crystal structure, with atoms drawn with arbitrary radii. The first coordination spheres of the U atoms are shown as green coordination polyhedra and those of the Ba atoms in gray. Symmetry transformations for the generation of equivalent atoms: U(1)#1 – x, 1 – y, 1 – z; F(1)#1– x, 1 – y, 1 – z; F(2)#1 – x, 1 – y, 1 – z; F(2)#2 1/2 – x, 1/2 + y, −1/2 + z; F(2)#3 1/2 – x, 1 – y, −1/2 + z; F(3)#1 1/2 + x, y, 3/2 – z; F(3)#2 1/2 + x, 3/2 – y, 3/2 – z; F(3)#3 1/2 + x, y, 3/2 – z; F(4)#1 – x, 1 – y, 1 – z; F(4)#2 x, 3/2 – y, z; F(5)#1 – x, 1 – y, 1 – z; F(5)#2 1 – x, 1 – y, 1 – z; F(5)#3 1 – x, 1/2 + y, 1 – z; F(6)#1 – x, 1 – y, 1 – z. The site occupancy factor of F(8) is only 0.36(4).
8. Selected Atom Distances d of Ba[U2F12]·1.36HF.
| atom distances d/Å | |||
|---|---|---|---|
| Ba(1)–F(2) | 2.676(7) | U(1)–F(1) | 2.060(7) |
| Ba(1)–F(3) | 2.748(7) | U(1)–F(2) | 2.068(7) |
| Ba(1)–F(4) | 2.695(7) | U(1)–F(3) | 2.062(7) |
| Ba(1)–F(5) | 2.686(8) | U(1)–F(4) | 2.068(7) |
| Ba(1)–F(7) | 2.924(11) | U(1)–F(5) | 2.061(7) |
| Ba(1)–F(8) | 2.90(3) | U(1)–F(6) | 2.260(7) |
| H(1)–F(7) | 1.3(2) | ||
The asymmetric unit contains a uranium atom U(1), which is located at the Wyckoff position 8d with site symmetry 1. In the first coordination sphere, the U atom is coordinated by seven fluorine atoms, which are located at Wyckoff position 8d. The two F atoms F(6) act μ-bridging between two U(1) atoms, and a dinuclear [U2F12]2– anion is formed. The U–F bond lengths agree within their tripled standard deviation with those of Sr[U2F12], with the U–F bond lengths of the μ-bridging F atoms are approximately 0.2 Å longer than the other U–F bond lengths.
The equivalent atoms are generated by symmetry transformations; for these, see the caption of Figure . The coordination sphere of the dinuclear [U2F12]2– anion can be described as two monocapped trigonal prisms with a shared edge.
The Ba atom is located at Wyckoff position 4c with site symmetry m. It is coordinated by eight fluorine atoms with a smaller distance of approximately 2.70 Å and two atoms with a larger distance of approximately 2.91 Å. The latter fluorine atoms F(7) and F(8) belong to HF molecules of crystallization, as can be seen in Figure . The site occupancy factor of F(8) is only 0.36(4), and therefore, the respective H atom could not be located. It is likely that the compound Ba[U2F12]·2HF exists, but during the removal of the solvent in vacuum, a part of the HF molecules of crystallization seem to be removed, too. The shape of the coordination polyhedron can be described as a distorted bifold-capped cube. In the homologue compound Sr[U2F12], the coordination number of the Sr2+ cation is eight with Sr–F distances of 2.479(4) Å.
Overall, a 3D network is formed by the corner shared coordination polyhedra of the barium and uranium atoms.
Structural-Chemical Classification of Alkali Metal Hexafluoridouranates(V)
The structural-chemical properties of alkali metal hexafluoridouranates(V) shall be compared with those of other representatives of the composition M[E VF6] (M = Li–Cs; E V = P–Bi, Pa, Np, Pu). Previous work is available for hexafluoridopnictates(V) and various hexafluorometallate(V) salts. Structural data for the actinoids, with the exception of uranium, are sparsely available for this class of compounds so that they can only be included in some places. Table lists structural parameters such as structure type, space group, lattice parameters, and the literature reference of the compounds under consideration. Data from high-pressure modifications were not listed (NaSbF6 (rhombohedral), KPF6 (cubic)). , The lithium salts with hexafluoridopnictate(V) and uranate(V) ions all crystallize in the trigonal crystal system, isotypic to LiSbF6, in space group R3̅ (No. 148). The coordination number of the cations is six.
9. Overview of Crystal Structures of Compounds with Composition M[E VF6] (M = Li, Na, K, Rb, and Cs; E V = P, As, Sb, Bi, Pa, U, Np, and Pu).
