ABSTRACT
Introduction
Platelet‐rich plasma (PRP) has been widely utilized in dermatological treatments, leading to the development of various administration methods. This study aims to evaluate and compare the effectiveness of PRP injections and microneedling techniques for treating androgenetic alopecia (AGA) in clinical practice.
Materials and Methods
A total of 40 participants diagnosed with AGA, aged between 18 and 50 years, were randomly assigned to receive either PRP injections or microneedling treatments. Each patient underwent two treatment sessions, with a 1‐month interval between them. Hair density and thickness measurements were performed using TrichoScan before treatment and 2 months after the final session. Statistical analysis was conducted using SPSS software.
Results
Among the participants, 28.57% were male and 71.43% were female, with an average age of 40.2 years (±7.7). In the PRP injection group, hair count increased by approximately 62.4% (from 17.40 ± 2.074 to 28.26 ± 6.229), and hair thickness improved by 58.6% (from 58.0 ± 25.01 to 92.0 ± 16.31). In the microneedling group, hair count increased by 88.4% (from 14.71 ± 3.988 to 27.71 ± 4.499), while hair thickness improved by 51.3% (from 49.0 ± 11.30 to 74.14 ± 22.42). Although both groups showed statistically significant improvements (p < 0.05), the intergroup differences in hair count and thickness were not statistically significant (p > 0.05).
Conclusion
The findings of this study confirm PRP as a viable treatment option for AGA. Both injection and microneedling methods provided therapeutic benefits. Additionally, microneedling appeared to enhance patient satisfaction and treatment tolerance.
Keywords: androgenetic alopecia, injection, microneedling, platelet‐rich plasma
1. Introduction
Androgenetic alopecia (AGA) is a prevalent hair loss condition affecting individuals worldwide [1, 2]. It manifests through a gradual decline in scalp hair density, displaying characteristic male and female patterns, along with disruptions in the hair growth cycle and follicular shrinkage [3, 4]. Various factors, including genetic predisposition, hormonal imbalances, environmental aspects, and psychological influences, contribute to the onset and progression of AGA [5, 6].
Both male and female pattern baldness become more common with age, impacting over 50% of people above 50 years old [3, 4, 5]. While hereditary and hormonal influences are the main causes of AGA in men, the condition in women is more multifaceted, involving numerous contributing factors. Hair loss in women can significantly affect their physical appearance, emotional health, and self‐esteem, as societal norms tend to be less accepting of female baldness [2, 7, 8]. Studies indicate that AGA is more commonly observed in Caucasians, with variations in prevalence based on age and ethnicity [5].
Several treatment methods have been explored for managing AGA, including medications, hair transplants, and physical therapies such as laser procedures and growth factor‐based interventions [9, 10]. However, the U.S. Food and Drug Administration (FDA) has approved only two pharmacological treatments for hereditary and hormonal hair loss: minoxidil and finasteride [3]. These medications require continuous, long‐term use, and hair regrowth often diminishes once treatment is discontinued [11]. Moreover, nonsurgical therapies have demonstrated limited success in managing AGA [8, 12]. Consequently, AGA remains a complex issue, requiring further research into more effective treatment alternatives [8].
Given the widespread occurrence of hereditary and hormonal hair loss, its effect on an individual's self‐perception, the slow hair regrowth associated with current treatments, their necessity for prolonged use, and their limited effectiveness with potential side effects, discovering a more efficient and practical therapy is essential [1, 13, 14]. AGA shares characteristics with tissue damage and repair processes, where growth factors stimulate cellular activity and attract necessary components for healing. This has led to the hypothesis that regenerative approaches, such as platelet‐rich plasma (PRP) therapy, could support hair follicle repair [11].
PRP is a component derived from plasma that contains an increased concentration of platelets, providing a rich source of growth factors such as transforming growth factor‐β (TGF‐β), vascular endothelial growth factor (VEGF), platelet‐derived growth factor (PDGF), insulin‐like growth factor (IGF), and epidermal growth factor (EGF) [11]. PRP has gained significant traction in medical, surgical, wound healing, and cosmetic applications [15, 16, 17]. More recently, it has emerged as a promising, minimally invasive option for promoting hair regrowth in AGA patients [10, 18]. PRP can be delivered via direct scalp injections or through microneedling [19]. The microneedling technique involves creating tiny skin injuries that enhance PRP absorption, leading to improved treatment outcomes; though some adverse effects may occur [20, 21].
