Abstract
Significance:
Skin lipids are essential for various skin functions including maintaining barrier integrity, regulating hydration, and providing protection against microbes and inflammatory irritants. Along with skin health, the role of lipids in the etiology of macroangiopathic diseases, such as atherosclerosis of arteries, is well recognized.
Recent Advances:
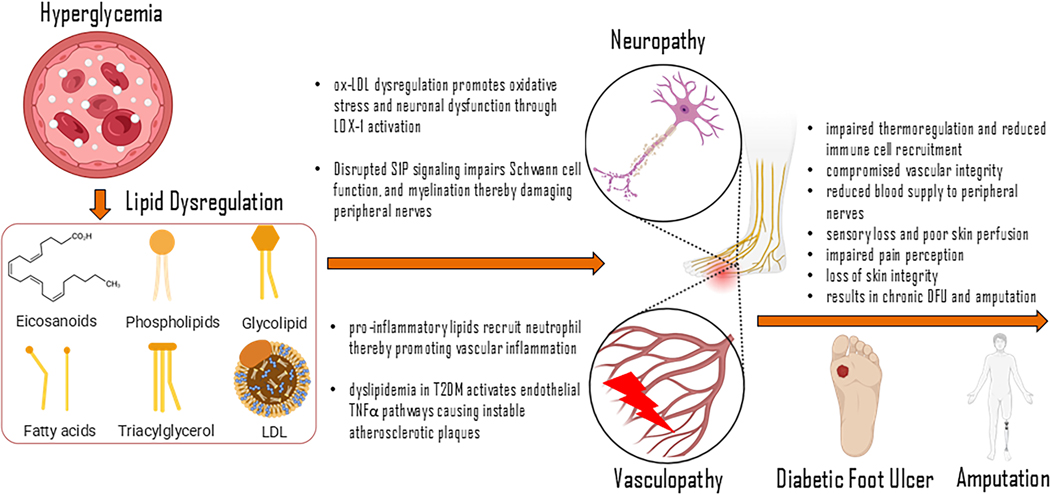
In diabetes, lipid dysregulation is evident and may contribute to the diverse complications of the disease. Diabetic vasculopathy primarily reflects the dysfunction and deterioration of existing blood vessels, as their preservation is key in preventing the progression of vascular disease and reducing the need for compensatory angiogenesis. In the peripheral diabetic skin of the limbs, diabetic vasculopathy runs alongside peripheral neuropathy. Although a causative link between the two is plausible, direct evidence in support of such claim is scanty.
Critical Issues:
Diabetic skin is known to be compromised in many ways, including weakened barrier functionality and diabetes-induced alterations in the extracellular matrix, likely stemming from chronic inflammation, which may directly affect vascular integrity and nerve health. Both, in the compromised skin and within wounds, microbial pathogens and their enzymes may metabolize host lipids, driving inflammatory reactions and exacerbating the pathogenesis of diabetic vasculopathy and related neuropathy.
Future Directions:
This review focuses on lipid mediators such as sphingolipids, resolvins, oxidized LDLs and their specific downstream signaling pathways to obtain a comprehensive understanding of diabetic complications relevant to wound healing. Through lipid-based strategies, this review hopes to inspire the development and utilization of individualized, precision-based approaches to manage diabetic vasculopathy and neuropathy.
1.0. BACKGROUND
Diabetes and the sequelae of its associated complications are some of the largest contributors to human mortality and morbidity in the Western world. For decades, the associated molecular cascades have been explored resulting in a highly intricate pathophysiology that ranges from micro-complications to macro-organ dysfunction. In particular, the neuropathic and vasculogenic derangements of diabetes are an area of interest due to the gamut of diseases they represent. Broadly termed vasculopathy, the disease processes of the blood vessels in the setting of diabetes are the underpinnings for many of the prototypical complications of diabetes. One of the significant complications of diabetic vasculopathy is the impairment of wound healing which tremendously reduces the quality of life of the affected patient. Most commonly, uncontrolled diabetes creates poor angiogenic conditions that result in nonhealing diabetic foot ulcers (DFU). These chronic wounds are characterized by impaired healing, influenced by a complex interplay of factors, including dysregulated inflammation, insufficient blood flow, and altered cellular functioning. Additionally, the compounding effects of diabetic neuropathy – a condition distinguished by progressive damage to sensory, motor, and autonomic nerves in patients with diabetes – further complicates wound healing and the potential of DFU formation1. In the United States, approximately 60–70% of patients with diabetes eventually suffer from diabetic neuropathy2. With damaged and entrapped nerves in the lower extremities, DFUs are more likely to form due to dysfunctions in protective mechanisms against wound development, such as impaired pain perception and general loss of skin integrity2. Conservative estimates place the prevalence of DFU at 6.3% globally3. A feared sequelae of DFU involves amputation, with an estimated 20% of patients with DFU eventually requiring amputation4. As impaired angiogenesis progresses, the wound environment becomes increasingly ischemic and hypoxic, resulting in chronic wound formation and necrosis4. This necrotic and persistent inflammatory state fosters a breeding ground for bacteria, causing severe tissue damage and ultimately leading to amputation4. Amputation has profound effects on quality of life, including increased total risk of morbidity and mortality, underscoring the necessity of its prevention5. While previous work from our laboratory has focused on the molecular cascade of impaired diabetic wounds, the emphasis has been on epigenetic modifications6–9. This previous focus aligned well with the paradigm that diabetes progresses as an extrinsic disease process caused by poor lifestyle6. However, there is an extensive need to examine the detailed pathophysiology of diabetic vasculopathy and neuropathy as it stands from a cellular cascade. In particular, the role of lipids, including skin lipids, and their molecular regulators has been recently investigated and shown to have great potential in not only the understanding of diabetic wounds and the impact of neuropathy and vasculopathy, but also in the therapeutic intervention of the former10–12.