| M | E V | structure type | space group (No.) | a/Å | b/Å | c/Å | V/Å3 | Lit. |
|---|---|---|---|---|---|---|---|---|
| Li | P | LiSbF6 | R3̅ (148) | 4.932 | a | 12.658 | 266.65 | |
| As | 5.016 | a | 13.028 | 283.87 | ||||
| Sb | 5.18 | a | 13.60 | 316.03 | ||||
| Bi | 5.181 | a | 13.99 | 325.22 | ||||
| U | 5.1902(7) | a | 14.265(3) | 332.78(1) | this work | |||
| Na | P | NaSbF6 | Fm3̅m (225) | 7.6140 | a | a | 441.41 | |
| As | LiSbF6 | R3̅ (148) | 5.3375 | a | 13.9645 | 344.53 | ||
| As | NaSbF6 | Fm3̅m (225) | 7.8608 | a | a | 485.74 | ||
| Sb | NaSbF6 | Fm3̅m (225) | 8.203 | a | a | 551.97 | ||
| Bi | LiSbF6 | R3̅ (148) | 5.468 | a | 15.16 | 392.54 | ||
| U | LiSbF6 | R3̅ (148) | 5.4101(8) | a | 15.746(3) | 399.12(1) | this work | |
| U | NaSbF6 | Fm3̅m (225) | 8.608 | a | a | 637.83 | ||
| Pa | ? | ? | 5.35 | a | 3.98 | 113.92 | ||
| K | P | KPF6 | Fm3̅m (225) | 7.7891 | a | a | 472.57 | |
| As | KAsF6 | R3̅ (148) | 7.348 | a | 7.274 | 340.13 | ||
| Sb | KNbF6 | P4̅2m (111) | 5.16 | a | 10.07 | 268.12 | , | |
| Sb | KSbF6 | Ia3̅ (206) | 10.15 | a | a | 1045.68 | ||
| Bi | KNbF6 | P4̅c2 (116) | 5.248 | a | 10.07 | 277.34 | ||
| Bi | KSbF6 | Ia3̅ (206) | 10.34 | a | a | 1105.51 | ||
| U | KUF6 | C2/m (12) | 11.4415(2) | 8.0345(1) | 5.5655(1) | 511.62(4) | this work | |
| Pa | ? | ? | 5.64 | 11.54 | 7.98 | 519.38 | ||
| Rb | P | KPF6 | Fm3̅m (225) | 7.887 | a | a | 490.61 | |
| As | KAsF6 | R3̅ (148) | 7.497 | a | 7.589 | 369.39 | ||
| As | CsPF6 | Fm3̅m (225) | 8.246 | a | a | 560.7 | ||
| Sb | KAsF6 | R3̅ (148) | 7.670 | a | 7.861 | 400.50 | ||
| Sb | Ca□B6 | Pm3̅m (221) | 5.287 | a | a | 147.8 | ||
| Bi | KAsF6 | R3̅ (148) | 7.712 | a | 7.889 | 406.34 | ||
| U | KUF6 | C2/m (12) | 11.797(2) | 8.0167(2) | 5.7272(1) | 541.64(2) | this work | |
| Pa | RbPaF6 | Cmme (67) | 8.0483 | 12.0253 | 5.8608 | 567.23 | ||
| Cs | P | KPF6 | Fm3̅m (225) | 8.197 | a | a | 550.76 | |
| As | KAsF6 | R3̅ (148) | 7.723 | a | 8.050 | 415.81 | ||
| As | CsPF6 | Fm3̅m (225) | 8.384 | a | a | 589.32 | ||
| Sb | KAsF6 | R3̅ (148) | 7.904 | a | 8.261 | 446.95 | ||
| Sb | Ca□B6 | Pm3̅m (221) | 5.474 | a | a | 164.03 | ||
| Bi | KAsF6 | R3̅ (148) | 7.930 | a | 8.274 | 450.60 | ||
| U | KAsF6 | R3̅ (148) | 8.019 | a | 8.437 | 469.9 | ||
| Pa | ? | ? | 6.14 | 12.56 | 8.06 | 621.57 | ||
| Np | KAsF6 | R3̅ (148) | 8.017 | a | 8.386 | 466.78 | ||
| Pu | ? | ? | 8.006 | a | 8.370 | 464.61 |
Hexagonal setting.
A partial transition to the cubic crystal system is already observed for the sodium salts. Except for the P, Bi, and Pa containing salts, polymorphism was observed in the sodium salts. In addition to the rhombohedral LiSbF6 type, the cubic NaSbF6 type (C.N(M) = 6), space group Fm3̅m (No. 225), occurs. The latter is related to the NaCl type. , The structural relationship between the rhombohedral and cubic phases can be shown with the help of a group–subgroup relationship in the form of a Bärnighausen family tree, see Figure . ,
9.
Bärnighausen tree with group–subgroup relations of aristotype NaSbF6 via α–CuZrF6 to hettotype LiSbF6.
Starting from the aristotype NaSbF6, space group F4/m3̅2/m (No. 225), a translationengleiche transition of index 2 leads to space group F2/m3̅ (No. 202) with the hettotype α-CuZrF6. ,, During the transition, the point symmetry is decreased from Oh to Th symmetry. Further symmetry reduction with a translationengleiche transition of index 4, under transformation of coordinates and axes into the hexagonal setting, leads to subgroup R3̅ (No. 148) with the hettotype LiSbF6. The lower site symmetry of 3̅ (S 6 symmetry) is then obtained for the positions.
Compared to the lighter homologues, the potassium salts show more diversity in the crystal systems and thus structure types, which can be attributed to the accessibility of different coordination numbers for the K+ ions. In addition to the cubic crystallizing representatives, with PF6 –, SbF6 –, or BiF6 – ions, tetragonal ones are also observed, which are isotypic to KNbF6 (C.N.(M): 8) or a distortion variant thereof. However, the uranium compound shows the greatest structural difference in the series. In addition to the monoclinic crystal system, instead of molecular UF6 – ions, one-dimensional infinite chains of the form ∞ {[UF4/1F4/2]−} are observed. In KPaF6, bridged anions are likely also formed, which can be assumed based on the lattice parameters similar to RbPaF6; single-crystal structure data are not available. − The coordination number of the K+ cation in KUF6 is 10.
The transition to rubidium salts shows no major anomalies for the hexafluoridopnictates(V). Cubic and rhombohedral variants are observed, which often crystallize in the KAsF6 structure type. The UF6 – and PaF6 – salts also show with bridged ∞ {[EF4/1F4/2]−} ions a different behavior. There is a modification of RbSbF6 that crystallizes in the CaB6 structure type, which is also related to the CsCl type and can be written as Ca□B6 type (C.N.(M): 6, □: unoccupied octahedral void). , Of the latter, a relationship with the KAsF6 structure type (C.N.(M): 12) can be shown via a group–subgroup relationship, see Figure . From the cubic aristotype Ca□B6, space group P4/m3̅2/m (No. 221), in which the octahedral voids on the 1b position are unoccupied, a translationengleiche transition of index 4 leads to subgroup R3̅2/m (No. 166). In the hettotype BaSiF6 the 1b position is occupied by Ba2+ cations. Further symmetry reduction by a translationengleiche transition of index 2 leads to subgroup R3̅ (No. 148), in which KAsF6 is represented.
10.
Bärnighausen tree with group–subgroup relation of the aristotype Ca□B6 via BaSiF6 to the hettotype KAsF6.
