Abstract
Background
Urinary incontinence is a common urinary tract disorder that has gradually become a significant health issue affecting middle-aged and elderly women worldwide in recent years. This study aimed to investigate the relationship between insulin resistance (IR) and female urge urinary incontinence (UUI).
Method
This study enrolled individuals aged 20 and older to investigate the relationship between IR and female UUI. The analysis leveraged data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2016. We employed multiple logistic regression models to assess the relationship between IR and female UUI. In addition, subgroup analyses, interaction tests, and smooth curve fitting were performed to evaluate the stability of this association.
Result
This study included a total of 2,961 participants, with a UUI prevalence rate of 28.20%. After adjusting for confounding variables, a positive correlation was found between the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and the prevalence of UUI (Model Ⅲ OR = 1.03,95% CI: 1.00, 1.06, P = 0.0264). Smoothed curve fitting also demonstrated a significant positive linear correlation between HOMA-IR and the risk of UUI in women. Subgroup analysis revealed that the association between HOMA-IR and UUI remained consistent across all subgroups, and no subgroup showed an interaction effect on this association (P for interaction > 0.05). However, when body mass index (BMI) was further added to the fully adjusted model, the association between HOMA-IR and the prevalence of UUI was substantially attenuated. It was no longer statistically significant (Model IV OR = 1.01, 95% CI: 0.98, 1.04, P = 0.4243). In model IV-based smooth curve fitting and subgroup analysis, the association between IR and UUI was weakened and not statistically significant.
Conclusion
In non-diabetic women, obesity is the primary modifiable risk factor for UUI. It is crucial to prioritize weight management and obesity prevention as core strategies for reducing the risk of UI in this population. Although IR remains an important indicator of metabolic dysfunction, interventions targeting UI should focus on comprehensive weight control rather than directly addressing IR.
Keywords: Insulin resistance, HOMA-IR, Prediabetic state, Obesity, Urge urinary incontinence, NHANES
Introduction
Urinary incontinence is a common urinary disorder that often occurs in middle-aged and older women. Female urinary incontinence is an unexpected leakage of urine that occurs in women without control [1]. This may occur due to diminished bladder control or abnormalities in the urinary voiding mechanism [1]. Urinary incontinence is categorized into various subtypes based on its etiology. Leakage due to increased abdominal pressure is referred to as stress incontinence and is often associated with urethral sphincter insufficiency [2]. Uncontrollable urinary leakage associated with a sudden strong urge to urinate is called urge incontinence, and overactive bladder syndrome is a common cause [3]. Mixed incontinence is related to both incontinence symptoms and has a more serious impact on the quality of life of female patients. Female urinary incontinence is a global health problem with a high prevalence, with approximately 40% of women experiencing incontinence in their lifetime [4, 5]. The prevalence of urinary incontinence increases with age and can range from 38 to 77% in women over the age of 60 [6, 7]. There are no significant racial differences in the prevalence of urinary incontinence [8]. Female urinary incontinence patients often suffer from perineal wetness and recurrent urinary tract infections, which may affect the quality of women’s daily life and bring psychological burdens and social difficulties [9–11]. Common risk factors for female urinary incontinence include pregnancy and childbirth, history of pelvic surgery, urinary tract infections, and obesity [12–15]. Insulin resistance (IR) is a phenomenon in which the biological response of body tissues to insulin is diminished, as evidenced by defects in the control of insulin-mediated glucose metabolism in target tissues, such as the liver, muscle, and brain [16–18]. IR is often a precursor and major driver of type 2 diabetes mellitus(T2DM), but not all IR develops into T2DM [18]. In addition, IR is also associated with the progression of diseases such as cardiovascular disease, Alzheimer’s disease, and non-alcoholic fatty liver disease [19–22]. By reducing IR in the human body, the progression of these diseases can be delayed. However, the association between IR and female UUI remains unclear. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) is an indicator of IR, primarily calculated by combining fasting blood glucose and insulin levels to reflect the body’s sensitivity to insulin. This study aims to investigate the relationship between IR and UUI in non-diabetic women.
Method
Subjects
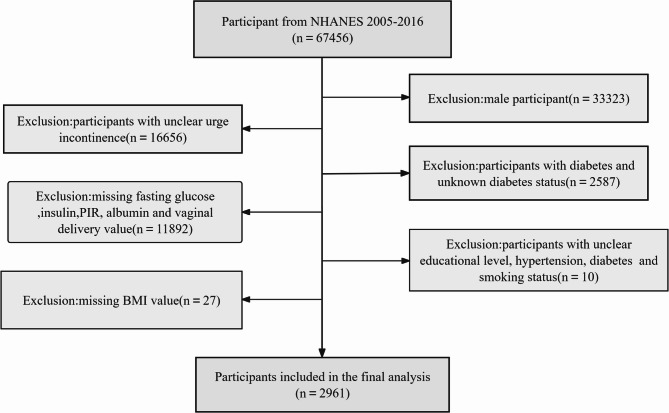
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive health assessment program launched by the CDC in the 1960 s that gathers data on the health, nutritional status, and disease risk factors of the U.S. population. Its dataset provides a nationally representative snapshot of the population, has undergone ethical review and approval by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and strictly adheres to the principles outlined in the Declaration of Helsinki. All participants also signed an informed consent form before participating in the NHANES survey. The study used data from 67,456 individuals from the 2005–2016 NHANES cycle. Based on the study criteria and study population, male patients (n = 33,323), individuals with unclear urge urinary incontinence status (n = 16,656), individuals with diabetes and unknown diabetes status(n = 2,587), individuals with missing fasting glucose, insulin, albumin, PIR, vaginal delivery value (n = 11,892) were excluded, and participants with unclear educational level, hypertension, diabetes and smoking status(n = 10). Exclusion of participants with missing BMI values(n=27). After applying the inclusion and exclusion criteria, we finally selected 2,961 participants for inclusion in this study (Fig. 1).