This study seeks to assess and compare the effectiveness of PRP administration through injection and microneedling in patients with AGA.
2. Materials and Methods
2.1. Study Design and Participants
This study involved 40 patients, aged 18–50, presenting with AGA at a dermatology clinic. Ethical approval was granted by the Research Ethics Committee (IR.TUMS.MEDICINE.REC.1400.031), and the study was officially registered (IRCT20200127046282N6).
Before enrollment, all participants were thoroughly briefed on the study's aims, procedures, possible benefits, risks, and follow‐up requirements. Each individual provided written informed consent. Participants were randomly allocated into two groups: one receiving PRP injections at hair loss sites (n = 20) and the other undergoing PRP application via microneedling (n = 20). Both groups received two treatment sessions, spaced 1 month apart, with follow‐up assessments conducted 2 months posttreatment. Additionally, all patients were prescribed standard AGA therapy—1 mg of finasteride daily for men and cyproterone compound tablets for women (administered from day 5 to day 21 of their menstrual cycle).
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
Diagnosis of AGA
Age between 18 and 50 years
Willingness to participate with informed consent
Hamilton scale scores of 2–5 for men and Ludwig scale scores of 1–3 for women
2.2.2. Exclusion Criteria
Platelet dysfunction or low platelet count
Use of anticoagulant medications
Presence of malignancies
Women with hyperprolactinemia or hormonal imbalances
Active infections or wounds at the treatment site
2.3. PRP Preparation and Administration
PRP was prepared following the American Association of Blood Banks' guidelines using a two‐step centrifugation process. Whole blood (10 mL) was collected in sodium citrate tubes and centrifuged using a 320 Universal device (Hettich, Germany). The initial centrifugation (160 g, 10 min) separated PRP from whole blood; followed by a second centrifugation (400 g, 10 min) to isolate platelet‐poor plasma. The platelet‐rich fraction was extracted using a sterile kit from Persian Bio‐Based Production (PBBP) Company.
A total of 2 cc of PRP was injected into androgen‐related scalp areas using a 30G needle.
2.4. Microneedling Procedure
A dermaroller with 1.5 mm needles was used to perform microneedling across the affected scalp regions in vertical, horizontal, and crosswise motions until pinpoint bleeding appeared. PRP was then applied topically to the treated areas. Each procedure lasted approximately 10–20 min per patient.
2.5. Assessment Methods
Evaluations were conducted at baseline and 2 months after the final treatment using the TricoScan device to measure hair density (n/cm2) and thickness. A designated scalp region was analyzed for quantitative assessment.
2.5.1. Pull Test
A standardized hair pull test was conducted by the same physician at each visit. Between 20 and 60 hairs were gently pulled near the scalp; shedding of more than 10% was considered a positive test result.
2.5.2. Patient Global Assessment Score
Participants rated their satisfaction using a four‐point scale:
0 = Poor
1 = Acceptable
2 = Good
3 = Excellent
2.6. Statistical Analysis
Quantitative variables, such as age, hair density, and hair thickness, were expressed as mean ± standard deviation, while categorical data were presented as percentages. Due to the limited sample size, the Wilcoxon nonparametric test was used to compare pre‐ and posttreatment hair density and thickness within each group. The Mann–Whitney test was applied to determine differences in changes between the two treatment groups. Statistical analysis was performed using SPSS software (Version 24), with significance set at p < 0.05.
No artificial intelligence–generated content (AIGC) tools, including large language models such as ChatGPT or similar, were used in the development, writing, or analysis of this manuscript. All content was prepared solely by the authors.
3. Results
3.1. Participant Demographics
Among the study participants, 28.57% were male, while 71.43% were female. The mean age of the group was 40.2 ± 7.7 years.
3.2. PRP Injection Outcomes
Hair count increased significantly from a baseline of 17.40 ± 2.074 to 28.26 ± 6.229 after PRP injection (p = 0.043). Similarly, hair thickness showed a statistically significant improvement, rising from 58.0 ± 25.01 to 92.0 ± 16.31 posttreatment (p = 0.043) (See Table 1, Figures 1 and 2).
TABLE 1.
