Abstract
Xyloglucan is a key polymer in the walls of growing plant cells. Using split pea stem segments and stem segments from which the epidermis had been peeled off, we demonstrate that the integration of xyloglucan mediated by the action of wall-bound xyloglucan endotransglycosylase suppressed cell elongation, whereas that of its fragment oligosaccharide accelerated it. Whole xyloglucan was incorporated into the cell wall and induced the rearrangement of cortical microtubules from transverse to longitudinal; in contrast, the oligosaccharide solubilized xyloglucan from the cell wall and maintained the microtubules in a transverse orientation. This paper proposes that xyloglucan metabolism controls the elongation of plant cells.
Xyloglucan, which occurs widely in the primary walls of higher plants, possesses a 1,4-β-glucan backbone with 1,6-α-xylosyl residues along the backbone. Because the 1,4-β-glucan backbone can bind specifically to cellulose microfibrils by hydrogen bonds (1), the xyloglucan probably contributes to the rigidity of the cell wall by cross-linking adjacent microfibrils (2). In fact, microfibrils seem to be coated with xyloglucan, which is located both on and between microfibrils throughout cell elongation (3). Masking xyloglucans in cell walls should prevent xyloglucan metabolism; in agreement with this prediction, an antibody specific to xyloglucan prevented an auxin-induced decrease in molecular size of xyloglucan and inhibited indole-3-acetic acid (IAA)-induced cell elongation in azuki hypocotyl segments (4). Xyloglucan endotransglycosylase (XET) has been proposed to participate in the dynamic changes of xyloglucan cross-linking (5, 6), but there is little direct evidence for this hypothesis. The question at issue concerns the structural function of xyloglucan, namely whether xyloglucan contributes to the extensibility of the wall by cross-linking adjacent microfibrils.
Fucose-containing xyloglucan oligosaccharides have been shown to inhibit auxin-induced elongation of pea stems (7, 8). Their inhibitory activity is approximately maximal at a concentration of 10−8 to 10−9 M. In the absence of auxin, a high concentration (>10−8 M) of xyloglucan oligosaccharide did not inhibit but slightly promoted cell elongation in pea stem segments (9). Thus, the oligosaccharides may provide either negative or positive feedback control during cell elongation. However, the positive feedback reaction has not been reproducibly observed in pea stems (T.T. and T.H., unpublished results) and also was not observed in maize primary roots (10). In the present communication, we examine whether xyloglucan oligosaccharides control the elongation growth of plant cells.
In cylindrical plant organs such as roots or stems, cells expand anisotropically, with the direction of most rapid growth parallel to the long axis of the organ, leading to elongation of the organ. It has been known for many years that the direction of elongation is perpendicular to the direction of net orientation of cellulose microfibrils (11). Therefore, parallel microfibrils must be separated during elongation. Because xyloglucans are thought to cross-link adjacent microfibrils, the separation of microfibrils during elongation has been thought to involve enzymes that cleave xyloglucan or loosen its binding to microfibrils (12, 13). On the other hand, xyloglucan and other wall polymers are constantly being secreted and incorporated into the wall. This action may slow down the elongation rate and even stop growth entirely. For this reason, xyloglucan metabolism should be precisely balanced between incorporation and breakdown.
This paper answers questions about the primary wall, whose composition and architecture controls plant cell growth. We found that xyloglucans are integrated into the wall, where the incorporation of xyloglucan or its fragment oligosaccharide results in opposite effects on cell growth. In addition, their integration affects not only the extracellular events in the wall but also intracellular events, which are related to microtubule reorientation. The significance of xyloglucan integration is in controlling cell growth.
Materials and Methods
Materials.
Seeds of Pisum sativum L. cv Alaska were imbibed in water overnight, planted in moistened vermiculite, and grown in darkness at 27°C for 1 wk. Third internodes, 2 cm in length, were selected. In some experiments, their cuticle was abraded with a carborundum slurry by rubbing the surface between thumb and forefinger. Segments of 10 mm and 15 mm were excised from the internodes, beginning 5 mm below the hook. The 10-mm segments were partially bisected longitudinally and split into halves (split stem segments). From the 15-mm segments, epidermal tissues were peeled off, and the remaining inner tissue segments were cut into 10-mm pieces (peeled stem segments). After a 20-min incubation in water, the split or the peeled stem segments were incubated in 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-dichlorophenoxyacetic acid (2,4-D) in the presence of whole xyloglucan or xyloglucan oligosaccharides. The lengths of surface-abraded and peeled stem segments were directly measured by using a microscope fitted with an eyepiece micrometer.