Fig. 1.
Participant inclusion and exclusion flowchart
Assessment of the exposed variable
HOMA-IR is a simple calculation based on fasting blood glucose and insulin levels used to assess the body’s insulin sensitivity. The higher the HOMA-IR index, the more severe the insulin resistance. The formula for calculating HOMA-IR is as follows:
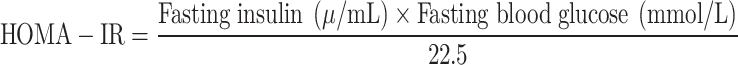 |
This study utilized HOMA-IR as an exposure variable to assess the degree of insulin resistance among participants, who were then divided into quartiles based on their HOMA-IR values for analysis.
Assessment of the covariates
The selection of potential covariates in this study was based on previous literature on cross-sectional studies of UUI. It was designed to include as many factors as possible that influence the relationship between HOMA-IR and the prevalence of female UUI. These covariates included demographics, lifestyle behaviors, physical measures, hematological indicators, and medical comorbidities. The covariates included in this study include age, race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, other race), albumin, total calcium, blood urea nitrogen (BUN), serum creatinine (SCr), osmolality, uric acid, vaginal delivery(≤ 2,>2 and ≤ 4,>4), body mass index(BMI). BMI was included as a key covariate given its strong potential to confound the relationship between insulin resistance and urinary incontinence, smoking (smoked at least 100 cigarettes in life), alcohol use (at least 12 alcoholic beverages in 1 year), hypertension (the doctor told you have high blood pressure), marital status (married, widowed, divorced, separated, never married, living with partner), education level (less than 9th grade, 9-11th grade, high school graduate, some college or AA degree, college graduate or above), and poverty income ratio (PIR ≤ 1, PIR>1 and ≤ 4, PIR>4).
Assessment of the outcome variable
Determination of urinary incontinence was based on a questionnaire for urology of renal conditions from the NHANES database. Urge incontinence was defined as the leakage of urine or loss of control over urination due to an urge or pressure to urinate within the past 12 months. Participants who answered “Yes” were diagnosed with urge incontinence, while those who answered “No” were considered healthy participants.
Statistical analysis
Statistical analyses were performed using EmpowerStats software (version 4.0) and RStudio software (version 4.3.1), available at http://www.empowerstats.net and http://cloud.r-project.org. Continuous variables with a normal distribution are presented as mean ± standard deviation. Categorical variables are expressed as percentages (%). One-way ANOVA was used to compare continuous variables with a normal distribution among HOMA-IR quartile groups. In contrast, the Kruskal-Wallis H test was used to compare skewed continuous variables. Chi-square analyses were employed to examine categorical variables. Logistic regression models were applied to investigate the association between HOMA-IR and the prevalence of female urge incontinence. Four different models were constructed to analyze the data: Model I unadjusted, Model II adjusted for age, race, and Model III adjusted for age, race, albumin, total calcium levels, osmolality, uric acid, BUN, SCr, vaginal delivery, smoking status, alcohol use, hypertension, marital status, education level and PIR. Model IV adjusted for all variables in Model III plus BMI. The addition of BMI in Model IV was to assess the extent to which the observed association was independent of overall adiposity. HOMA-IR were further divided into quartiles to apply trend tests and analyze the linear trend of the association between IR and prevalence of female UUI. The consistency of associations between these subgroups was verified through interaction tests. In addition, the smooth curve fitting models used in our study were based on generalized additive models (GAMs), which are non-parametric regression techniques designed to capture potential linear relationships.
Result
Baseline characteristics of the population
Following the exclusion of ineligible participants, the final analysis included 2,961 participants (Table 1). And 28.20% of the participants in this study suffered from UUI. Eligible participants were grouped into four quartiles of HOMA-IR: Q1 (0.99 ± 0.29), Q2 (1.82 ± 0.25), Q3 (2.95 ± 0.45), and Q4 (7.01 ± 4.38). The prevalence of UUI in the study population trended upward from Q1 to Q4. Participants in Group Q4 had poorer economic conditions and higher rates of obesity. They were more likely to have hypertension and less likely to drink alcohol. Participants in group Q4 had higher uric acid and osmolality levels, as well as lower albumin levels. Participants in Group Q4 had lower levels of education, and a higher proportion lived alone. Except for age, BUN, SCr, total calcium levels, and marital status, all variables showed significant differences among the four groups (P < 0.05).
Table 1.