The cesium salts show differences in relation to the other alkali metal actinoid compounds. The fluoridouranate, -neptunate, and -plutonate crystallize in the rhombohedral crystal system with molecular EF6 – ions, while the protactinate likely crystallizes isotypic to RbPaF6 in the orthorhombic system with infinite strands of anions. , This raises the question of the driving force behind the interconnection of the anions with respect to the electron configuration. On the one hand, the diamagnetic Pa(V) compounds ([Rn]5f0) show bridged anions from potassium to cesium, which, however, cannot be attributed to a Pa···Pa interaction. On the other hand, the paramagnetic U(V) compounds ([Rn]5f1) show bridged anions in the potassium and rubidium salt and molecular anions in the cesium salt. The cesium hexafluoridopnictates(V) crystallize, just like the lighter homologues, in either the cubic or trigonal crystal system.
Finally, the relationships and data shown in Table are summed up graphically in a structure field diagram in Figure , see also the literature. The observed structure types of the corresponding ME(V)F6 compounds are plotted as a function of the effective ionic radii of the cation. The diagram is divided into three colored areas, which are intended to show the relationship to the aristotypes, i.e., the NaCl and CsCl, or the KUF6 structure type. Table lists selected crystallographic data, such as the Wyckoff position of the alkali metal ion M or the coordination number of M of the observed structure types.
11.
Structure field diagram of fluorides with composition ME(V)F6 (M = Li, Na, K, Rb, and Cs; E V = P, As, Sb, Bi, and U), with color-coded areas to show the structural relationships. The effective ionic radii of the cations r M were chosen in accordance with the coordination number; those of the E(V)-Ions are for C.N. 6.
10. Summary of Crystal-Chemical Data for the Observed Structure Types.
| structure type | crystal system | Wyckoff position (M) | site symmetry | C.N.(M): |
|---|---|---|---|---|
| NaSbF6 [127] | cubic | 4b | 3̅. | 6 |
| KSbF6 [134] | cubic | 8b | .3̅. | 6 |
| KPF6 [128] | cubic | 4b | m3̅m | - |
| CsPF6 [137] | cubic | 4a | m3̅m | - |
| Ca□B6 [138] | cubic | 1b | m3̅m | 6 |
| KAsF6 [130] | trigonal | 3b | 3̅. | 12 |
| LiSbF6 [89] | trigonal | 3b | 3̅. | 6 |
| KNbF6 [132] | tetragonal | 2c | 4̅.. | 8 |
| KUF6 | monoclinic | 4h | 2 | 10 |
Disordered structures.
Single-crystal studies of this work.
Conclusions
We presented the synthesis of the compounds MUF6 (M = Li–Cs, Tl) by the reaction of β-uranium(V) fluoride with the respective fluoride MF in aHF. This route also allowed us to obtain these compounds in the form of single crystals. The oxonium salt H3OUF6 was obtained by the reaction of β-UF5 with silica glass wool in aHF. Other forms of silicon dioxide, such as amorphous or annealed SiO2, led to the formation of unidentified solid side products. AgUF6 was synthesized in aHF from Ag and UF6 in the presence of CO.
The salts LiUF6 and NaUF6 (rhombohedral) crystallize in the trigonal crystal system and are isotypic to LiSbF6 with molecular UF6 – ions in the solid state. The crystal structures are related to the NaSbF6 structure type, which presents a hettotype of the NaCl structure type. This was shown by a group–subgroup relation.
H3OUF6 crystallizes in the cubic crystal system and is an isotypic KSbF6. Molecular UF6 – ions are present in the solid. Previous studies had not been able to discriminate between a cubic primitive or body-centered Bravais lattice, as the indexing of the reflections in the powder X-ray pattern does not allow for discrimination.
KUF6, RbUF6, and TlUF6 crystallize in the monoclinic crystal system and contain more complex structural motives compared with the compounds mentioned above. In the solids, the anions are one-dimensional (1D) infinite strands of the type ∞ {[UF4/1F4/2]−}. The U atoms have a coordination number of 8 and the coordination polyhedron is a distorted trigonal dodecahedron. There was uncertainty in the literature regarding the anions present and the crystal system of these salts. Various orthorhombic space groups were assigned to the compounds, but these could not be confirmed in this work. On the one hand, no structural model from the single-crystal structure investigations could be satisfactorily refined in orthorhombic space groups and, on the other hand, the powder X-ray diffractograms showed reflection splitting, which indicated the monoclinic crystal system. However, the data described in the literature could also have been obtained on other modifications of the compounds, but no evidence for such polymorphism could be found in this work.
A coordination of the U atoms similar to that in KUF6, RbUF6, and TlUF6 was observed in AgUF6. By single-crystal X-ray diffraction, we could establish the space group to be P42/m. Attempts to determine and refine the structure in the previously proposed space group P42/mcm resulted in a completely inappropriate structure model. AgUF6 crystallizes in the CaTbF6 structure type. The coordination sphere of the Ag+ cations is octahedron-like or distorted pentagonal antiprismatic if longer Ag–F distances are considered. The alkali metal cations in KUF6 and RbUF6 show a distorted pentagonal-prismatic coordination sphere.
The dodecafluoridodiuranate(V) Ba[U2F12]·1.36HF was synthesized, and its crystal structure was determined. In contrast to the only other known compound containing the [U2F12]2– dianion, Sr[U2F12], the barium compound contains HF molecules of crystallization.
Experimental Section
General
All operations were performed on a Monel steel Schlenk line, which was passivated with fluorine at various temperatures and pressures before use. Reaction vessels were made out of fluoropolymer (perfluoroalkoxy alkanes, PFA) and sealed with a suitable needle valve (Swagelok). The vessels were baked out in vacuum (∼10–3 mbar) at ca. 100 °C several times and passivated with diluted F2 (F2/Ar 20:80, v/v, Solvay). Alkali metal and alkaline earth metal fluorides had been purchased from Merck and purified according to literature methods. Solid starting materials were stored and handled in an Ar-filled (Ar 5.0, Nippon Gases) glovebox (MBraun).