To prepare pea xyloglucan, we used the expanded tissues from epicotyls previously sprayed with 2.5 mM 2,4-D, because this tissue yielded a homogenous xyloglucan fraction with a mean molecular mass of 50 kDa (14). The epicotyl tissue (5 kg) was sequentially extracted three times with 0.1 M EDTA (pH 7.0) at 85°C for 3 h, three times with 4% KOH/0.1% NaBH4 at 35°C for 3 h, and three times with 24% KOH/0.1% NaBH4 at 35°C for 3 h. Xyloglucan was purified from the 24% KOH extract. The extract was neutralized with acetic acid and dialyzed against distilled water for 3 days. The sample was treated with salivary α-amylase for 12 h at 40°C and with proteinase K (50 mg) for 24 h at 40°C, and dialyzed against distilled water. After dialysis and freeze-drying, the sample was dissolved in 1 M CaCl2 solution and precipitated with 3% I2 in 4% KI solution. After 2 h at 4°C, the precipitate was collected by centrifugation and dissolved in hot water, and the mixture was treated with aqueous Na2S2O3. The resulting solution was dialyzed against distilled water and passed through a column (5.0 × 10 cm) of DEAE-Sephadex A-25 (Amersham Biosciences; phosphate type) to remove acidic contaminants, and the sample was again dialyzed against distilled water and freeze-dried (yield, 10.0 g). For the preparation of xyloglucan fragment oligosaccharides, pea xyloglucan prepared as above or amyloid xyloglucan (from Tamarindus indica) provided from Dainippon Pharmaceutical (Osaka, Japan) was hydrolyzed with Streptomyces endoglucanase, and the digest was fractionated on a Bio-Gel P-4 column (3 × 120 cm). The xyloglucan oligosaccharides XXXG and XXXF were obtained from the digest of pea xyloglucan, and XLLG and XXLG from that of amyloid xyloglucan.
Preparation of an Antibody Against Pea XET.
Recombinant pea xyloglucan endotransglycosylase was expressed in Escherichia coli cells harboring the pET-15b expression vector fused with full-length PsEXT cDNA (GenBank accession no. AB042531). The recombinant protein was purified by using a His-bound resin and SDS/PAGE and injected with Freund's adjuvant into a rabbit. The antiserum was precipitated with ammonium sulfate at 20 to 50% saturation. In the experiment with split stem segments, the antibody was dialyzed against 2 mM sodium phosphate buffer (pH 6.2) just before incubation.
Determination of Bound and Solubilized Xyloglucan.
For the determination of xyloglucan bound, ten peeled stem segments (10 mm) were incubated for 40 min in 3.5 ml of 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-D plus xyloglucan. The amount of xyloglucan remaining in the incubation mixture was determined by the iodine-sodium sulfate method (15). Bound xyloglucan was calculated from the reduction of xyloglucan in the incubation mixture after the segments were added. For the determination of solubilized xyloglucan, ten peeled stem segments (10 mm) were incubated for 40 min in 3.5 ml of 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-D plus XXXG. Xyloglucan in the incubation mixture was measured by the iodine-sodium sulfate method and regarded as solubilized xyloglucan (15).
Xyloglucan Derivatives.
To prepare fluorescent xyloglucan (16), we dissolved 10 mg of CNBr and 20 mg of pea xyloglucan in 1 ml of water adjusted to pH 11.0 by addition of NaOH. The activated polysaccharide was incubated with 4 mg fluoresceinamine overnight at room temperature. The fluorescein-labeled xyloglucan was purified by gel filtration on Sephadex G-50 (Amersham Biosciences). Calculations showed that 1 μmol of fluorescein was incorporated into 150 μmol of sugar residues, which corresponds to a substitution of 2.7 mol of fluorescein per mol of xyloglucan (xyloglucan molecular weight = 50,000).
To prepare fluorescent XXXG (17), lissamine rhodamine sulfonyl chloride was conjugated to the aminodeoxyglucitol end of reductively aminated XXXG. The reaction products were subjected to gel filtration on Bio-Gel P-2, and the fluorescent carbohydrate fraction was further purified by paper chromatography by using Whatman 3MM paper with 1-propanol/ethyl acetate/water, 3:2:1 (vol/vol), and eluted with water from the excised paper.
Pretreatment of Stem Segments.
For the pretreatment with antibody, the split stem segments were incubated for 30 min in 5 mM PBS containing the antiserum and washed three times with PBS. They were again incubated with polyclonal goat anti-rabbit IgG for 30 min and washed three times with PBS. After the pretreatment, the segments were incubated with fluorescent xyloglucan. For pretreatment with taxol or 6-dimethylaminopurine, apical 15-mm stem segments were incubated in 10 μM taxol or 1 mM 6-dimethylaminopurine. After incubation for 40 min, the epidermal layers were peeled off the segments, and the peeled stem segments were cut into 10-mm lengths. The 10-mm peeled stem segments were further preincubated in the solution for 20 min and incubated in xyloglucan or XXXG in the presence of 10 μM taxol or 1 mM 6-dimethylaminopurine.
Determination of Mechanical Properties of Pea Stems.
The effects of xyloglucan or XXXG on the dynamic elastic modulus (E′), loss modulus (E"), and elongation rate of peeled stem segments were examined by the tensile forced oscillation method (18) using an automatic dynamic viscoelastometer TMA/SS6100 (Seiko Instruments, Tokyo). The elastic modulus is defined as the stress/strain ratio at constant deformation. The loss elastic modulus is defined as the stress 90o out of phase with the strain divided by the strain. It is a measure of the energy dissipated or loss as heat during sinusoidal deformation. These experiments were carried out in 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-D and either 0.2 mM xyloglucan or 9 mM XXXG at 23°C after preincubation in buffer containing 5 μM 2,4-D for 20 min. The experimental conditions were 0.05 Hz frequency and 5 mm span, and the static tensile force was 4 ± 3 g amplitude strain. The tensile force was loaded in the longitudinal direction of peeled stem segments.
Microscopic Observations.