Baseline characteristics for the participants
| HOMA-IR quartile | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| N | 733 | 734 | 750 | 744 | |
| HOMA-IR | 0.99 ± 0.29 | 1.82 ± 0.25 | 2.95 ± 0.45 | 7.01 ± 4.38 | < 0.001 |
| Age (years) | 48.43 ± 17.32 | 49.05 ± 16.99 | 49.99 ± 17.48 | 49.28 ± 17.12 | 0.378 |
| Albumin(g/L) | 41.60 ± 3.70 | 41.25 ± 3.50 | 40.89 ± 3.57 | 40.07 ± 3.56 | < 0.001 |
| BUN(mmol/L) | 4.12 ± 1.77 | 4.29 ± 2.24 | 4.24 ± 1.83 | 4.20 ± 1.75 | 0.346 |
| SCr(umol/L) | 68.31 ± 29.79 | 68.01 ± 23.44 | 66.66 ± 16.67 | 67.58 ± 17.92 | 0.518 |
| Total calcium (mmol/L) | 2.33 ± 0.09 | 2.33 ± 0.09 | 2.34 ± 0.09 | 2.33 ± 0.09 | 0.344 |
| Uric acid(umol/L) | 261.17 ± 63.05 | 276.48 ± 67.66 | 291.30 ± 71.11 | 324.69 ± 82.60 | < 0.001 |
| Osmolality(mmol/kg) | 276.12 ± 5.06 | 277.22 ± 4.94 | 277.15 ± 5.01 | 277.33 ± 5.08 | < 0.001 |
| Race (%) | < 0.001 | ||||
| Mexican American | 85 (11.60%) | 98 (13.35%) | 144 (19.20%) | 151 (20.30%) | |
| Other Hispanic | 39 (5.32%) | 84 (11.44%) | 75 (10.00%) | 68 (9.14%) | |
| Non-Hispanic White | 450 (61.39%) | 375 (51.09%) | 343 (45.73%) | 298 (40.05%) | |
| Non-Hispanic Black | 107 (14.60%) | 128 (17.44%) | 155 (20.67%) | 188 (25.27%) | |
| Other Race | 52 (7.09%) | 49 (6.68%) | 33 (4.40%) | 39 (5.24%) | |
| Education level (%) | < 0.001 | ||||
| < high school graduate | 147 (20.05%) | 173 (23.57%) | 198 (26.40%) | 229 (30.78%) | |
| High school graduate | 148 (20.19%) | 168 (22.89%) | 177 (23.60%) | 181 (24.33%) | |
| >high school graduate | 438 (59.75%) | 393 (53.54%) | 375 (50.00%) | 334 (44.89%) | |
| Marital status (%) | 0.055 | ||||
| Married or living with a partner | 478 (65.21%) | 455 (61.99%) | 453 (60.40%) | 435 (58.47%) | |
| Living alone | 255 (34.79%) | 279 (38.01%) | 297 (39.60%) | 309 (41.53%) | |
| PIR (%) | < 0.001 | ||||
| ≤ 1 | 134 (18.28%) | 127 (17.30%) | 176 (23.47%) | 206 (27.69%) | |
| >1 and ≤ 4 | 366 (49.93%) | 392 (53.41%) | 415 (55.33%) | 407 (54.70%) | |
| >4 | 233 (31.79%) | 215 (29.29%) | 159 (21.20%) | 131 (17.61%) | |
| BMI (%) | < 0.001 | ||||
| ≤ 25 | 473 (64.53%) | 278 (37.87%) | 142 (18.93%) | 47 (6.32%) | |
| >25 and ≤ 30 | 204 (27.83%) | 268 (36.51%) | 273 (36.40%) | 172 (23.12%) | |
| >30 | 56 (7.64%) | 188 (25.61%) | 335 (44.67%) | 525 (70.56%) | |
| Hypertension (%) | < 0.001 | ||||
| Yes | 143 (19.51%) | 220 (29.97%) | 272 (36.27%) | 336 (45.16%) | |
| No | 590 (80.49%) | 514 (70.03%) | 478 (63.73%) | 408 (54.84%) | |
| Smoke (%) | 0.022 | ||||
| Yes | 327 (44.61%) | 272 (37.06%) | 293 (39.07%) | 292 (39.25%) | |
| No | 406 (55.39%) | 462 (62.94%) | 457 (60.93%) | 452 (60.75%) | |
| Alcohol use (%) | < 0.001 | ||||
| Yes | 508 (69.30%) | 469 (63.90%) | 450 (60.00%) | 397 (53.36%) | |
| No | 225 (30.70%) | 265 (36.10%) | 300 (40.00%) | 347 (46.64%) | |
| Vaginal delivery (%) | 0.033 | ||||
| ≤ 2 | 484 (66.03%) | 471 (64.17%) | 461 (61.47%) | 466 (62.63%) | |
| >2 and ≤ 4 | 205 (27.97%) | 201 (27.38%) | 207 (27.60%) | 201 (27.02%) | |
| >4 | 44 (6.00%) | 62 (8.45%) | 82 (10.93%) | 77 (10.35%) | |
| Urge urinary incontinence (%) | < 0.001 | ||||
| Yes | 165 (22.51%) | 200 (27.25%) | 223 (29.73%) | 248 (33.33%) | |
| No | 568 (77.49%) | 534 (72.75%) | 527 (70.27%) | 496 (66.67%) |
Normally distributed continuous variables are shown as Mean ± SD. Categorical variables are displayed as percentages (%). The Kruskal-Wallis test was used to determine P-values for constant variables. Fisher’s exact test computed P-values for categorical variables with expected frequencies of less than 10
BUN blood urea nitrogen, SCr serum creatinine, PIR poverty income ratio
Association of HOMA-IR with UUI
HOMA-IR and HOMA-IR quartiles were positively associated with the prevalence of UUI in both unadjusted and partially adjusted models (Table 2). After adjusting for some covariates, the risk of UUI was independently increased by 3% for each 1-unit increase in HOMA-IR (Model III, OR = 1.03,95% CI: 1.00, 1.06, P = 0.0264). In addition, the Q4 cohort was 49% more likely to experience UUI than the Q1 cohort (Model III, OR = 1.49, 95% CI: 1.14–1.94, P = 0.0034). However, when BMI was further added to the fully adjusted model (Model IV), the association between HOMA-IR and the prevalence of UUI was substantially attenuated. It was no longer statistically significant (Model IV, OR = 1.01, 95% CI: 0.98, 1.04, P = 0.4243) (Table 3). Similarly, the increased risk observed in the highest HOMA-IR quartile (Q4) was also attenuated after adjusting for BMI (Model IV, OR = 1.14, 95% CI: 0.85–1.53, P = 0.3666).