2.0. Diabetes and its Complications
The preponderance of diabetes and its neuropathic and vascular complications cannot be understated. It is important to contextualize these disorders in comparison to the effect that diabetes has on the world and particularly the western hemisphere. According to the National Diabetes Statistics Report of 2020, 30 million people over the age of 18 living with type 2 diabetes mellitus (T2DM), and another 68 million live with the ascribed diagnosis of ‘prediabetes’. Along with the current incidence, the rising prevalence of the disease is most worrisome13. With the exponential increase in the rates of diabetes in younger populations, it is becoming one of the largest detractors of life expectancy in the healthcare system. T2DM is characterized by derangements in insulin responsiveness and glycemic control. Consequently, this creates an environment of chronic inflammation and radical oxidization that harms small and large neurovascular bundles resulting in diabetic neuropathy and vasculopathy. This progression contributes to a wide range of diabetic pathologies, most notably diabetic wounds.
2.1. Diabetic Neuropathy
Destruction of nerves, better known as neuropathy, is prevalent among a majority of patients with diabetes,14 often presenting initially as a symmetric, stocking-glove pattern of sensory loss in the distal extremities15. Diabetic neuropathy largely affects the lower extremities, impairing sensory, autonomic and motor nerve functions, thereby fostering an environment prone to wound formation16. Sensory deficits diminish a patient’s ability to perceive touch, pressure, and pain signals. Over time, unnoticed and minor injuries in the foot accumulate, often progressing to chronic and severe wounds that are challenging to treat17. Autonomic neuropathy also contributes to wound development, causing impairments in vasodilatation and sudomotor functioning, processes essential for skin health and barrier integrity18. Lack of blood flow and diminished sweat production result in dry, cracked skin that is more susceptible to injury and infection19. Dysfunctional thermoregulation and increased arteriovenous shunting further compound the risk of tissue breakdown and ulcer formation by disrupting normal skin perfusion and delaying wound healing20. In motor neuropathy, a process characterized by intrinsic muscle atrophy and tissue misalignment, it can result in anatomical deformities such as clawfoot or Charcot foot. These deformities often result in abnormal pressure points, increasing the risk of ulceration21. Additionally, diabetic peripheral entrapment neuropathy plays a significant role in neuropathic complications, particularly in regions where nerves are prone to compression, such as the tarsal tunnel or the common peroneal nerve at the fibular head22–24. Chronic hyperglycemia-induced connective tissue changes, along with ischemic damage, contribute to nerve entrapment, exacerbating neuropathic pain and functional deficits25–28. Together, these neuropathic conditions heighten the susceptibility to wound formation, stemming from a cycle of repeated injury, compressional ischemia, poor tissue repair, and increased risk for pathology16. Current standard of care (SOC) targets plantar pressure offloading, debridement of ischemic tissue, physiological topical dressings, infection treatment, biofilm suppression, and revascularization when peripheral arterial insufficiency is present. Based on The International Working Group on the Diabetic Foot (IWGDF) guidelines, use of non-removable knee-high devices remains the standard as a first-choice offloading choice29. For topical dressings, IWGDF recently highlights the use of sucrose-octasulfate impregnated dressings to combat non-healing ulcers, in addition to judicious hyperbaric oxygen therapy and sharp debridement30. These recommendations remain the current gold standard DFU treatment. Recent evidence highlights the clinical significance of decompression for peripheral nerve entrapment in fibro-osseous tunnels has been shown in pain relief27, neurophysiologic recovery28, and minimizing DFU recurrence risk26. Autonomic dysfunction is rejuvenated by nerve decompression and can restore skin transcutaneous oxygen pressure (TcPO2) and peripheral arterial flow volume25.