To examine split stem segments containing fluorescent derivatives, the segments were incubated in 300 μl of 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-D plus 0.2 mM fluorescent xyloglucan or 9 mM fluorescent XXXG in the dark at 23°C with shaking. After 6 h, the segments were washed three times with water, and 100- to 150-μm sections were cut in the transverse direction by using a microtome. The sections were again washed twice with water and examined under a Zeiss Axioskop microscope equipped with epifluorescence illumination. The fluorescence micrographs were taken under the same condition in dim light to trace the shape of the sections.
To examine the surfaces of peeled stem segments with high-resolutional scanning electron microscopy, samples were prepared by the following method. For the plunge freezing method, the segment was washed with water for 3 h at room temperature, rapidly frozen in liquid propane (−185°C), and freeze-dried. The dried segment was coated with ≈1 nm thick platinum/carbon by rotary shadowing at an angle of 60° by using a freeze etching apparatus (JEOL JFD-9010). Samples were examined under a Hitachi (Tokyo) field emission scanning electron microscope FE-SEM (Hitachi S-4500) at an accelerating voltage of 1.5 kV.
To examine the orientation of cortical microtubules, peeled stem segments were fixed in buffer (5% formaldehyde/5 mM EGTA/8% Nonidet P-40 in 20 mM phosphate buffer, pH 6.8) at room temperature for 2 h. The segments were then frozen in 20 mM PBS on a freezing stage, and 100- to 150-μm sections were cut in the longitudinal direction by using a microtome. The sections were treated with monoclonal mouse anti-chicken tubulin (Sigma) diluted 1:500 in PBS for 1 hr, rinsed with PBS, and treated with FITC-conjugated anti-mouse Ig (Sigma) diluted 1:50 in PBS for 1 h. After washing with PBS buffer, the orientation of the cortical microtubules of a sliced section was examined under an Olympus (New Hyde Park, NY) laser confocal microscope. To measure whether microtubule orientation is transverse, inclined, or longitudinal, we used about 30 micrographs of cortical microtubules, including the outermost first to fifth cell layers for each analysis.
Results
Cell Elongation.
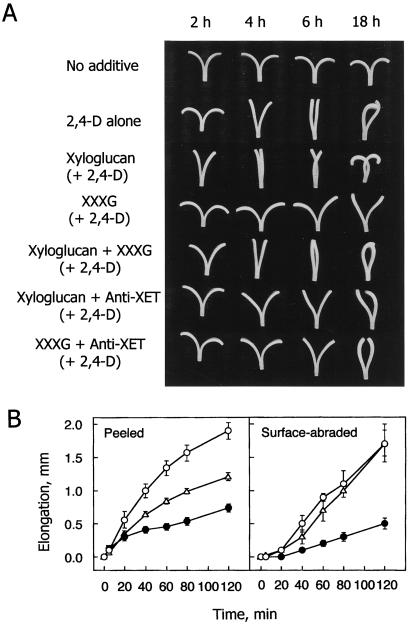
Pea stem segments partially bisected longitudinally, i.e., split stem segments (19), were incubated in 2 mM Mes/KOH (pH 6.2) buffer containing 5 μM 2,4-D plus or minus whole xyloglucan (0.2 mM) or a xyloglucan oligosaccharide, 9 mM XXXG (Glc/Xyl = 4:3; ref. 20). With no addition, splitting the section caused the halves to rapidly bow outward forming a “V” shape (Fig. 1A). During subsequent incubation, the arms of the “V” separated further as the inner tissue elongated relatively more than did the outer tissue. When split segments were incubated in 2,4-D, greater growth of the epidermis compared with the inner tissue caused the halves of the segments to grow together, straightening the segments by 6 hr and reversing the bend with long incubation. When segments were incubated in 2,4-D plus whole xyloglucan, the auxin-mediated straightening was enhanced, whereas incubating in oligosaccharide blocked the effect of auxin. These effects of xyloglucans can be interpreted by hypothesizing that the whole polymer stiffened the inner tissue whereas the oligosaccharide made that tissue more extensive. The response of the segments to whole xyloglucan + XXXG + 2,4-D was intermediate between whole xyloglucan + 2,4-D and XXXG + 2,4-D, indicating that the action of whole xyloglucan might be competitive with that of XXXG. A straightforward prediction of the hypothesis is that the xyloglucans were incorporated into the cell wall by virtue of XET activity. Consistent with this result, an antibody against pea XET suppressed to some extent the effects of either whole xyloglucan or oligosaccharide (Fig. 1A). The antibody against pea XET was found to bind to both wall-bound and intracellular XETs to give a single 32-kDa signal by Western blot analysis. Furthermore, in crude preparations from pea stems, XET activity could be inhibited by the antibody in a dose-dependent way, up to a maximal inhibition of 70%. It should be noted that preimmune serum affected neither XET activity nor the suppression and acceleration of the cell elongation in the inner tissue in the presence of xyloglucan or XXXG (results not shown).
Figure 1.
Effects of xyloglucan and its fragment oligosaccharide (XXXG) on cell elongation. (A) Time course for the elongation of split stem segments. (B) Time course for the elongation of peeled and outer-surface-abraded segments. Filled circles, 0.2 mM whole xyloglucan plus 5 μM 2,4-D; open circles, 9 mM XXXG plus 5 μM 2,4-D; triangles, 5 μM 2,4-D alone. Three independent experiments were performed for the determination of stem length with five stem segments each. Error bars indicate standard deviations.