Comparison of measured parameters at baseline and after treatment in PRP injection group.
| Variables | Time | Mean ± SD | Percentiles | p | ||
|---|---|---|---|---|---|---|
| 25 | 50 (median) | 75 | ||||
| n = 5 | ||||||
| (Triple ×60) hair number in 4*5 Mm | Before | 17.40 ± 2.074 | 15.5 | 17.0 | 19.5 | 0.043 |
| After | 28.6 ± 6.229 | 24.0 | 27.0 | 34.0 | ||
| (Kpl ×150) hair thickness in Mm | Before | 58.0 ± 25.01 | 36.0 | 61.0 | 78.5 | 0.043 |
| After | 92.0 ± 16.31 | 78.5 | 87.0 | 108.0 | ||
FIGURE 1.
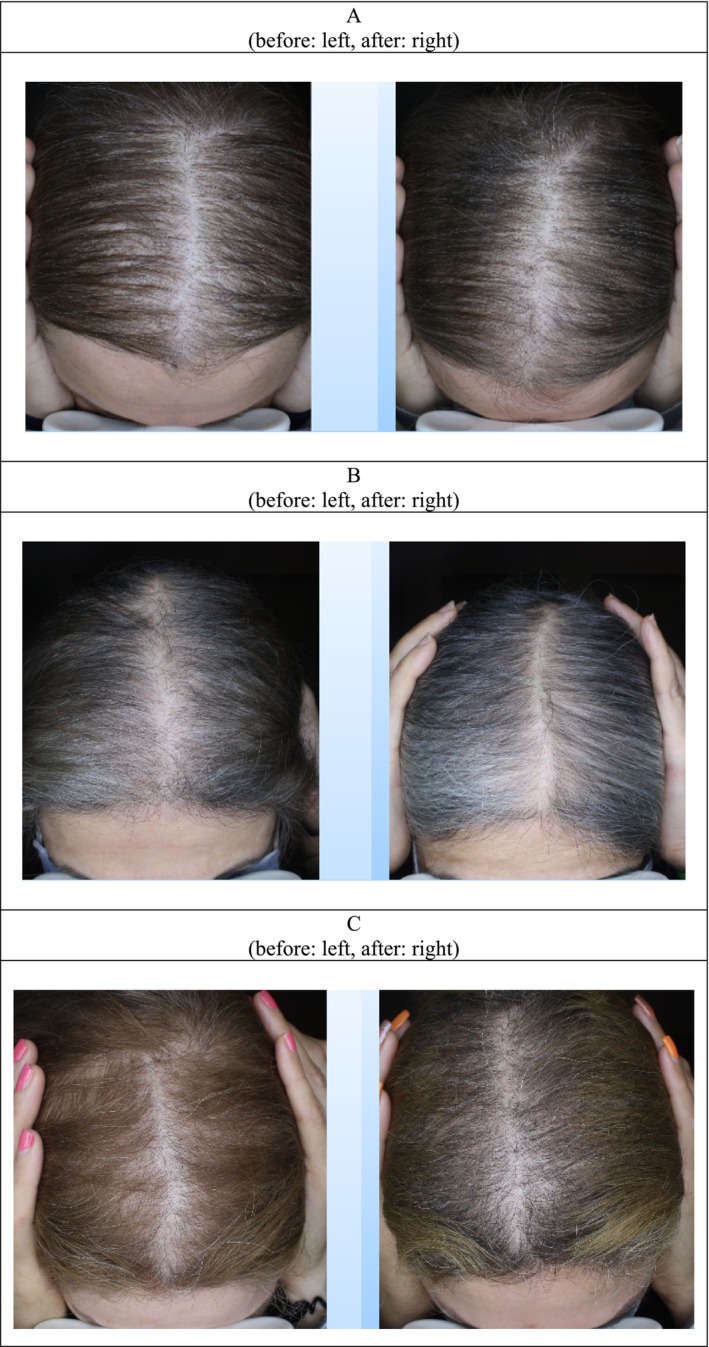
Image of before and 2 months after treatment by PRP injection in three patients' (A–C).
FIGURE 2.

Image of before (A, C) and 2 months after (B, D) of treatment by PRP injection (60× and 150× lens).
3.3. PRP Microneedling Outcomes
For patients undergoing PRP with microneedling, hair count rose from 14.71 ± 3.988 before treatment to 27.71 ± 4.499 after treatment, demonstrating a significant increase (p = 0.018). Hair thickness also improved from 49.0 ± 11.30 to 74.14 ± 22.42 (p = 0.018) (See Table 2).
TABLE 2.