Table 2.
Odds ratios accompanied by 95% confidence intervals for HOMA-IR and the prevalence of UUI
| Urge urinary incontinence | Model Ⅰ | Model Ⅱ | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| HOMA-IR | 1.04 | (1.02, 1.07) | 0.0008 | 1.04 | (1.02, 1.07) | 0.0008 |
| HOMA-IR | ||||||
| Q1 | Reference | Reference | ||||
| Q2 | 1.29 | (1.02, 1.64) | 0.0361 | 1.26 | (0.98, 1.61) | 0.0699 |
| Q3 | 1.46 | (1.15, 1.84) | 0.0016 | 1.36 | (1.07, 1.74) | 0.0124 |
| Q4 | 1.72 | (1.37, 2.17) | < 0.0001 | 1.66 | (1.30, 2.11) | < 0.0001 |
Multiple regression equations for the four models were developed
Model I unadjusted
Model II adjusted for age and race
Table 3.
Odds ratios accompanied by 95% confidence intervals for HOMA-IR and the prevalence of UUI
| Urge urinary incontinence | Model Ⅲ | Model Ⅳ | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| HOMA-IR | 1.03 | (1.00, 1.06) | 0.0264 | 1.01 | (0.98, 1.04) | 0.4243 |
| HOMA-IR | ||||||
| Q1 | Reference | Reference | ||||
| Q2 | 1.21 | (0.94, 1.56) | 0.1367 | 1.09 | (0.84, 1.41) | 0.5287 |
| Q3 | 1.29 | (1.00, 1.65) | 0.0507 | 1.06 | (0.81, 1.39) | 0.6530 |
| Q4 | 1.49 | (1.14, 1.94) | 0.0034 | 1.14 | (0.85, 1.53) | 0.3666 |
Model III adjusted for age, race, albumin, total calcium levels, osmolality, uric acid, BUN, SCr, vaginal delivery, smoking status, alcohol use, hypertension, marital status, education level, and PIR
Model IV adjusted for all variables in Model III plus BMI
BUN blood urea nitrogen, SCr serum creatinine, PIR poverty income ratio, OR odds ratio, CI confidence interval, BMI body mass index
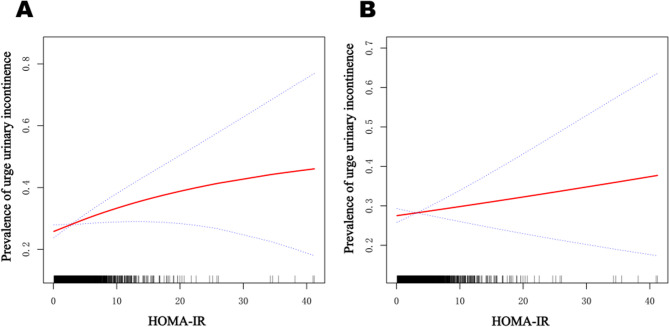
Smooth curve fitting of HOMA-IR with the prevalence of UUI
As shown in Fig. 2 A, the smoothed curves indicate a significant positive linear association between HOMA-IR and the prevalence of UUI. In Fig.2 B, after adjusting for the covariate BMI, the positive linear correlation between HOMA-IR and the prevalence of UUI weakened.
Fig. 2.