Consistent with the observations on split stems, the elongation of stem segments with the epidermal tissue peeled off (called peeled stem segments hereafter) was suppressed by the addition of 0.2 mM whole xyloglucan but accelerated by the addition of 9 mM XXXG (Fig. 1B). Likewise, elongation of abraded stem segments with epidermal cells remaining was also suppressed by the addition of xyloglucan, although the promotion of elongation by oligosaccharide was minimal (Fig. 1B). The elongation of peeled segments was accelerated by a hydrolysate of whole xyloglucan produced by a pea xyloglucanase (ref. 21; data not shown) presumably because of the presence of xyloglucan oligosaccharides.
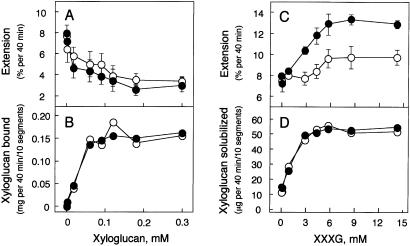
To determine whether the exogenous xyloglucans in these experiments were substrates for XET, we used peeled stem segments and assayed either the amount of whole xyloglucan bound to the segments or the amount of xyloglucan solubilized. The amount of xyloglucan bound to the segments increased with the concentration of xyloglucan. Similarly, the amount of xyloglucan solubilized increased with the concentration of XXXG (Fig. 2). Elongation in the presence of 2,4-D was suppressed by whole xyloglucan with an in situ Km of 25 μM, and accelerated by XXXG with a in situ Km of 2.7 mM. Nevertheless, on a mass basis, the Km values were nearly the same (1.25 mg/ml for whole xyloglucan and 2.87 mg/ml for XXXG). The results show that the exogenous whole xyloglucan bound to peeled stem segments, whereas the exogenous XXXG caused the solubilization of endogenous xyloglucan from the walls of peeled stem segments. The presence of 2,4-D did not affect the amount of either exogenous whole xyloglucan bound or endogenous xyloglucan solubilized, but caused a slight increase in the suppression of extension by whole xyloglucan and a marked increase in the acceleration of extension with XXXG. A synergistic action appeared to occur between XXXG and auxin during cell elongation.
Figure 2.
Effects of various concentrations of xyloglucan and its fragment oligosaccharide (XXXG) on the elongation of peeled stem segments. The level of extension is shown in the presence of whole xyloglucan (A), of XXXG (C), the amount of exogenous whole xyloglucan bound (B), and endogenous xyloglucan solubilized (D). Filled circles, presence of 5 μM 2,4-D; open circles, without auxin. Error bars indicate standard deviations.
Mechanical Properties of Stem Segments.
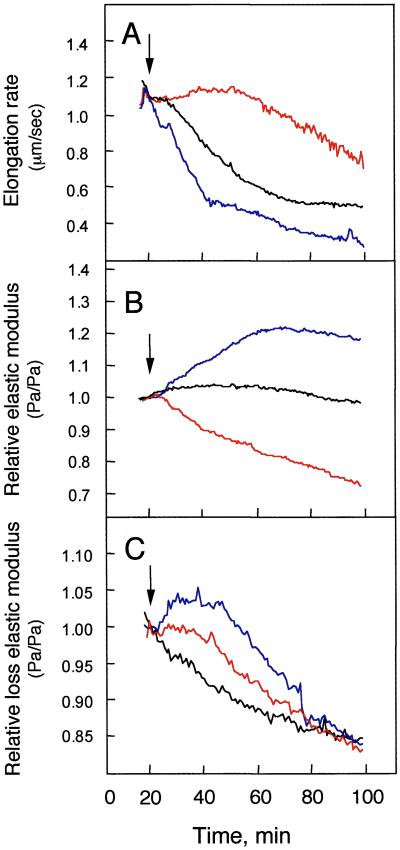
The effect of either whole xyloglucan or the oligosaccharide on the mechanical properties of the cell wall was tested by using peeled stem segments (Fig. 3). The stem segments were stabilized for 20 min in 2 mM Mes/KOH buffer (pH 6.2) containing 5 μM 2,4-D and then placed in buffer containing 5 μM 2,4-D plus either 0.2 mM whole xyloglucan or 9 mM XXXG for 80 min. Because conditions for making these measurements differed from those used above, we measured the elongation rate of the segments, confirming the previous results (Fig. 3A). Compared with auxin alone, whole xyloglucan increased the elastic modulus (E′) of the segment and the oligosaccharide decreased the modulus (Fig. 3B). This result is consistent with the effects of the xyloglucans on elongation documented above. On the other hand, the loss elastic modulus (E") had a wide peak at 37 min in the presence of whole xyloglucan, but a tailing peak at 28 min in the presence of XXXG (Fig. 3C). The results showed that the polysaccharide and its oligosaccharide gave rigidity and loosening properties, respectively, because of irreversible wall plasticity with different time peaks during incubation.
Figure 3.
Effects of xyloglucan and its fragment oligosaccharide (XXXG) on mechanical properties of peeled stem segments. (A) Elongation rate; (B) relative elastic modulus; (C) relative loss elastic modulus. Arrows indicate the time of addition of either 0.2 mM whole xyloglucan (blue) or 9 mM XXXG (red) or none (black) to the incubation mixture containing 5 μM 2,4-D. The patterns (A–C) of mechanical properties were all of the same in five independent peeled stem segments.
Integration of Xyloglucan and Its Fragment Oligosaccharide into Cell Walls.