Comparison of measured parameters at baseline and after treatment in PRP microneedling group.
| Variables | Time | Mean ± SD | Percentiles | p | ||
|---|---|---|---|---|---|---|
| 25 | 50 (median) | 75 | ||||
| n = 7 | ||||||
| (Triple ×60) hair number in 4 × 5 Mm | Before | 14.71 ± 3.988 | 12.0 | 13.0 | 18.0 | 0.018 |
| After | 27.71 ± 4.499 | 25.0 | 30.0 | 32.0 | ||
| (Kpl ×150) hair thickness in Mm | Before | 49.0 ± 11.30 | 37.0 | 53.0 | 58.0 | 0.018 |
| After | 74.14 ± 22.42 | 47.0 | 84.0 | 92.0 | ||
3.4. Comparison of PRP Injection vs. Microneedling
Tables 1 and 2 present hair density and thickness changes for both treatment groups, confirming significant gains in both variables.
Hair count improvements were more prominent in the microneedling group; though this difference was not statistically significant (p = 0.219).
Hair thickness increased slightly more in the PRP injection group, but the difference compared to microneedling was not statistically significant (p = 0.570) (See Table 3, Figures 3 and 4).
TABLE 3.
Comparison of the rate of parameter changes in PRP injection and microneedling groups.
| Variables | Group | Mean ± SD | Percentiles | p | ||
|---|---|---|---|---|---|---|
| 25 | 50 (median) | 75 | ||||
| (Triple ×60) hair number in 4 × 5 Mm | PRP | 64.69 ± 30.29 | 35.15 | 66.67 | 93.26 | 0.219 |
| PRP + Micro | 95.18 ± 39.84 | 66.67 | 88.24 | 150.0 | ||
| (Kpl ×150) hair thickness in Mm | PRP | 76.64 ± 59.60 | 29.35 | 42.62 | 140.95 | 0.570 |
| PRP + Micro | 51.80 ± 39.73 | 23.68 | 50.79 | 69.811 | ||
FIGURE 3.

Image of before and 2 months after treatment by PRP microneedling in three patients' (A, B).
FIGURE 4.

Image of before (A, C) and 2 months after (B, D) of treatment by PRP microneedling (60× and 150× lens).
3.5. Patient Satisfaction and Pain Perception
Treatment satisfaction was higher among microneedling patients, with 69% rating their results as “excellent” (score 3), compared to 58% in the PRP injection group.
Pain tolerance was also higher in the microneedling group, with 63% reporting good pain tolerance, while 49% in the PRP injection group expressed similar comfort levels. Although microneedling appeared to cause slightly less discomfort than direct PRP injections, the difference was not statistically significant.
4. Discussion
Although pharmaceutical treatments for AGA are available, their long‐term use and potential side effects make them less appealing to many patients [11]. As a result, PRP has emerged as an alternative therapeutic option, showing promise not only for AGA but also for various dermatological conditions [15, 22, 23]. Different PRP delivery techniques have been explored, with studies reporting varying levels of effectiveness [10].
This study compared PRP injection and microneedling methods for treating AGA to determine their relative effectiveness. Both approaches significantly enhanced hair count and thickness, but no statistically significant difference was observed between them. Hair count increased by an average of 64.69 in the PRP injection group and 95.18 in the microneedling group; though the difference was not significant (p > 0.05), likely due to the small sample size and data variations. A similar pattern was noted for hair thickness.
The therapeutic benefits of PRP stem from its rich composition of growth factors, including PDGF, insulin‐like growth factor‐1 (IGF‐1), and VEGF, which play key roles in counteracting AGA‐related changes [24, 25, 26, 27]. Since AGA is associated with factors like reduced angiogenesis, scalp microinflammation, excessive sebum production, and decreased IGF‐1 levels, PRP components may help mitigate these effects, thereby supporting hair growth [24, 28, 29, 30].
Previous research suggests PRP exerts its effects through anti‐inflammatory properties, regulation of scalp oil production, stimulation of follicular activity, and overall enhancement of hair growth [24, 31]. AGA has also been linked to dysfunction in the Wnt/beta‐catenin signaling pathway [32], which PRP has been shown to activate through PDGF stimulation, promoting dermal papilla cell proliferation [33].
Findings from other studies align with our results. Rodriguez et al. demonstrated PRP's efficacy in treating male‐pattern baldness by comparing PRP with saline injections. Their evaluation, conducted at baseline, 15 days, and 3 months posttreatment using TricoScan, showed significant improvements in hair density and growth in the PRP group [34]. Similarly, Kumar Jha et al. assessed PRP microneedling in patients with mild to moderate AGA and observed increased hair follicle numbers, improved hair diameter, and high patient satisfaction after 3 months of treatment [19]. Additionally, Gentile et al. reported that microneedling combined with autologous nonactivated PRP effectively stimulated hair regrowth in AGA patients [35].