Smooth curve fitting of HOMA-IR with the prevalence of UUI. Smooth curve fitting of HOMA-IR with the prevalence of UUI. A adjusted for age, race, albumin, total calcium levels, osmolality, uric acid, BUN, SCr, vaginal delivery, smoking status, alcohol use, hypertension, marital status, education level, and PIR. B adjusted for all variables in Figure 2 A plus BMI. The red line represents the odds ratio, while the blue line denotes the 95% confidence interval range. Abbreviation: BUN: blood urea nitrogen, SCr: serum creatinine, PIR: poverty income ratio, BMI: body mass index
Subgroup analysis of the correlation between HOMA-IR and UUI
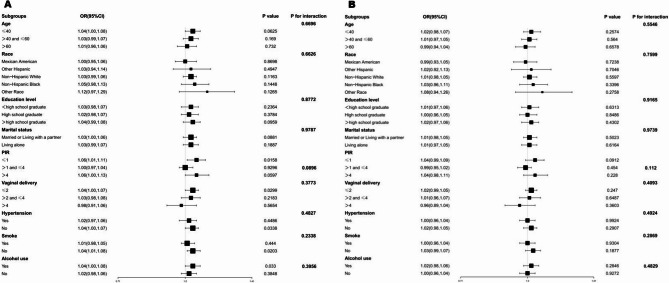
Subgroup analyses and interaction tests were performed using the Model III framework without BMI adjustment to explore whether the observed association varied across different population strata (Fig. 3 A). The positive association was consistent across all subgroups, and no significant interaction effects were detected. In contrast, when the subgroup analysis was repeated with full adjustment for BMI in addition to all other covariates (Fig. 3 B), the association between HOMA-IR and UUI was consistently attenuated across all subgroups and lost statistical significance. No significant interaction effects were observed in the BMI-adjusted model (P for interaction > 0.05).
Fig. 3.
Subgroup analysis of the correlation between HOMA-IR and UUI. These analyses explored whether the association between HOMA-IR and UUI varied across specific factors, including age, race, vaginal delivery, smoking status, alcohol use, hypertension, PIR, education level, and marital status. Abbreviations: PIR: poverty income ratio; OR: odds ratio; CI: confidence interval
Discussion
This cross-sectional study analyzed data from 2,961 adult women in the National Health and Nutrition Examination Survey (NHANES) database between 2005 and 2016 to investigate the association between IR and UUI in women. Our study found that while a significant association between HOMA-IR and UUI was observed in initial models, this association was completely attenuated and became non-significant after accounting for BMI. This key finding suggests that the apparent link between IR and UUI is not independent but is largely explained by the confounding effect of obesity. Obesity is widely recognized as the core driver and primary modifiable risk factor for IR. Obesity is characterized by a chronic low-grade inflammatory state, which directly triggers the onset and progression of IR [23]. Obesity-induced inflammation typically originates in adipose tissue, leading to impaired insulin signaling through the promotion of adipocyte dysfunction and systemic metabolic imbalance [24]. Obesity increases lipolysis, leading to elevated plasma free fatty acid levels, which in turn interfere with insulin action in peripheral tissues [25]. Additionally, in an obese state, adipose tissue dysfunction manifests as adipocyte hypertrophy, macrophage infiltration, and persistent low-grade inflammation, characterized by the secretion of pro-inflammatory factors such as TNF-α and IL-6, which disrupt insulin signaling pathways and contribute to the development of IR [26–28]. Clinically, the causal association between obesity and IR has been confirmed by numerous studies, and obesity is considered the primary underlying driver of the global prevalence of T2DM [29]. Especially in children and adolescents, obesity is not only the primary modifiable risk factor for IR but also significantly increases the risk of cardiovascular disease and T2DM in adulthood [30]. Obesity is also a risk factor for UUI. The sustained increase in intra-abdominal pressure in obese patients is an important pathological basis for the onset of urinary incontinence [31]. This sustained pressure exerts a downward force on the bladder, reducing its functional capacity and compliance, which leads to a strong urge to urinate even when the bladder contains only a small amount of urine [32]. Furthermore, long-term pressure can affect the pelvic floor support structure, causing excessive stretching and weakening of the pelvic floor muscles and connective tissue, and potentially damaging the pelvic floor nerves that control the bladder [32]. Hormonal changes also play a key role in obesity-related UUI. As an endocrine organ, adipose tissue secretes adipokines such as leptin, which may influence bladder function through neuroendocrine pathways. Leptin can stimulate sympathetic nervous system activity, which may directly increase the excitability and involuntary contractions of the detrusor muscle in the bladder [33]. The most important pathogenic mechanism is the systemic inflammation caused by obesity. Obesity is associated with low-grade systemic inflammation and the release of pro-inflammatory cytokines. These inflammatory mediators may directly damage the neurovascular structure of the pelvic floor by producing reactive oxygen species [10, 34]. Studies have shown that UUI patients experience more severe storage symptoms and a decline in quality of life, which is consistent with inflammation-induced bladder dysfunction [10]. Based on the findings of this study and an analysis of existing literature, the association between IR and UUI warrants a re-examination of the confounding effects of obesity. Multiple studies have shown that IR is positively correlated with the severity of UI, particularly in female patients with T2DM, where elevated HOMA-IR indices are significantly associated with UI prevalence [35]. However, the populations in these studies generally have