Caution! HF is highly toxic. Therefore, proper protective equipment must be worn, and appropriate emergency treatment procedures must be available in the case of contact. Uranium compounds are radioactive, and appropriate or required measures for their handling need to be taken.
The preparation of β-UF5 was carried out by the photoreduction of UF6 with CO in a UV reactor. , The purity of the obtained β-uranium(V) fluoride was analyzed by powder X-ray diffraction and IR spectroscopy; see the Supporting Information, Figures S11 and S12.
To prepare the alkali metal hexafluoridouranates(V), one FEP Schlenk tube each was dried at 150 °C in a drying oven and transferred to the glovebox. There it was charged with equimolar quantities of alkali metal fluoride and β-uranium(V) fluoride, attached to a Schlenk line outside of the glovebox, and then aHF was distilled onto the solids in a vacuum under cooling with liquid nitrogen. Exemplary quantities used are listed in Table S1. The reaction vessel was heated in an air bath until the hydrogen fluoride melted; as soon as it became a liquid, the majority of the solids dissolved, turning the solution blue. At room temperature, the reaction mixtures were homogenized by vortexing, and after a short time, the solvent was distilled into a separate FEP Schlenk tube at a low flow rate under vacuum. This led to the formation of crystalline products, photographs of which are shown in Figures S1–S3. Several vacuum inert gas purges were carried out to remove traces of the solvent. Typical batch sizes for the syntheses of the various hexafluoridouranates(V) are given in the Supporting Information, Table S1.
AgUF6 was obtained as a side product from the reaction of Ag powder with UF6 in aHF under a CO atmosphere. A PFA-vessel with an approximate volume of 4 mL was loaded with Ag powder (103.6 mg, 0.96 mmol) and closed with a PFA valve. At −196 °C, aHF (1 mL) and UF6 (880.0 mg, 2.50 mmol) were condensed into the vessel, and CO (2 bar) was added. The reaction was warmed to −40 °C, and a colorless solution and a colorless solid were observed. For several times, all volatile compounds were pumped off at −196 °C and fresh CO was added. After 4 weeks, the reaction vessel was warmed to room temperature, and all volatile compounds were removed in static vacuum. The obtained colorless solid (254.0 mg, 57% with respect to the used Ag) was dried for only a short time in dynamic vacuum, to prevent a potential carbonyl compound from decomposition. A few colorless needle-like crystals were found beneath greater agglomerates of yellowish solid, which were identified as AgUF6. Unfortunately, no powder X-ray diffraction pattern could be obtained.
For the synthesis of Ba[U2F12]·1.36HF, an FEP Schlenk tube was charged with BaF2 (26.3 mg, 0.15 mmol) and UF5 (50 mg, 0.15 mmol) and 2 mL of aHF was condensed onto the solids in vacuum under cooling with liquid nitrogen. The vessel was heated to 80 °C for 2 h, and the solution changed its color to blue. Subsequent storage at −40 °C yielded greenish plate-like crystals. The solvent was removed under static vacuum at room temperature, and Ba[U2F12]·1.36HF was isolated (isolated yield: 35.8 mg, 0.04 mmol, 27% with respect to BaF2). Unfortunately, no powder X-ray pattern could be obtained.
CCDC 2468468 (LiUF6), 2468469 (NaUF6), 2468470 (AgUF6), 2468471 (RbUF6), 2468472 (TlUF6), 2468473 (KUF6), 2468474 (H3OUF6), and 2468676 (Ba[U2F12]·1.36HF) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
Supplementary Material
Acknowledgments
We thank Solvay for the kind donations of F2, Urenco for UF6, and Dr. S. I. Ivlev for helpful discussions. This study is dedicated to the late Prof. Dr. Hartmut Bärnighausen
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.5c03005.
Typical batch sizes of the syntheses, photographs of most compounds, selected crystallographic data of the structure determinations, powder X-ray diffraction patterns of most bulk phase syntheses, and the characterization of the UF5 used as a starting material (PDF)
The authors declare no competing financial interest.
References
- Gary Eller P., Larson A. C., Peterson J. R., Ensor D. D., Young J. P.. Crystal Structures of α-UF5 and U2F9 and Spectral Characterization of U2F9. Inorg. Chim. Acta. 1979;37:129–133. doi: 10.1016/S0020-1693(00)95530-0. [DOI] [Google Scholar]
- Taylor J. C., Waugh A. B.. Neutron Diffraction Study of Beta-Uranium Pentafluoride between 77 and 403K. J. Solid State Chem. 1980;35:137–140. doi: 10.1016/0022-4596(80)90485-5. [DOI] [Google Scholar]
- Selbin J., Ortego J. D.. The Chemistry of Uranium (V) Chem. Rev. 1969;69(5):651–671. doi: 10.1021/cr60261a004. [DOI] [Google Scholar]