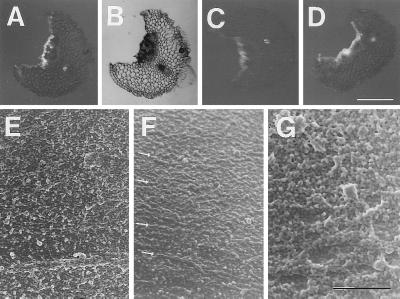
When split stem segments were incubated with 0.2 mM fluorescent whole xyloglucan in the presence of 5 μM 2,4-D, fluorescence remained at the cut surface of the segments even after the segments had been washed with water (Fig. 4 A and B). Fluorescence was predominantly at the surface of the cut cells, being strongest at cut regions within the stele. Additionally, some vascular bundles interior to the cut were also stained. The incorporation of the fluorescence (92%) was nearly eliminated in segments pretreated with an antibody against pea XET, but the staining pattern was not changed (Fig. 4C). It should be noted that preimmune serum did not affect the incorporation into inner tissue in the presence of whole xyloglucan (data not shown). Fluorescence was also incorporated into the cut surface incubated with fluorescent XXXG in an apparently identical pattern as observed with the whole xyloglucan (Fig. 4D).
Figure 4.
Integration of xyloglucan and its fragment oligosaccharide (XXXG) at the surfaces of split and peeled stem segments. (A) Thin sections of a split stem segment after incubation with fluorescent whole xyloglucan plus 2,4-D for 6 h. (B) The same by light microscopy. (C) Thin sections of split stem segments after the treatment of the segments with an antibody against pea XET followed by incubation with fluorescent whole xyloglucan plus 2,4-D for 6 h. (D) Thin sections of split stem segments after incubation with fluorescent XXXG plus 2,4-D for 6 h. Bar = 0.5 mm (A–D). (E) Rapidly frozen surfaces of peeled stem segments after incubation with 2,4-D alone. (F) Rapidly frozen surfaces of peeled stem segments after incubation with XXXG and 2,4-D. The arrows indicate microfibrillar textures. (G) Rapidly frozen surfaces of peeled stem segments after incubation with whole xyloglucan and 2,4-D. Bar = 1 μm (E–G). The micrographs (E–G) show transverse sections perpendicular to the direction of elongation.
The surface of the cell wall in the exposed cortical parenchyma of the peeled segments was examined at high resolution with field-emission scanning electron microscopy. In segments treated with 5 μM 2,4-D alone, followed by rapid freezing, amorphous material was observed overlaying a microfibrillar texture (Fig. 4E). In contrast, in segments treated with the oligosaccharide, less of this amorphous material was seen, and the microfibrils were more visible (arrows in Fig. 4F); whereas, in segments treated with the polymer, the amorphous material was ubiquitous and thoroughly obscured the microfibrils (Fig. 4G). These results are in agreement with the findings (Fig. 2) that exogenous whole xyloglucan binds to peeled stem segments, whereas exogenous XXXG solubilizes endogenous xyloglucan from the walls of such segments.
Microtubule Arrangement.
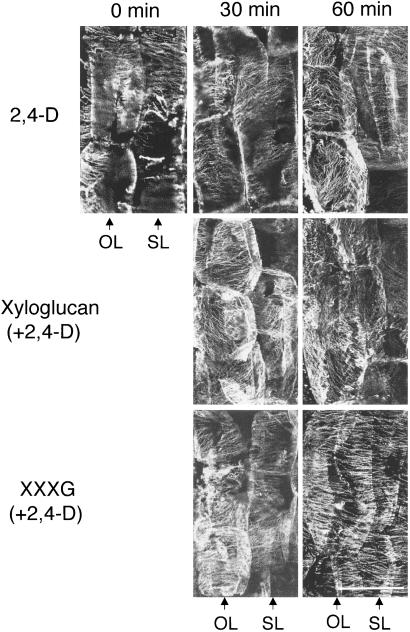
The arrangement of cortical microtubules was analyzed in the cortical parenchyma of peeled stem segments. At 1 hr after the addition of 5 μM 2,4-D, obliquely and longitudinal microtubules were found in 50% of the outermost cell layer of parenchyma, whereas essentially 100% of cells in the second layer had transverse microtubules (Fig. 5). After the addition of 0.2 mM whole xyloglucan plus 5 μM 2,4-D, oblique and longitudinal microtubules were formed within 30 min in 75% of the outermost layer of cells and, by 1 hr, were found in nearly all cells in both layers. In contrast, the addition of 9 mM XXXG plus 5 μM 2,4-D maintained transverse microtubules in all layers of cells for at least 60 min. In the control (2,4-D alone), the reorientation of microtubules followed the decreasing elongation rate (Fig. 3), which shows that reorienting microtubules did not cause the elongation rate to decline. In segments treated with whole xyloglucan, microtubule reorientation occurred faster than in the control. The elongation rate declined faster than in control also, but the time course suggested that the decline in the elongation rate still preceded the reorientation of microfibrils. In the segments treated with the oligosaccharide, the orientation of microtubules and the decline in the elongation were both delayed for 1 h, again suggesting that the reorientation follows and does not govern the prevailing elongation rate.
Figure 5.
Effects of xyloglucan and its fragment oligosaccharide (XXXG) on the arrangement of cortical microtubules. The left side of the photo shows surface parenchyma (the outermost first layer cells) of peeled stem segments. Arrows: OL, outermost cell layer; SL, second cell layer. Bar = 20 μm.