high obesity rates, and obesity itself has been established as an independent risk factor for UI[36–38]. For example, obese women have twice the risk of UUI compared to women of normal weight, and weight loss can significantly improve UI symptoms [37, 38]. In diabetic populations, the association between IR and UI may be overestimated, as T2DM patients often have obesity [35, 39]. Another cross-sectional study involving 17,474 US participants assessed IR using the metabolic score for insulin resistance(METS-IR) and found a relationship between IR and UI [40]. However, the METS-IR is an indicator composed of BMI, and obesity may play a major role in the link between the METS-IR and UI. Additionally, this study found that the association between IR and UUI in non-diabetic populations was also mediated by obesity. Furthermore, even in postmenopausal women, the association between IR indicators such as the triglyceride-glucose index and SUI may be partially mediated by obesity [41]. Our study found that obesity is a more critical modifiable risk factor for UUI in non-diabetic women. Obesity is a key risk factor and contributing mechanism for IR, directly leading to the onset and progression of UI. Obesity increases the prevalence of UI, including SUI and UUI, which may result from the combined effects of mechanical and metabolic factors [42–44]. Studies have shown that weight loss is positively correlated with improvements in UI symptoms, and weight reduction can significantly reduce the frequency of UI episodes, thereby improving patients'quality of life [37, 45, 46]. Specifically, behavioral weight management and low-calorie diets are recommended as first-line treatment strategies, particularly for obese patients with UI, as weight management can effectively reduce the overall risk of UI, not just targeting IR [46–48]. The guidelines also recommend that patients with a BMI of 30 kg/m² or greater prioritize weight loss interventions before considering surgery for UI [47]. Treatment should shift from targeting IR to comprehensive weight management. Weight management may not only indirectly alleviate IR issues but also more comprehensively address the multifactorial mechanisms of obesity-related UI. The strength of this study lies in its use of the NHANES database, which provides a representative and diverse sample of individuals in the United States across various age groups, genders, racial backgrounds, and socioeconomic statuses. Participants were selected through random sampling, ensuring a diverse sample that accurately reflects the overall health of the U.S. population. However, we must also recognize some limitations. Given the investigation's cross-sectional design, it was impossible to establish a causal link between insulin resistance and female urge urinary incontinence. This study relies on personal questionnaires to diagnose UI, which may be subject to recall bias. Furthermore, the NHANES questionnaire did not specify the frequency or severity of UUI episodes. The diagnostic criteria for UUI did not explicitly exclude other potential causes, such as urinary tract infections or neurological disorders, which may share similar symptoms. This could have led to the inclusion of cases with underlying conditions confounding the pure UUI population. Our case definition was based on patients'self-reported symptoms of urinary urgency accompanied by UI. This definition may have inadvertently included patients with MUI in the study, rather than those with purely UUI. Some relevant confounders were adjusted; residual confounders may still be present.
Acknowledgements
The authors thank the investigators and participants of the National Health and Nutrition Examination Survey, the parent study, who made this report possible.
Abbreviations
- UUI
Urge urinary incontinence
- MUI
Mixed urinary incontinence
- SUI
Stress urinary incontinence
- NHANES
National health and nutrition examination survey
- HOMA-IR
Homeostatic model assessment of insulin resistance
- PIR
Poverty income ratio
- BUN
Blood urea nitrogen
- SCr
Serum creatinine
- OR
Odds ratio
- CI
Confidence interval
- T2DM
Type 2 diabetes mellitus
- METS-IR
Metabolic score for insulin resistance
Authors’ contributions
Shuxin Li, Tong Yang, and Xin Lian wrote the main manuscript text, and E.F. prepared Figs. 1, 2 and 3. All authors reviewed the manuscript.
Funding
WU JIEPING Medical Foundation (NO.3D4240299428).
Data availability
The data used in this research are publicly available at https://www.cdc.gov/nchs/nhanes.
Declarations
Ethics approval and consent to participate
This study utilized de-identified, publicly available data from the National Health and Nutrition Examination Survey, which was conducted by the Centers for Disease Control and Prevention. The National Center approved the NHANES protocol for the Health Statistics Research Ethics Review Board. All participants provided written informed consent. Detailed approval information is available on the NHANES website: https://www.cdc.gov/nchs/nhanes/irba98.htm.
Conflict of interest
The authors declared no conflicts of interest.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuxin Li and Tong Yang are co-first authors who contributed equally to this work.
References
- 1.Aoki Y, Brown HW, Brubaker L, Cornu JN, Daly JO, Cartwright R. Urinary incontinence in women. Nat Rev Dis Primers. 2017;3:17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferson FA, Linder BJ. Evaluation and management of female stress urinary incontinence. Mayo Clin Proc. 2024;99(11):1802–14. [DOI] [PubMed] [Google Scholar]