- Bacher, W. ; Jacob, E. . Gmelin Handbuch Der Anorganischen Chemie Uran Ergänzungsband C8; Springer-Verlag: Berlin, Heidelberg, NY, 1980. [Google Scholar]
- Penneman R. A., Sturgeon G. D., Asprey L. B.. Fluoride Complexes of Pentavalent Uranium. Inorg. Chem. 1964;3(1):126–129. doi: 10.1021/ic50011a029. [DOI] [Google Scholar]
- Ryan J. L.. Halide Complexes of Pentavalent Uranium. J. Inorg. Nucl. Chem. 1971;33(1):153–177. doi: 10.1016/0022-1902(71)80019-2. [DOI] [Google Scholar]
- Halstead, G. W. ; Eller, P. G. ; Paine, R. T. . 35. Uranium(V) Fluorides and Alkoxides. In Inorganic Syntheses; Fackler, J. P. , Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1982; Vol. 21, pp 162–167. [Google Scholar]
- Ogle, P. R. ; Geichman, J. R. ; Trond, S. S. . Reactions of Molybdenum, Tungsten, and Uranium Hexafluorides with Nitrogen Compounds. Part I. Nitrous and Nitric Oxides, GAT-T-552(Rev.1), 4828160; Goodyear Atomic Corp, 1959; http://www.osti.gov/servlets/purl/4828160-fdj8mU/. (accessed February 10, 2016). [Google Scholar]
- Geichman, J. R. ; Smith, E. A. ; Swaney, L. R. ; Ogle, P. R. . Hexafluorides of Molybdenum, Tungsten and Uranium. II. Reactions with Liquid and Gaseous Dinitrogen Tetroxide, GAT-T-970, 4787827; Goodyear Atomic Corp, 1962; http://www.osti.gov/servlets/purl/4787827-0ERS3B/. (accessed February 09, 2016). [Google Scholar]
- Geichman, J. R. ; Swaney, L. R. ; Ogle, P. R. . Hexafluorides of Molybdenum, Tungsten and Uranium. III. Reactions with Nitrogen Dioxide and Nitrogen Oxyhalides, GAT-T-971, 4726485; Goodyear Atomic Corp., 1962; http://www.osti.gov/servlets/purl/4726485-Ia6IMy/. (accessed February 10, 2016). [Google Scholar]
- Geichman J. R., Smith E. A., Trond S. S., Ogle P. R.. Hexafluorides of Molybdenum, Tungsten, and Uranium. I. Reactions with Nitrous and Nitric Oxides. Inorg. Chem. 1962;1(3):661–665. doi: 10.1021/ic50003a042. [DOI] [Google Scholar]
- Geichman J. R., Smith E. A., Ogle P. R.. Hexafluorides of Molybdenum, Tungsten, and Uranium. II. Reactions with Nitryl Fluoride, Nitrosyl Fluoride, and Nitrosyl Chloride. Inorg. Chem. 1963;2(5):1012–1015. doi: 10.1021/ic50009a031. [DOI] [Google Scholar]
- Scheibe B., Lippert S., Rudel S. S., Buchner M. R., Burghaus O., Pietzonka C., Koch M., Karttunen A. J., Kraus F.. NOUF6 Revisited: A Comprehensive Study of a Hexafluoridouranate(V) Salt. Chem. - Eur. J. 2016;22(34):12145–12153. doi: 10.1002/chem.201602265. [DOI] [PubMed] [Google Scholar]
- Rampy, G. A. Ammonium Uranium(V) Fluoride And Potassium Uranium(V) Fluoride--A Preliminary Report, GAT-T-697, Goodyear Atomic Corp., 1959; https://www.osti.gov/scitech/biblio/4211023-ammonium-uranium-fluoride-potassium-uranium-fluoride-preliminary-report. (accessed February 09, 2016). [Google Scholar]
- Nguyen N., Dianoux A. J., Marquet-Ellis H., Plurien P.. Contribution à l′étude d’un Fluorure Complexe d’uranium Pentavalent et d’ammonium. Préparation et Susceptibilité Magnétique. C. R. Hebd. Seances Acad. Sci. 1965;260:1963–1966. [Google Scholar]
- Geichman, J. R. ; Swaney, L. R. ; Ogle, P. R. . Chemical Properties Of Nitrosyl Hexafluorouranate(V), GAT-T-808, Report GAT-T-808; Goodyear Atomic Corp., 1963. [Google Scholar]
- Sturgeon G. D., Penneman R. A., Kruse F. H., Asprey L. B.. Synthesis and Crystallographic Properties of Single Crystals of Alkali Uranium(V) Fluoride Complexes. Inorg. Chem. 1965;4(5):748–750. doi: 10.1021/ic50027a033. [DOI] [Google Scholar]
- Jache A. W., Cady G. H.. Solubility of Fluorides of Metals in Liquid Hydrogen Fluoride. J. Phys. Chem. A. 1952;56(9):1106–1109. doi: 10.1021/j150501a018. [DOI] [Google Scholar]
- Grüttner, B. ; Dove, M. F. A. ; Clifford, A. F. . Chemistry in Nonaqueous Ionizing Solvents - Vol. II Chemistry in Anhydrous, Prototropic Inorganic Solvents - Part 1 Inorganic Chemistry in Liquid Hydrogen Cyanide and Liquid Hydrogen Fluoride; Jander, G. ; Spandau, H. ; Addison, C. C. , Eds.; Friedr. Vieweg & Sohn:: Braunschweig, 1971; Vol. 1. [Google Scholar]
- Adelhelm M., Bacher W., Höhn E. G., Jacob E.. Dicarbonylgold(I)-hexafluorouranat(VI), Au(CO)2UF6. Chem. Ber. 1991;124(7):1559–1561. doi: 10.1002/cber.19911240712. [DOI] [Google Scholar]
- Bougon R., Plurien P.. TlUF6 Und AgUF6 Compt Rend. C. R. Hebd. Seances Acad. Sci. 1965;260:4217–4218. [Google Scholar]
- Asprey L. B., Penneman R. A.. Properties of Uranium(V) in Hydrofluoric Acid Solution; the New Compounds HUF6.2.5H2O and HUF6.1.25H2O. Inorg. Chem. 1964;3(5):727–729. doi: 10.1021/ic50015a028. [DOI] [Google Scholar]
- Masson J. P., Desmoulin J. P., Charpin P., Bougon R.. Synthesis and Characterization of a New Uranium(V) Compound: Oxonium Hexafluorouranate(V) Inorg. Chem. 1976;15(10):2529–2532. doi: 10.1021/ic50164a042. [DOI] [Google Scholar]