When the cortical microtubules of peeled stem segments were fixed in transverse orientation by pretreatment for 20 min with taxol, the microtubules were not affected by the addition of either whole xyloglucan or XXXG (data not shown). Nevertheless, elongation of segments in the presence of taxol was suppressed by whole xyloglucan and accelerated by XXXG (Table 1). Similarly, when the cortical microtubules were fixed in a longitudinal orientation by pretreatment for 20 min with the kinase inhibitor, 1 mM 6-dimethylaminopurine (22), microtubules were not further affected by treatment with either type of xyloglucan (data not shown). Nevertheless, elongation of segments in the presence of dimethylaminopurine was suppressed by the whole polymer and accelerated by the oligosaccharide (Table 1). These findings confirm the argument made above on the basis of time courses that microtubule reorientation follows (or accompanies) but does not cause changes in elongation rate. Interestingly, growth stimulation caused by XXXG was enhanced in the presence of taxol, and growth suppression caused by the whole xyloglucan was more severe in the presence of dimethylaminopurine, findings that indicate that microtubule orientation may enhance in the regulation of elongation.
Table 1.
Effects of taxol and 6-dimethylaminopurine on the elongation of peeled stem segments
| Treatment | Elongation, mm
|
||
|---|---|---|---|
| 30 min | 60 min | 120 min | |
| No pretreatment, +5 μM 2,4-D | 0.30 ± 0.05 | 0.50 ± 0.10 | 0.97 ± 0.23 |
| 10 μM Taxol (+5 μM 2,4-D) | |||
| Control | 0.20 ± 0.05 | 0.30 ± 0.13 | 0.77 ± 0.35 |
| + Xyloglucan | 0.17 ± 0.09 | 0.23 ± 0.11 | 0.43 ± 0.08 |
| + XXXG | 0.60 ± 0.13 | 1.07 ± 0.29 | 1.43 ± 0.31 |
| 1 mM 6-dimethylaminopurine (+ 5 μM 2,4-D) | |||
| Control | 0.10 ± 0.07 | 0.17 ± 0.11 | 0.26 ± 0.15 |
| + Xyloglucan | <0.10 | <0.10 | <0.10 |
| + XXXG | 0.43 ± 0.16 | 0.78 ± 0.24 | 1.00 ± 0.26 |
Each value was the mean of two experimental replicates measured in six stem segments ± SD.
Discussion
Integration of Xyloglucans.
Here, we have demonstrated the suppression and acceleration of elongation by the integrations of whole xyloglucan and xyloglucan oligosaccharide, respectively. Plant cell growth may thus be controlled by the molecular size of free xyloglucans and the way they are integrated. The first integration could occur at the surface of the plasma membrane, where xyloglucan secreted via secretory vesicles is likely to encounter nascent elementary cellulose fibrils. The xyloglucans could attach to the elementary fibrils by hydrogen bonds and become bundled up with the glucose chains to form a microfibril (2, 23). For any xyloglucan molecule thus intercalated into a microfibril, its terminus would be anchored inside the microfibril. The second integration would occur as xyloglucans are secreted into the cell wall and become transglycosylated by XET, which is in the apoplastic space bound to xyloglucan (24, 25). Although carboxymethylcellulose binds to cellulose by hydrogen bonding (26, 3), the cellulose derivative did not affect the mechanical properties of peeled stem segments (data not shown). This result confirms that the changes in the mechanical properties by xyloglucan are due to endotransglycosylation between exogenous xyloglucan and anchored xyloglucan rather than to hydrogen bonding between exogenous xyloglucan and cellulose microfibrils. The mechanical responses obtained could be due to cell wall modifications, but they also could be mediated by responses of the cell.
Integration would allow random insertion of newly synthesized xyloglucans into wall xyloglucan to form chimeric molecules. This integration is in agreement with the finding in cells of Rosa that XET-catalyzed interpolymeric transglycosylation integrates newly synthesized [13C]xyloglucan into the cell wall xyloglucan (27, 28). Large amounts of XET may exist in cell walls; XET constitutes 1.2% of total apoplastic proteins in Azuki hypocotyls (6). Thus, the availability of donor and/or acceptor substrates probably determines the suppression and acceleration of cell elongation. McQueen-Mason et al. (29) reported that exogenous XET activity alone is neither sufficient nor necessary for extension of heat-inactivated walls of cucumber hypocotyls; this finding may be because combining XET and xyloglucan oligosaccharide is required to induce extension.
Potential Endotransglycosylation Between Cellulose Microfibrils.
The transferase-type of XET in the primary wall has been proposed to form an enzyme-acceptor complex as well as an enzyme-donor complex at the onset of the cleavage of the glucan backbone (30, 31). When whole xyloglucan was added to segments, XET bound to the non-reducing end of xyloglucan, forming an enzyme-acceptor complex in the cell walls. This complex could catch the internal region of exogenous whole xyloglucan, cleave it, and transfer this newly generated reducing end to the non-reducing end of the endogenous one. Thus, the xyloglucan in the wall becomes a larger molecule during endotransglycosylation. In accord with this model, exogenous whole xyloglucan was incorporated into the cell walls of the peeled stem segment (Fig. 2). Because most of the whole xyloglucan serves as a donor rather than acceptor, the other end (non-reducing end) of the exogenous xyloglucan might become entangled in the cell wall matrix or, alternatively, might become further linked to endogenous xyloglucan after binding another XET enzyme-donor complex. It is possible that the above two endotransglycosylase reactions occur at both non-reducing and reducing ends of exogenous xyloglucan to form tethers between cellulose microfibrils. Nevertheless, cell elongation was not accelerated but suppressed by the presence of exogenous whole xyloglucans, probably because the exogenous whole xyloglucan seldom serves as an acceptor because of the limited number of its non-reducing ends to cleave a xyloglucan tether.