Although microneedling has been recognized as a beneficial technique for AGA treatment [36], its superiority over PRP injections remains uncertain. Shruti Gupta observed increased hair density in patients treated with PRP microneedling, but the improvement was not statistically significant [19]. Microneedling may enhance PRP effectiveness by inducing micro‐injuries that improve absorption, stimulate collagen production, and enhance blood circulation, all of which contribute to hair growth [21].
Contrary to most studies, Shapiro et al. investigated PRP's impact on AGA by randomly injecting either PRP or saline into different scalp regions of 35 patients. After three monthly treatment sessions and follow‐up at 3 months, they found no significant difference in hair density between the PRP and control groups [37]. Such discrepancies may result from variations in study design, methodology, sample size, and participant characteristics.
A recent study by Özcan et al. (2022) compared PRP application using Dermapen microneedling with intradermal point‐by‐point injection in male patients with AGA. The trial included 62 male participants with Norwood–Hamilton grades II–V, who received four PRP sessions (the first three at 2‐week intervals and the fourth after 1 month). The results demonstrated significant improvements in hair count, density, terminal hair number, and average hair length in both groups after treatment (all p < 0.05). However, the Dermapen microneedling group showed significantly better outcomes in terms of anagen hair count, telogen hair count, and average hair length compared to the injection group (p < 0.05). These findings align with our study, where the microneedling group also exhibited greater improvements in hair count, albeit without reaching statistical significance. Incorporating this recent evidence supports the potential superiority of automated microneedling over point‐by‐point PRP injection for enhancing hair regrowth parameters in patients with AGA [38].
Another key aspect of this study was assessing patient satisfaction and pain tolerance. While the microneedling group reported slightly higher satisfaction and better pain tolerance than the PRP injection group, the differences were not statistically significant. Yepuri et al. found that microneedling caused less discomfort than conventional treatments [39], and Nagaratna et al. reported similar findings when PRP was combined with microneedling [40].
Despite certain limitations, such as a relatively small sample size and a short follow‐up duration, this study provides valuable insights into PRP's effectiveness for AGA treatment. By analyzing both individual treatment outcomes and intergroup comparisons, we were able to assess the potential advantages of each method.
5. Conclusion
This study supports PRP as an effective treatment for AGA, leading to significant increases in hair count and thickness. Both PRP injection and microneedling approaches yielded positive outcomes, with microneedling showing a greater improvement in hair count; although the difference was not statistically significant. Further large‐scale studies are needed to determine whether microneedling provides superior results.
Author Contributions
Contributions to the current study include M.R., E.B., A.J., and S.Z. in study idea and design, in the literature review, and drafting and revising the manuscript critically for important intellectual content. M.N. and SO.Z. are involved in drafting the revised manuscript and literature review, and in the analysis and interpretation of the revised version and drafting the manuscript. A.J. is involved in the proposal preparation, statistics, analysis, and drafting the revised manuscript. S.Z. and M.R. are involved in study supervision, data gathering, and literature review. All authors have read and approved the final version to be published and agreed to be accountable for all aspects of the work. All authors agreed on the order in which their names are listed in the revised manuscript.
Ethics Statement
All collected data were kept confidential and analyzed without the use of specific names. The study adhered to the ethical principles outlined in the Helsinki Declaration. The project was registered at Tehran University of Medical Sciences under registration number IRCT20200127046282N6, with the scientific title “Phase I Clinical Trial: Comparing the Efficacy, Safety, Tolerability, and Satisfaction of Platelet‐Rich Plasma (PRP) Injections and Microneedling for the Treatment of Androgenetic Alopecia.” It was approved by the Research Council under ethics code number IR.TUMS.MEDICINE.REC.1400.031.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors express their gratitude to the Skin and Stem Cell Research Center at Tehran University of Medical Sciences and the Persian Bio‐Based Production (PBBP) Company in Tehran, Iran, for their technical and editorial assistance.
Nilforoushzadeh M. A., Roohaninasab M., Behrangi E., et al., “Phase I Clinical Trial: Evaluating the Efficacy, Safety, and Patient Satisfaction of Platelet‐Rich Plasma (PRP) Injections and Microneedling for Androgenetic Alopecia Treatment,” Journal of Cosmetic Dermatology 24, no. 9 (2025): e70408, 10.1111/jocd.70408.