- Scheibe B., Pietzonka C., Mustonen O., Karppinen M., Karttunen A. J., Atanasov M., Neese F., Conrad M., Kraus F.. The [U2F 12]2– Anion of Sr[U2F 12 ] Angew. Chem., Int. Ed. 2018;57(11):2914–2918. doi: 10.1002/anie.201800743. [DOI] [PubMed] [Google Scholar]
- Müller, U. Anorganische Strukturchemie, 6., Aufl.,; Studium; Vieweg + Teubner: Wiesbaden, 2009. [Google Scholar]
- Aroyo M. I., Kirov A., Capillas C., Perez-Mato J. M., Wondratschek H.. Bilbao Crystallographic Server. II. Representations of Crystallographic Point Groups and Space Groups. Acta Crystallogr., Sect. A: Found. Crystallogr. 2006;62(2):115–128. doi: 10.1107/S0108767305040286. [DOI] [PubMed] [Google Scholar]
- Rosenzweig A., Cromer D. T.. The Crystal Structure of CsUF 6 . Acta Crystallogr. 1967;23(5):865–867. doi: 10.1107/S0365110X67003858. [DOI] [Google Scholar]
- Eastman M. P., Gary Eller P., Halstead G. W.. Electron Paramagnetic Resonance and Crystal Structure Study of Bis(Triphenylphosphine)Iminium Hexafluorouranate(V) J. Inorg. Nucl. Chem. 1981;43(11):2839–2842. doi: 10.1016/0022-1902(81)80627-6. [DOI] [Google Scholar]
- Howard C. J., Taylor J. C., Waugh A. B.. Crystallographic Parameters in α-UF5 and U2F9 by Multiphase Refinement of High-Resolution Neutron Powder Data. J. Solid State Chem. 1982;45(3):396–398. doi: 10.1016/0022-4596(82)90185-2. [DOI] [Google Scholar]
- Levy J. H., Taylor J. C., Waugh A. B.. Neutron Powder Structural Studies of UF6, MoF6 and WF6 at 77 K. J. Fluorine Chem. 1983;23(1):29–36. doi: 10.1016/S0022-1139(00)81276-2. [DOI] [Google Scholar]
- International Union of Crystallography . International Tables for Crystallography: Vol. A: Space-Group Symmetry, 5th ed.; Hahn, T. , Ed.; Springer: Dordrecht, 2005. [Google Scholar]
- Thewlis J.. Unit-Cell Dimensions of Lithium Fluoride Made from Li 6 and Li 7 . Acta Crystallogr. 1955;8(1):36–38. doi: 10.1107/S0365110X55000091. [DOI] [Google Scholar]
- Ostwald W.. Studien über die Bildung und Umwandlung fester Körper: 1. Abhandlung: Übersättigung und Überkaltung. Z. Phys. Chem. 1897;22U(1):289–330. doi: 10.1515/zpch-1897-2233. [DOI] [Google Scholar]
- Stranski I. N., Totomanow D.. Die Ostwaldsche Stufenregel. Naturwissenschaften. 1932;20(50):905. doi: 10.1007/BF01504912. [DOI] [Google Scholar]
- Pathak P. D., Trivedi J. M., Vasavada N. G.. Thermal Expansion of NaF, KBr and RbBr and Temperature Variation of the Frequency Spectrum of NaF. Acta Crystallogr., Sect. A. 1973;29(4):477–479. doi: 10.1107/S0567739473001142. [DOI] [Google Scholar]
- Shannon R. D.. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr., Sect. A: Found. Adv. 1976;32(5):751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- Charpin P.. Uranium(V) Hexafluoride Complexes. Crystal Structure of Uranium(V) Hexafluoride Complexes of Silver, Potassium, Ammonium, Rubidium, and Thallium. C. R. Hebd. Seances Acad. Sci. 1965;260(7):1914–1916. [Google Scholar]
- Asprey L. B., Kruse F. H., Rosenzweig A., Penneman R. A.. Synthesis and X-Ray Properties of Alkali Fluoride-Protactinium Pentafluoride Complexes. Inorg. Chem. 1966;5(4):659–661. doi: 10.1021/ic50038a034. [DOI] [Google Scholar]
- Burns J. H., Levy H. A., Keller O. L. Jr. The Crystal Structure of Rubidium Hexafluoroprotactinate(V),RbPaF6. Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater. 1968;24:1675–1680. doi: 10.1107/S0567740868004838. [DOI] [Google Scholar]
- Halides of the Transition Elements -Halides of the Lanthanides and Actinides; Brown, D. , Ed.; Wiley: London, New York, Sydney, 1968. [Google Scholar]
- Müller, U. Symmetry Relationships between Crystal Structures: Applications of Crystallographic Group Theory in Crystal Chemistry; IUCr Texts on Crystallography; Oxford University Press: Oxford, 2013. [Google Scholar]
- Johnson N. W.. Convex Polyhedra with Regular Faces. Can. J. Math. 1966;18:169–200. doi: 10.4153/CJM-1966-021-8. [DOI] [Google Scholar]
- Lima-de-Faria J., Hellner E., Liebau F., Makovicky E., Parthé E.. Nomenclature of Inorganic Structure Types. Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr., Sect. A:Found. Crystallogr. 1990;46(1):1–11. doi: 10.1107/S0108767389008834. [DOI] [Google Scholar]
- De Sio S. M., Wilson R. E.. Structural and Spectroscopic Studies of Fluoroprotactinates. Inorg. Chem. 2014;53(3):1750–1755. doi: 10.1021/ic402877a. [DOI] [PubMed] [Google Scholar]
- Bode H., Teufer G. Ü.. ber Strukturen von Hexafluorozirkonaten Und Hexafluorohafnaten. Z. Anorg. Allg. Chem. 1956;283:18–25. doi: 10.1002/zaac.19562830105. [DOI] [Google Scholar]