- 3.Okada C, Kim JI, Roselli N, Kadam Halani P, Melamed ML, Abraham N. Food insecurity is associated with urge urinary incontinence: an analysis of the 2005–2010 National Health and Nutrition Examination Survey. J Urol. 2023;210(3):481–91. [DOI] [PubMed] [Google Scholar]
- 4.Naidu D, Modi R, Kulkarni M, Gunasaegaram V, Rolnik DL, Freites J, Vereeck S, Pendharkar G, Webb J, Rosamilia A et al. Intravaginal electrical stimulation of the pelvic floor for women with urinary incontinence - a systematic review of randomised controlled trials. Am J Obstet Gynecol. 2025:S0002–9378(25):00163–2. [DOI] [PubMed]
- 5.Rolland TJ, Peterson TE, Singh RD, Rizzo SA, Boroumand S, Shi A, et al. Exosome biopotentiated hydrogel restores damaged skeletal muscle in a Porcine model of stress urinary incontinence. NPJ Regen Med. 2022;7(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlögl M, Gordon A. Hearts, minds and souls-it is time for geriatricians to bring more to continence management. Age Ageing. 2021;50(5):1508–11. [DOI] [PubMed] [Google Scholar]
- 7.Schlögl M, Umbehr MH, Habib MH, Wagg A, Gordon AL, Harwood R. Promoting continence in older people. Age Ageing. 2022. 10.1093/ageing/afac199. [DOI] [PubMed] [Google Scholar]
- 8.Akbar A, Liu K, Michos ED, Brubaker L, Markossian T, Bancks MP, et al. Racial differences in urinary incontinence prevalence and associated bother: the multi-ethnic study of atherosclerosis. Am J Obstet Gynecol. 2021;224(1):80.e81-80.e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui NY, Wiseman JB, Cella D, Bradley CS, Lai HH, Helmuth ME, et al. Mental health, sleep and physical function in treatment seeking women with urinary incontinence. J Urol. 2018;200(4):848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai HH, Wiseman JB, Helmuth ME, Smith AR, Amundsen CL, Cameron AP, Glaser AP, Hendrickson WK, Kirkali Z, Kenton K. Phenotyping of urinary urgency patients without urgency incontinence, and their comparison to urgency incontinence patients: findings from the LURN study. J Urol. 2023;209(1):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women: a review. JAMA. 2017;318(16):1592–604. [DOI] [PubMed] [Google Scholar]
- 12.Bohlin KS, Ankardal M, Lindkvist H, Milsom I. Factors influencing the incidence and remission of urinary incontinence after hysterectomy. Am J Obstet Gynecol. 2017;216(1):e5351-9. [DOI] [PubMed] [Google Scholar]
- 13.Gan ZS, Sundaram K, Smith AL. Factors associated with urinary incontinence in nulliparous female elite athletes: an exploratory, cross-sectional study using dynamic pelvic magnetic resonance imaging and questionnaire data. J Urol. 2025. 10.1097/JU.0000000000004455. [DOI] [PubMed] [Google Scholar]
- 14.Thomas-White KJ, Kliethermes S, Rickey L, Lukacz ES, Richter HE, Moalli P, Zimmern P, Norton P, Kusek JW, Wolfe AJ, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol. 2017;216(1):55.e51-55.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamerton TJ, Mielke GI, Brown WJ. Urinary incontinence, body mass index, and physical activity in young women. Am J Obstet Gynecol. 2021;225(2):e164161-164113. [DOI] [PubMed] [Google Scholar]
- 16.James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021;22(11):751–71. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-García A, Torrecilla-Parra M, Fernández-de Frutos M, Martín-Martín Y, Pardo-Marqués V, Ramírez CM. Posttranscriptional regulation of insulin resistance: implications for metabolic diseases. Biomolecules. 2022;12(2):208. [DOI] [PMC free article] [PubMed]
- 18.da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8(19):e14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Las Heras N, Lahera V. Relevance of mitochondrial dysfunction in heart disease associated with insulin resistance conditions. Pflugers Arch. 2022;474(1):21–31. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Yan H, Jin A, Meng X, Lin J, Li H, Wang Y, Pan Y. Adipose tissue specific insulin resistance and prognosis of nondiabetic patients with ischemic stroke. Diabetol Metab Syndr. 2023;15(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramasubbu K, Devi Rajeswari V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced ages on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem. 2023;478(6):1307–24. [DOI] [PubMed] [Google Scholar]
- 22.Uehara K, Santoleri D, Whitlock AEG, Titchenell PM. Insulin regulation of hepatic lipid homeostasis. Compr Physiol. 2023;13(3):4785–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue J, Gao J, Gu Y, Wang A, Yu S, Li B, Yin Y, Wang J, Su W, Zhang H, et al. Human umbilical cord-derived mesenchymal stem cells alleviate insulin resistance in diet-induced obese mice via an interaction with splenocytes. Stem Cell Res Ther. 2022;13(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priyanka A, Sindhu G, Shyni GL, Preetha Rani MR, Nisha VM, Raghu KG. Bilobalide abates inflammation, insulin resistance and secretion of angiogenic factors induced by hypoxia in 3T3-L1 adipocytes by controlling NF-κB and JNK activation. Int Immunopharmacol. 2017;42:209–17. [DOI] [PubMed] [Google Scholar]
- 25.Kimura T, Pydi SP, Wang L, Haspula D, Cui Y, Lu H, et al. Adipocyte G(q) signaling is a regulator of glucose and lipid homeostasis in mice. Nat Commun. 2022;13(1):1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojta I, Chacińska M, Błachnio-Zabielska A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020. 10.3390/nu12051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Tang Y, Luo Y, Gao Y, He L. Role and mechanism of specialized pro-resolving mediators in obesity-associated insulin resistance. Lipids Health Dis. 2024;23(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta AP, Syed AA, Garg R, Goand UK, Singh P, Riyazuddin M, et al. Pancreastatin inhibitor PSTi8 attenuates hyperinsulinemia induced obesity and inflammation mediated insulin resistance via MAPK/NOX3-JNK pathway. Eur J Pharmacol. 2019;864:172723. [DOI] [PubMed] [Google Scholar]