When XXXG was added to segments, XET bound to the internal region of xyloglucan (forming an enzyme-donor complex) in the cell walls could cleave the xyloglucan and transfer the newly generated reducing end to the non-reducing end of XXXG. If endogenous xyloglucan exists as a tether between cellulose microfibrils, the reaction with XXXG could result in the cleavage of the tether. When the endogenous xyloglucans are endotransglycosylated many times with XXXG, their molecular sizes in the wall would be decreased. In fact, exogenous XXXG caused the solubilization of some endogenous xyloglucan in the cell walls of peeled stem segments (Fig. 2). However, if XET exists bound to the non-reducing end of xyloglucan in the wall, this enzyme-acceptor complex could not form an enzyme-acceptor-donor complex with XXXG because XXXG is not recognized as a donor. It should be noted that the endotransglycosylation of xyloglucan occurs in the 1,4-linked glucose backbone at every fourth glucose unit. However, in addition to XXXG, other xyloglucan oligosaccharides stimulated elongation comparably in the split and peeled segments, including XLLG (Glc/Xyl/Gal = 4:3:2), XXLG (Glc/Xyl/Gal = 4:3:1), and XXFG (Glc/Xyl/Gal/Fuc = 4:3:1:1) (data not shown).
Xyloglucan Fragments as Active Oligosaccharides.
The acceleration of cell elongation by the xyloglucan oligosaccharide may be involved in acid growth (32). Because the oligosaccharide-induced elongation of peeled stem segments occurred rapidly, endotransglycosylation and expansin action may occur synergistically in the primary wall as proposed for tomato fruit ripening (33). Synergy was also observed between auxin and XXXG, although XXXG accelerates cell elongation even in the absence of auxin. The effect of XXXG on cell elongation was clearly observed in peeled stem segments (Figs. 1B, 2C, and 3B), and, although similar conclusions were reached in two reports (9, 34), in one other, a stimulation of elongation by xyloglucan oligosaccharides in the absence of auxin was not observed (10). The lack of reproducibility in those reports is probably due to the presence of waxy cuticle on the outer surface of epidermal tissue, which prevented the uptake of XXXG. In fact, fluorescent derivatives were hardly incorporated into the walls of epidermal cells (Fig. 4 A–D). The effect was further confirmed when high concentrations of the oligosaccharides were used for incubation: the Km value for cell elongation in peeled stem segments was 2.7 mM for XXXG, which is higher than the concentrations of oligosaccharides used by the above three research groups (10−4 to 10−10 M).
We did not observe any inhibitory effect of XXFG at a low concentration of 10−4 to 10−9 M on cell elongation in either split or peeled stem segments of pea in the presence of 2,4-D, although this fucose-containing xyloglucan oligosaccharide has been shown to inhibit 2,4-D-induced elongation of pea stem segments (7, 8). However, a high concentration of XXFG accelerated the cell elongation of peeled stem segments in the presence of 2,4-D, which was identical to the effect of XXXG. The reported inhibitory effect of the fucose-containing oligosaccharide may result not from a direct interaction with XET but instead from a signal transduction cascade, such as the induction of wall-associated peroxidase (35) and/or α-fucosidase activities (36).
Rearrangement of Cortical Microtubules.
Although cortical microtubules are known to become rearranged in response to plant hormones, gravity, physical stimuli, light, and mechanical stresses (37), the mechanisms underlying these changes are not known. We show here that microtubule reorientation is triggered by the integration of whole xyloglucan into the cell wall. The integration of whole xyloglucan probably confers a strong tensile stress to the walls of peeled stem segments because oblique and longitudinal microtubules appeared to be formed in a short time. The phenomenon could be explained by the newly formed xyloglucan cross-links preventing loosening between preexisting transverse microfibrils. In other words, microtubule reorientation from a transverse to longitudinal direction could be a result of the mechanical prevention of cell elongation (38). In addition, the integration of whole xyloglucan appeared to be strong enough to suppress cell elongation even when cortical microtubules remain transverse, as indicated by the taxol results. When the microtubules were longitudinally fixed by pretreatment with 6-dimethylaminopurine, the integration of whole xyloglucan almost completely suppressed cell elongation. On the basis of our experiments with whole xyloglucan, we hypothesize that cell elongation is controlled by xyloglucan, the integration of which directly suppresses wall extension and then indirectly changes the arrangement of the microtubules.
Cell elongation was accelerated by maintaining the transverse cortical microtubules in the presence of xyloglucan oligosaccharide (XXXG). The integration of oligosaccharide probably caused cleavage of xyloglucan tethers between preexisting transverse microfibrils, and the increased rate of extension may have, in turn, stabilized microtubules. Cell elongation was accelerated by the addition of oligosaccharide even in the presence of longitudinal cortical microtubules fixed by pretreatment with 6-dimethylaminopurine. This result may be explained by the oligosaccharide greatly reducing the ability of newly synthesized microfibrils to become incorporated into the load-bearing texture of the cell wall.