- Alam, S. M. N. ; Haas, Z. J. In Coverage and Connectivity in Three-Dimensional Networks, Proceedings of the 12th Annual International Conference on Mobile Computing and Networking; ACM Press: New York, 2006; pp 346–357 10.1145/1161089. [DOI] [Google Scholar]
- Ryan R. R., Penneman R. A., Asprey L. B.. Single-Crystal X-Ray Study of b-Uranium Pentafluoride. The Eight Coordination of U(V) Acta Crystallogr., Sect.B: Struct. Crystallogr. Cryst. 1976;32:3311–3313. doi: 10.1107/s0567740876010182. [DOI] [Google Scholar]
- Scheibe B., Rudel S. S., Buchner M. R., Karttunen A. J., Kraus F.. A 1D Coordination Polymer of UF5 with HCN as a Ligand. Chem. - Eur. J. 2017;23(2):291–295. doi: 10.1002/chem.201605293. [DOI] [PubMed] [Google Scholar]
- Mulak J., Zolnierek Z.. Ground State of the U5+ Ion in CsUF6 and Its Paramagnetic Susceptibility. Bull. Acad. Polym. Sci., Ser. Sci. Chim. 1972;20:1081–1086. [Google Scholar]
- Davey W. P.. Precision Measurements of Crystals of the Alkali Halides. Phys. Rev. 1923;21(2):143–161. doi: 10.1103/PhysRev.21.143. [DOI] [Google Scholar]
- Vouillon J. C., Sebaoun A.. Le Système Binaire Eau-Fluorure de Rubidium (Domaine Riche En Sel) Bull. Soc. Chim. Fr. 1969:2604–2608. [Google Scholar]
- Malm J. G.. The Preparation and Properties of AgUF6 and AgUOF5. J. Inorg. Nucl. Chem. 1980;42(7):993–994. doi: 10.1016/0022-1902(80)80389-7. [DOI] [Google Scholar]
- Ketelaar J. A. A.. Die Kristallstruktur Des Thallofluorids. Z. Kristallogr. - Cryst. Mater. 1935;92(1–6):30–38. doi: 10.1524/zkri.1935.92.1.30. [DOI] [Google Scholar]
- Charpin P., Michel J., Rigny P.. Etude structurale de l′heptafluorouranate de nitrosyle. J. Inorg. Nucl. Chem. 1976;28:131–134. doi: 10.1016/0022-1902(76)80613-6. [DOI] [Google Scholar]
- Christe K. O., Charpin P., Soulie E., Bougon R., Fawcett J., Russell D. R.. Structure and Vibrational Spectra of Oxonium Hexafluoroarsenates(V) and -Antimonates(V) Inorg. Chem. 1984;23(23):3756–3766. doi: 10.1021/ic00191a019. [DOI] [Google Scholar]
- Hoskins B. F., Linden A., O’Donnell T. A.. Controlled Hydrolysis of the Hexafluorides of Molybdenum, Tungsten and Rhenium: Structure of Oxonium (.Mu.-Fluoro)Bis(Tetrafluorooxotungstate(VI)) Inorg. Chem. 1987;26(14):2223–2228. doi: 10.1021/ic00261a012. [DOI] [Google Scholar]
- Mootz D., Wiebcke M.. Fluorides and Fluoro Acids. 10. Crystal Structures of Acid Hydrates and Oxonium Salts. 23. Crystal Structure of the Low-Temperature Form of Oxonium Hexafluoroarsenate(V) Inorg. Chem. 1986;25(17):3095–3097. doi: 10.1021/ic00237a034. [DOI] [Google Scholar]
- Zhang D., Rettig S. J., Trotter J., Aubke F.. Superacid Anions: Crystal and Molecular Structures of Oxonium Undecafluorodiantimonate(V), [H3O][Sb2F11], Cesium Fluorosulfate, CsSO 3 F, Cesium Hydrogen Bis(Fluorosulfate), Cs[H(SO3F)2], Cesium Tetrakis(Fluorosulfato)Aurate(III), Cs[Au(SO3F)4], Cesium Hexakis(Fluorosulfato)Platinate(IV), Cs2[Pt(SO3F)6], and Cesium Hexakis(Fluorosulfato)Antimonate(V), Cs[Sb(SO3F)6 . Inorg. Chem. 1996;35(21):6113–6130. doi: 10.1021/ic960525l. [DOI] [Google Scholar]
- Mootz D., Steffen M.. Kristallstrukturen von Säurehydraten und Oxoniumsalzen. XX. Die Oxoniumtetrafluoroborate H3OBF4, [H5O2]BF4 und [H(CH3OH)2]BF4. Z. Anorg. Allg. Chem. 1981;482(11):193–200. doi: 10.1002/zaac.19814821124. [DOI] [Google Scholar]
- Graudejus O., Müller B. G.. Zur Kristallstruktur von O2+MF6- (M = Sb, Ru, Pt, Au) Z. Anorg. Allg. Chem. 1996;622(6):1076–1082. doi: 10.1002/zaac.19966220623. [DOI] [Google Scholar]
- Larson E. M., Abney K. D., Larson A. C., Eller P. G.. Structure of Oxonium Hexafluoroantimonate(V) Acta Crystallogr., Sect. B:Struct. Sci. 1991;47(2):206–209. doi: 10.1107/S0108768190011806. [DOI] [Google Scholar]
- Bode H., Voss E.. Die Kristallstruktur des Kaliumhexafluoroantimonats (V) Z. Anorg. Allg. Chem. 1951;264(2–4):144–150. doi: 10.1002/zaac.19512640208. [DOI] [Google Scholar]
- Bärnighausen H.. Group-Subgroup Relations between Space Groups: A Useful Tool in Crystal Chemistry. 1980;9:139–175. [Google Scholar]
- Davey W. P., Wick F. G.. Minutes of the Annual Meeting, Chicago, December 28–30, 1920. Phys. Rev. 1921;17(3):403–404. doi: 10.1103/PhysRev.17.367. [DOI] [Google Scholar]
- International Tables for Crystallography: Symmetry Relations between Space Groups, 2nd ed.; Wondratschek, H. ; Müller, U. , Eds.; International Union of Crystallography: Chester, England, 2011; Vol. A1 10.1107/97809553602060000110. [DOI] [Google Scholar]
- Kroumova E., Perez-Mato J. M., Aroyo M. I.. WYCKSPLIT: A Computer Program for Determination of the Relations of Wyckoff Positions for a Group-Subgroup Pair. J. Appl. Crystallogr. 1998;31(4):646. doi: 10.1107/S0021889898005524. [DOI] [Google Scholar]