- 29.Vu J, Ying W. Isolation and analysis of stromal vascular cells from visceral adipose tissue. Bio-protocol. 2017;7(16):e2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagi VM, Chiarelli F. Obesity and insulin resistance in children. Curr Opin Pediatr. 2020;32(4):582–8. [DOI] [PubMed] [Google Scholar]
- 31.Durigon Keller K, La Rosa VL, Cerentini TM, Machado de Souza C, Langlois Costa F, da Rosa P, da Silva Klahr P, de Almeida Pereira E. Quality of Life and Urinary Incontinence Symptoms in Women Undergoing Bariatric Surgery: A Combined Case-Cohort Study. Female Pelvic Med Reconstr Surg. 2020;26(11):e62–7. [DOI] [PubMed] [Google Scholar]
- 32.Pan J, Liang E, Cai Q, Zhang D, Wang J, Feng Y, Yang X, Yang Y, Tian W, Quan C, et al. Progress in studies on pathological changes and future treatment strategies of obesity-associated female stress urinary incontinence: a narrative review. Transl Androl Urol. 2021;10(1):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milsom I. M Gyhagen 2023 Does the climacteric influence the prevalence, incidence and type of urinary incontinence? Climacteric 26 2 75–9. [DOI] [PubMed] [Google Scholar]
- 34.Soriano A, Andy U, Hassani D, Whitmore K, Harvie H, Malykhina AP, et al. Relationship of bladder pain with clinical and urinary markers of neuroinflammation in women with urinary urgency without urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27(2):e418-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Zou J, Wang Z, Wang M, Yuan Y, Lv H. Correlation between insulin resistance and urinary incontinence in female patients with type 2 diabetes mellitus. Int Urogynecol J. 2024;35(2):431–40. [DOI] [PubMed] [Google Scholar]
- 36.Abufaraj M, Xu T, Cao C, Siyam A, Isleem U, Massad A, Soria F, Shariat SF, Sutcliffe S, Yang L. Prevalence and trends in urinary incontinence among women in the united states, 2005–2018. Am J Obstet Gynecol. 2021;225(2):e166161-166112. [DOI] [PubMed] [Google Scholar]
- 37.Purwar B, Cartwright R, Cavalcanti G, Digesu GA, Fernando R, Khullar V. The impact of bariatric surgery on urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2019;30(8):1225–37. [DOI] [PubMed] [Google Scholar]
- 38.Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev. 2018;19(12):1735–45. [DOI] [PubMed] [Google Scholar]
- 39.Fwu CW, Schulman IH, Lawrence JM, Kimmel PL, Eggers P, Norton J, Chan K, Mendley SR, Barthold JS. Association of obesity, metabolic syndrome, and diabetes with urinary incontinence and chronic kidney disease: analysis of the National health and nutrition examination survey, 2003–2020. J Urol. 2024;211(1):124–33. [DOI] [PubMed] [Google Scholar]
- 40.Cao S, Meng L, Lin L, Hu X, Li X. The association between the metabolic score for insulin resistance (METS-IR) index and urinary incontinence in the United States: results from the national health and nutrition examination survey (NHANES) 2001–2018. Diabetol Metab Syndr. 2023;15(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Hu W, Li L. Association between triglyceride-glucose index and its correlation indexes and stress urinary incontinence in postmenopausal women: evidence from NHANES 2005–2018. Lipids Health Dis. 2024;23(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Body mass index, abdominal fatness, weight gain and the risk of urinary incontinence: a systematic review and dose-response meta-analysis of prospective studies. BJOG. 2019;126(12):1424–33. [DOI] [PubMed] [Google Scholar]
- 43.Marcelissen T, Anding R, Averbeck M, Hanna-Mitchell A, Rahnama’i S, Cardozo L. Exploring the relation between obesity and urinary incontinence: pathophysiology, clinical implications, and the effect of weight reduction, ICI-RS 2018. Neurourol Urodyn. 2019;38(Suppl 5):S18-24. [DOI] [PubMed] [Google Scholar]
- 44.Fuselier A, Hanberry J, Margaret Lovin J, Gomelsky A. Obesity and stress urinary incontinence: impact on pathophysiology and treatment. Curr Urol Rep. 2018;19(1):10. [DOI] [PubMed] [Google Scholar]
- 45.Yazdany T, Jakus-Waldman S, Jeppson PC, Schimpf MO, Yurteri-Kaplan LA, Ferzandi TR, Weber-LeBrun E, Knoepp L, Mamik M, Viswanathan M, et al. American urogynecologic society systematic review: the impact of weight loss intervention on lower urinary tract symptoms and urinary incontinence in overweight and obese women. Female Pelvic Med Reconstr Surg. 2020;26(1):16–29. [DOI] [PubMed] [Google Scholar]
- 46.Persu C, Cartas RN, Ciofu I, Mastalier B, Cauni VM. Is surgical treatment for obesity able to cure urinary incontinence in women?-a prospective single-center study. Life (Basel). 2023. 10.3390/life13091897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheridan W, Da Silva AS, Leca BM, Ostarijas E, Patel AG, Aylwin SJ, et al. Weight loss with bariatric surgery or behaviour modification and the impact on female obesity-related urine incontinence: a comprehensive systematic review and meta-analysis. Clin Obes. 2021;11(4):e12450. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Chen H, Bai Y, Zhang T, Bai W, Jiang B. Ketogenic diet may be a new approach to treatment stress urinary incontinence in obese elderly women: report of five cases. BMC Womens Health. 2022;22(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this research are publicly available at https://www.cdc.gov/nchs/nhanes.