Acknowledgments
We thank Kozo Kanayama, Kenkichi Murakami, Keiji Takabe, Junji Sugiyama, Hiroh Shibaoka, Stephen C. Fry, and Tobias I. Baskin for valuable discussions. This work was supported by Research Grant BDP-02-II-2 from the Ministry of Agriculture, Forestry and Fisheries.
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- XET
xyloglucan endotransglycosylase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hayashi T, Ogawa K, Mitsuishi Y. Plant Cell Physiol. 1994;35:1199–1205. [PubMed] [Google Scholar]
- 2.Hayashi T. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:139–168. [Google Scholar]
- 3.Hayashi T, Maclachlan G. Plant Physiol. 1984;75:596–604. doi: 10.1104/pp.75.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoson T, Masuda Y, Sone Y, Misaki A. Plant Physiol. 1991;96:551–557. doi: 10.1104/pp.96.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry S C, Smith R C, Renwick K F, Martin D J, Hodge S K, Matthews K J. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishitani K, Tominaga R. J Biol Chem. 1992;267:21058–21064. [PubMed] [Google Scholar]
- 7.York W S, Darvill A G, Albersheim P. Plant Physiol. 1984;75:295–297. doi: 10.1104/pp.75.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall G J, Fry S C. Planta. 1988;175:412–416. doi: 10.1007/BF00396348. [DOI] [PubMed] [Google Scholar]
- 9.McDougall G J, Fry S C. Plant Physiol. 1990;93:1042–1048. doi: 10.1104/pp.93.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y, Cosgrove D J. J Exp Bot. 2000;51:1543–1553. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- 11.Green P B. Annu Rev Plant Physiol. 1980;31:51–82. [Google Scholar]
- 12.Cosgrove D J. Nature (London) 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 13.Pauly M, Albersheim P, Darvill A, York W S. Plant J. 1999;20:629–639. doi: 10.1046/j.1365-313x.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Wong Y, Maclachlan G. Plant Physiol. 1984;75:605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooiman P. Recl Trav Chim Pays Bas. 1960;79:675–678. [Google Scholar]
- 16.Hayashi T, Marsden M P F, Delmer D P. Plant Physiol. 1987;83:384–389. doi: 10.1104/pp.83.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry S C. Plant J. 1997;11:1141–1150. [Google Scholar]
- 18.Ferry J D. Viscoelastic Properties of Polymers. 2nd Ed. New York: Wiley; 1970. [Google Scholar]
- 19.Van Overbeek J, Went F W. Bot Gaz. 1937;99:22–41. [Google Scholar]
- 20.Fry S C, York W S, Albersheim P, Darvill A, Hayashi T, Joseleau J P, Kato Y, Lorences E P, Maclachlan G A, McNeil M, et al. Physiol Plant. 1993;89:1–3. [Google Scholar]
- 21.Matsumoto T, Sakai F, Hayashi T. Plant Physiol. 1997;114:661–667. doi: 10.1104/pp.114.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno K. Plant Cell Physiol. 1994;35:1149–1157. [Google Scholar]
- 23.Whitney S E C, Brigham J E, Darke A H, Reid J S G, Gidley M J. Plant J. 1995;8:491–504. [Google Scholar]
- 24.Sulova Z, Takacova M, Steele N M, Fry S C, Farkas V. Biochem J. 1998;15:1475–1480. doi: 10.1042/bj3301475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele N M, Fry S C. Biochem J. 2000;340:207–211. [PMC free article] [PubMed] [Google Scholar]
- 26.Haigler C H, Benziman M. In: Cellulose and Other Natural Polymer Systems. Brown R M Jr, editor. New York: Plenum; 1982. pp. 273–297. [Google Scholar]
- 27.Thompson J E, Smith R C, Fry S C. Biochem J. 1997;327:699–708. doi: 10.1042/bj3270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson J E, Fry S C. Plant J. 2001;26:23–34. doi: 10.1046/j.1365-313x.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- 29.Mcqueen-Mason S J, Fry S C, Durachko M D, Cosgrove D J. Planta. 1993;190:327–331. doi: 10.1007/BF00196961. [DOI] [PubMed] [Google Scholar]
- 30.Nishitani K. J Plant Res. 1995;108:137–148. [Google Scholar]
- 31.Takeda T, Fukumi S, Hayashi T. Biosci Biotech Biochem. 1996;60:1950–1955. doi: 10.1271/bbb.60.1950. [DOI] [PubMed] [Google Scholar]
- 32.Rayle D L, Cleland R E. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose J K C, Bennett A B. Trends Plant Sci. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 34.Cutillas-Iturralde A, Lorences E P. Plant Physiol. 1997;113:103–109. doi: 10.1104/pp.113.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warneck H M, Haug T, Seitz H U. J Exp Bot. 1996;47:1897–1904. [Google Scholar]
- 36.Vargas-Rechia C, Reicher F, Sierakowski M R, Heyraud A, Driguez H, Lienart Y. Plant Physiol. 1998;116:1013–1021. doi: 10.1104/pp.116.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nick P. Inter Rev Cytol. 1998;184:33–80. [Google Scholar]
- 38.Zandomeni K, Schopfer P. Protoplasma. 1994;182:96–101. doi: 10.1007/BF01403471. [DOI] [PubMed] [Google Scholar]