Funding: The authors received no specific funding for this work.
Mohammad Ali Nilforoushzadeh and Masoumeh Roohaninasab contributed equally to preparing this article and are co‐firstauthors.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [S.Z.] upon reasonable request; also, all additional files are included in the manuscript.
References
- 1. Alves R., “Androgenetic Alopecia: A Review and Emerging Treatments,” Journal of Clinical Research in Dermatology 4, no. 4 (2017): 1–13. [Google Scholar]
- 2. Lolli F., Pallotti F., Rossi A., et al., “Androgenetic Alopecia: A Review,” Endocrine 57, no. 1 (2017): 9–17. [DOI] [PubMed] [Google Scholar]
- 3. Sadeghzadeh Bazargan A., Tavana Z., Dehghani A., et al., “The Efficacy of the Combination of Topical Minoxidil and Oral Spironolactone Compared With the Combination of Topical Minoxidil and Oral Finasteride in Women With Androgenic Alopecia, Female and Male Hair Loss Patterns: A Blinded Randomized Clinical Trial,” Journal of Cosmetic Dermatology 23, no. 2 (2024): 543–551, 10.1111/jocd.15979. [DOI] [PubMed] [Google Scholar]
- 4. Starace M., Orlando G., Alessandrini A., and Piraccini B. M., “Female Androgenetic Alopecia: An Update on Diagnosis and Management,” American Journal of Clinical Dermatology 21, no. 1 (2020): 69–84. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka Y., Aso T., Ono J., Hosoi R., and Kaneko T., “Androgenetic Alopecia Treatment in Asian Men,” Journal of Clinical and Aesthetic Dermatology 11, no. 7 (2018): 32–35. [PMC free article] [PubMed] [Google Scholar]
- 6. Lohia K., Doshi B. R., and Manjunathswamy B. S., “Hair Loss Severity and Its Impact on Quality of Life in Patients Suffering From Androgenic Alopecia: A One‐Year Cross‐Sectional Study,” Clinical Dermatology Review 5, no. 1 (2021): 59–64. [Google Scholar]
- 7. Lam S. M., “Hair Loss and Hair Restoration in Women,” Facial Plastic Surgery Clinics of North America 28, no. 2 (2020): 205–223. [DOI] [PubMed] [Google Scholar]
- 8. Coleman E., “Types and Treatment of Hair Loss in Men and Women,” Plastic Surgical Nursing 40, no. 4 (2020): 222–235. [DOI] [PubMed] [Google Scholar]
- 9. York K., Meah N., Bhoyrul B., and Sinclair R., “A Review of the Treatment of Male Pattern Hair Loss,” Expert Opinion on Pharmacotherapy 21, no. 5 (2020): 603–612. [DOI] [PubMed] [Google Scholar]
- 10. Chen J. X., Justicz N., and Lee L. N., “Platelet‐Rich Plasma for the Treatment of Androgenic Alopecia: A Systematic Review,” Facial Plastic Surgery 34, no. 6 (2018): 631–640. [DOI] [PubMed] [Google Scholar]
- 11. Roohaninasab M., Goodarzi A., Ghassemi M., Sadeghzadeh‐Bazargan A., Behrangi E., and Najar Nobari N., “Systematic Review of Platelet‐Rich Plasma in Treating Alopecia: Focusing on Efficacy, Safety, and Therapeutic Durability,” Dermatologic Therapy 34, no. 2 (2021): e14768. [DOI] [PubMed] [Google Scholar]
- 12. Sadick N. S., “New‐Generation Therapies for the Treatment of Hair Loss in Men,” Dermatologic Clinics 36, no. 1 (2018): 63–67. [DOI] [PubMed] [Google Scholar]
- 13. Varothai S. and Bergfeld W. F., “Androgenetic Alopecia: An Evidence‐Based Treatment Update,” American Journal of Clinical Dermatology 15, no. 3 (2014): 217–230, 10.1007/s40257-014-0077-5. [DOI] [PubMed] [Google Scholar]
- 14. Gupta A. K., Mays R. R., Dotzert M. S., Versteeg S. G., Shear N. H., and Piguet V., “Efficacy of Non‐Surgical Treatments for Androgenetic Alopecia: A Systematic Review and Network Meta‐Analysis,” Journal of the European Academy of Dermatology and Venereology 32, no. 12 (2018): 2112–2125. [DOI] [PubMed] [Google Scholar]