- Allmann, R. Röntgenpulverdiffraktometrie: Rechnergestützte Auswertung, Phasenanalyse und Strukturbestimmung, 2., korrgierte und erw. Aufl.; Springer: Berlin, 2003. [Google Scholar]
- Charpin P.. Structure Cristalline Des Hexafluorures Complexes d’uraniurn V et d’argent, de Potassium, d’ammonium, de Rubidium Ou de Thallium. C. R. Hebd. Seances Acad. Sci. 1965;260:1914–1916. [Google Scholar]
- Musil, F. J. ; Ogle, P. R. ; Beu, K. E. . Powder X-Ray Diffraction and Related Data on NOUF6 and NOMoF6, GAT-T-553, Technical Report GAT-T-553; Goodyear Atomic Corp., 1958. [Google Scholar]
- Röhr C., Kniep R.. Die Kristallstrukturen von Li[PF6] Und Li[AsF6]: Zur Kristallchemie von Verbindungen A[EVF6]/The Crystal Structures of Li[PF6] and Li[AsF6]: On the Crystal Chemistry of Compounds A[EVF6] Z. Naturforsch., B: J. Chem. Sci. 1994;49(5):650–654. doi: 10.1515/znb-1994-0514. [DOI] [Google Scholar]
- Mazej Z., Hagiwara R.. Hexafluoro-, Heptafluoro-, and Octafluoro-Salts, and [MnF5n+1]– (N = 2, 3, 4) Polyfluorometallates of Singly Charged Metal Cations, Li+–Cs+, Cu+, Ag+, In+ and Tl+ J. Fluorine Chem. 2007;128(4):423–437. doi: 10.1016/j.jfluchem.2006.10.007. [DOI] [Google Scholar]
- Sowa H.. Pressure-Induced Fm-3m R-3 Phase Transition in NaSbF6. Acta Crystallogr., Sect. B: Struct. Sci. 1997;53(1):25–31. doi: 10.1107/S010876819601066X. [DOI] [Google Scholar]
- Sowa H., Ahsbahs H.. Single-Crystal Study of the Pressure-Induced NaCl to CsCl Type Phase Transition in KPF6 and the Crystal Structure of the High-Pressure Phase. Z. Kristallogr. - Cryst. Mater. 1999;214(11):751–757. doi: 10.1524/zkri.1999.214.11.751. [DOI] [Google Scholar]
- Burns J. H.. The Crystal Structure of Lithium Fluoroantimonate(V) Acta Crystallogr. 1962;15(11):1098–1101. doi: 10.1107/S0365110X62002935. [DOI] [Google Scholar]
- Bragg W. L.. The Analysis of Crystals by the X-Ray Spectrometer. Proc. R. Soc. London, Ser. A. 1914;89(613):468–489. doi: 10.1098/rspa.1914.0015. [DOI] [Google Scholar]
- Propach V., Steffens F.. Über Die Strukturen Der CuZrF6-Modifikationen - Neutronenbeugungsuntersuchungen an Den Kristallpulvern/A Neutron Diffraction Study on the Modifications of CuZrF6. Z. Naturforsch., B: Anorg. Chem., Org. Chem. 1978;33(3):268–274. doi: 10.1515/znb-1978-0304. [DOI] [Google Scholar]
- Biswal M., Body M., Legein C., Corbel G., Sadoc A., Boucher F.. Structural Investigation of α- and β-Sodium Hexafluoroarsenate, NaAsF6, by Variable Temperature X-Ray Powder Diffraction and Multinuclear Solid-State NMR, and DFT Calculations. J. Phys. Chem. C. 2012;116(21):11682–11693. doi: 10.1021/jp3040727. [DOI] [Google Scholar]
- Yang C., Qu B. Y., Pan S. S., Zhang L., Zhang R. R., Tong P., Xiao R. C., Lin J. C., Guo X. G., Zhang K., Tong H. Y., Lu W. J., Wu Y., Lin S., Song W. H., Sun Y. P.. Large Positive Thermal Expansion and Small Band Gap in Double-ReO3-Type Compound NaSbF6. Inorg. Chem. 2017;56(9):4990–4995. doi: 10.1021/acs.inorgchem.7b00002. [DOI] [PubMed] [Google Scholar]
- Asprey L. B., Keenan T. K., Penneman R. A., Sturgeon G. D.. Alkali Fluoride Complexes of Pentavalent Neptunium. Inorg. Nucl. Chem. Lett. 1966;2(1):19–21. doi: 10.1016/0020-1650(66)80084-3. [DOI] [Google Scholar]
- Morss, L. R. ; Williams, C. W. ; Carnall, W. T. . Stability and Electronic Spectrum of Cesium Plutonium Hexafluoride. In Plutonium Chemistry, ACS Symposium Series; American Chemical Society; 1983; Vol. 216, pp 199–210 10.1021/bk-1983-0216.ch013. [DOI] [Google Scholar]
- Gafner G., Kruger G. J.. Potassium Arsenic Hexafluorine: A Redetermination. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1974;30(1):250–251. doi: 10.1107/S0567740874002597. [DOI] [Google Scholar]
- Pauling L., Weinbaum S.. Kürzere Originalmitteilungen Und Notizen. Z. Kristallogr. - Cryst. Mater. 1934;87:181–182. doi: 10.1524/zkri.1934.87.1.181. [DOI] [Google Scholar]
- Hoard J. L., Vincent W. B.. Structures of Complex Fluorides. 1 Barium Fluosilicate and Barium Fluogermanate. J. Am. Chem. Soc. 1940;62(11):3126–3129. doi: 10.1021/ja01868a060. [DOI] [Google Scholar]
- Advances in Chemistry. In Lanthanide/Actinide Chemistry; Fields, P. R. ; Moeller, T. , Eds.; American Chemical Society: Washington D.C., 1967; Vol. 71. [Google Scholar]
- Brauer, G. Handbuch der Präparativen Anorganischen Chemie in drei Bänden; Baudler, M. ; Brauer, G. ; Fehér, F. ; Huber, F. ; Klement, R. ; Kwasnik, W. ; Schenk, P. W. ; Schmeisser, M. ; Steudel, R. , Eds.; Ferdinand Enke Verlag: Stuttgart, 1975. [Google Scholar]
- Halstead G. W., Eller P. G., Asprey L. B., Salazar K. V.. Convenient Multigram Syntheses of Uranium Pentafluoride and Uranium Pentaethoxide. Inorg. Chem. 1978;17(10):2967–2969. doi: 10.1021/ic50188a059. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.