- 15. Samadi P., Sheykhhasan M., and Khoshinani H. M., “The Use of Platelet‐Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review,” Aesthetic Plastic Surgery 43, no. 3 (2019): 803–814. [DOI] [PubMed] [Google Scholar]
- 16. Jafarzadeh A., Pour Mohammad A., Keramati H., Zeinali R., Khosravi M., and Goodarzi A., “Regenerative Medicine in the Treatment of Specific Dermatologic Disorders: A Systematic Review of Randomized Controlled Clinical Trials,” Stem Cell Research & Therapy 15, no. 1 (2024): 176, 10.1186/s13287-024-03800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jafarzadeh A., PourMohammad A., and Goodarzi A., “A Systematic Review of the Efficacy, Safety and Satisfaction of Regenerative Medicine Treatments, Including Platelet‐Rich Plasma, Stromal Vascular Fraction and Stem Cell‐Conditioned Medium for Hypertrophic Scars and Keloids,” International Wound Journal 21, no. 4 (2024): e14557, 10.1111/iwj.14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paul D. and Velez M. W., “History of PRP,” in Aesthetic Clinician's Guide to Platelet Rich Plasma (Springer, 2021), 1–7. [Google Scholar]
- 19. Gupta S., Revathi T. N., Sacchidanand S., and Nataraj H. V., “A Study of the Efficacy of Platelet‐Rich Plasma in the Treatment of Androgenetic Alopecia in Males,” Indian Journal of Dermatology, Venereology and Leprology 83 (2017): 412. [DOI] [PubMed] [Google Scholar]
- 20. Stoll S., Dietlin C., and Nett‐Mettler C. S., “Microneedling as a Successful Treatment for Alopecia X in Two P Omeranian Siblings,” Veterinary Dermatology 26, no. 5 (2015): 387–388. [DOI] [PubMed] [Google Scholar]
- 21. Fertig R. M., Gamret A. C., Cervantes J., and Tosti A., “Microneedling for the Treatment of Hair Loss?,” Journal of the European Academy of Dermatology and Venereology 32, no. 4 (2018): 564–569. [DOI] [PubMed] [Google Scholar]
- 22. Evans A. G., Mwangi J. M., Pope R. W., et al., “Platelet‐Rich Plasma as a Therapy for Androgenic Alopecia: A Systematic Review and Meta‐Analysis,” Journal of Dermatological Treatment 33 (2021): 1–14. [DOI] [PubMed] [Google Scholar]
- 23. Dubin D. P., Lin M. J., Leight H. M., et al., “The Effect of Platelet‐Rich Plasma on Female Androgenetic Alopecia: A Randomized Controlled Trial,” Journal of the American Academy of Dermatology 83, no. 5 (2020): 1294–1297. [DOI] [PubMed] [Google Scholar]
- 24. Roohaninasab M., Jafarzadeh A., Sadeghzadeh‐Bazargan A., et al., “Evaluation of the Efficacy, Safety and Satisfaction Rates of Platelet‐Rich Plasma, Non‐Cross‐Linked Hyaluronic Acid and the Combination of Platelet‐Rich Plasma and Non‐Cross‐Linked Hyaluronic Acid in Patients With Burn Scars Treated With Fractional CO2 Laser: A Randomized Controlled Clinical Trial,” International Wound Journal 21, no. 10 (2024): e70065, 10.1111/iwj.70065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomita Y., Akiyama M., and Shimizu H., “PDGF Isoforms Induce and Maintain Anagen Phase of Murine Hair Follicles,” Journal of Dermatological Science 43, no. 2 (2006): 105–115. [DOI] [PubMed] [Google Scholar]
- 26. Li J., Yang Z., Li Z., Gu L., Wang Y., and Sung C., “Exogenous IGF‐1 Promotes Hair Growth by Stimulating Cell Proliferation and Down Regulating TGF‐β1 in C57BL/6 Mice In Vivo,” Growth Hormone & IGF Research 24, no. 2–3 (2014): 89–94. [DOI] [PubMed] [Google Scholar]
- 27. Jafarzadeh A., Mohammad A. P., and Goodarzi A., “A Systematic Review of Case Series and Clinical Trials Investigating Regenerative Medicine for the Treatment of Vitiligo,” Journal of Cosmetic Dermatology 24 (2024): e16660, 10.1111/jocd.16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michel L., Reygagne P., Benech P., et al., “Study of Gene Expression Alteration in Male Androgenetic Alopecia: Evidence of Predominant Molecular Signalling Pathways,” British Journal of Dermatology 177, no. 5 (2017): 1322–1336. [DOI] [PubMed] [Google Scholar]
- 29. Panchaprateep R. and Asawanonda P., “Insulin‐Like Growth Factor‐1: Roles in Androgenetic Alopecia,” Experimental Dermatology 23, no. 3 (2014): 216–218. [DOI] [PubMed] [Google Scholar]
- 30. Chew E. G. Y., Tan J. H. J., Bahta A. W., et al., “Differential Expression Between Human Dermal Papilla Cells From Balding and Non‐Balding Scalps Reveals New Candidate Genes for Androgenetic Alopecia,” Journal of Investigative Dermatology 136, no. 8 (2016): 1559–1567. [DOI] [PubMed] [Google Scholar]
- 31. Anitua E., Pino A., Martinez N., Orive G., and Berridi D., “The Effect of Plasma Rich in Growth Factors on Pattern Hair Loss: A Pilot Study,” Dermatologic Surgery 43, no. 5 (2017): 658–670. [DOI] [PubMed] [Google Scholar]
- 32. Jafari M. A., Bazgir G., Hosseini‐Baharanchi F. S., Jafarzadeh A., and Goodarzi A., “Efficacy and Safety of Laser Therapy and Phototherapy in Cicatricial and NonCicatricial Alopecia: A Systematic Review Study,” Health Science Reports 7, no. 11 (2024): e70180, 10.1002/hsr2.70180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta A. K. and Carviel J., “A Mechanistic Model of Platelet‐Rich Plasma Treatment for Androgenetic Alopecia,” Dermatologic Surgery 42, no. 12 (2016): 1335–1339. [DOI] [PubMed] [Google Scholar]
- 34. Rodrigues B. L., Montalvão S. A. L., Cancela R. B. B., et al., “Treatment of Male Pattern Alopecia With Platelet‐Rich Plasma: A Double‐Blind Controlled Study With Analysis of Platelet Number and Growth Factor Levels,” Journal of the American Academy of Dermatology 80, no. 3 (2019): 694–700. [DOI] [PubMed] [Google Scholar]
- 35. Gentile P., Dionisi L., Pizzicannella J., De Angelis B., de Fazio D., and Garcovich S., “A Randomized Blinded Retrospective Study: The Combined Use of Micro‐Needling Technique, Low‐Level Laser Therapy and Autologous Non‐Activated Platelet‐Rich Plasma Improves Hair Regrowth in Patients With Androgenic Alopecia,” Expert Opinion on Biological Therapy 20, no. 9 (2020): 1099–1109. [DOI] [PubMed] [Google Scholar]
- 36. Dhurat R., Sukesh M. S., Avhad G., Dandale A., Pal A., and Pund P., “A Randomized Evaluator Blinded Study of Effect of Microneedling in Androgenetic Alopecia: A Pilot Study,” International Journal of Trichology 5, no. 1 (2013): 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shapiro J., Ho A., Sukhdeo K., Yin L., and Lo Sicco K., “Evaluation of Platelet‐Rich Plasma as a Treatment for Androgenetic Alopecia: A Randomized Controlled Trial,” Journal of the American Academy of Dermatology 83, no. 5 (2020): 1298–1303. [DOI] [PubMed] [Google Scholar]
- 38. Ozcan K. N., Sener S., Altunisik N., and Turkmen D., “Platelet Rich Plasma Application by Dermapen Microneedling and Intradermal Point‐By‐Point Injection Methods, and Their Comparison With Clinical Findings and Trichoscan in Patients With Androgenetic Alopecia,” Dermatologic Therapy 35, no. 1 (2022): e15182, 10.1111/dth.15182. [DOI] [PubMed] [Google Scholar]
- 39. Chandrashekar B. S., Yepuri V., and Mysore V., “Alopecia Areata‐Successful Outcome With Microneedling and Triamcinolone Acetonide,” Journal of Cutaneous and Aesthetic Surgery 7, no. 1 (2014): 63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chatnallikar N., Asha G. S., Leelavthy B., and Revathi T. N., “Safety and Efficacy of Microneedling With Autologous Platelet‐Rich Plasma in Chronic and Stable Alopecia Areata,” Journal of Pakistan Association of Dermatologists 28, no. 1 (2018): 59–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [S.Z.] upon reasonable request; also, all additional files are included in the manuscript.