Summary box 1:
Diabetic neuropathy drives DFU formation through sensory loss, foot deformities, and poor skin perfusion
Standard of care includes offloading, infection control, and revascularization
Nerve decompression improves pain scores, nerve functioning, skin oxygenation, and a reduction in ulcer recurrence
2.2. Diabetic Vasculopathy
In diabetic vasculopathy, the loss of angiogenesis creates a burden of chronic ischemia which is directly related to the ability of the body to heal wounds31. However, the effects of chronic diabetes go beyond adjacent vascular structures. The same microcosm of derangements described earlier occurs throughout the body. This can affect vessels of any size, ranging from small tributaries in the lower extremities to large named vessels such as the aorta. As a result, every cutaneous structure is at risk of developing into non-healing wounds due to arterial insufficiency. In the peripheral vessels, this disease process is referred to as Peripheral Arterial Disease (PAD), which most frequently leads to amputation and is associated with high morbidity32,33.
3.0. Overview of Angiogenesis and Vasculogenesis
Angiogenesis, the formation of new blood vessels, is a complex and dynamic process crucial for tissue growth and wound healing. Understanding the intricacies of angiogenesis requires a detailed examination of endothelial cell behavior and vascular maturation mechanisms. Endothelial cells (ECs), the building blocks of blood vessels, arise from specialized progenitor cells during embryonic development34. Within the literature surrounding adult ECs, the focus has been on their role in vasculogenesis. These ECs have been shown to have a phenotypic switch to become tip cells, which is important for guiding the cell toward angiogenic factors. This push is arguably the first formation of an early vascular plexus35. Vasculogenesis, the generation of new vessels, involves the coalescence and assembly of endothelial cells into a primitive vascular plexus. This process is concurrent with angiogenesis, where new vessels sprout from existing ones. Studies have shown the integral role of metabolism in the formation of new vascular structures. Manipulation of the glycolytic and glycogenic pathways promotes the propagation of these new vessels. In particular, the inhibition of apoptosis of these named “tip” cells is directly involved in creating new vascular plexuses35,36. Animal models studying adult angiogenesis confirm this model where these tip cells and “stalk” cells evolve from ECs with appropriate angiogenic factors35,36. These factors, combined with certain metabolic environment, create earliest signs of early vascular plexus and de-novo angiogenesis37. Additionally, endothelial cells exhibit distinct behaviors during angiogenesis, including the formation of podia and filopodia, which extend into avascular spaces to initiate vessel formation. Additionally, regulatory signaling pathways involving vascular endothelial growth factor (VEGF), NOTCH, fibroblast growth factor 2 (FGF2), and others play critical roles in orchestrating endothelial cell behavior and vessel formation38. Vascular maturation is essential for ensuring the proper function and stability of newly formed blood vessels. This process involves intricate steps such as lumen formation, vascular remodeling, and the establishment of vascular identity. Lumen formation, for instance, requires endothelial cells to undergo sprouting, intracellular vacuolation, and vacuole coalescence to create a patent vessel lumen. Vascular identity, including arterial, venous, and lymphatic specification, is regulated by complex gene expression patterns and transcriptional control agents39.
3.1. Molecular Processes of Vasculogenesis and Angiogenesis
The key stages of angiogenesis involve regulatory control over cell proliferation, migration, and vascular permeability, enabling vascular permeation. As described above, VEGF and its dominant receptors, VEGFR1 and VEGFR2,23 have been reported to play an integral role in mediating this process. Studies on inducible hypoxia have shown that VEGF expression, regulated by hypoxia inducible factor (HIF), promotes VEGF signaling and VEGFR1 activation23,24. Additionally, research has identified VEGFR2 as specifically promoting cellular mitogenesis and permeability in the context of angiogenesis23. Together VEGFR1 and VEGFR2 drive angiogenesis through complex cellular cascades that result in the formation of filopodia and vascular permeation. Ongoing studies have also identified other VEGF-associated receptors, such as Flk-1 and Flt-1, as deeply intertwined in the molecular regulation of angiogenesis. These novel receptors are particularly implicated in cases of aberrant neovascularization, like diabetic angiogenesis and retinal neovascularization23.
The underlying pathology of vasculopathy should be placed in the context of normal physiology as it pertains to blood flow. The human vascular system contains a complex and expansive set of vessels that take oxygen, nutrient-rich blood and deliver it to tissues and organs. Simultaneously, these vessels transfer oxidative waste and gas back via venules and lymphatics31. This system is finely refined, and most of the blood vessels of the human body are formed during embryonic development or in other precise instances (such as in the uterus during the menstrual cycle)40. Conversely, in a healthy adult, new angiogenesis or vasculogenesis is rare and is highly specific, refined, and heavily incorporated into the native arterial architecture.
4.0. Lipids in the Vascular and Neural Systems
Lipids are a broad group of organic compounds composed of different types of lipoproteins and include other lipophilic signaling molecules such as fatty acids, eicosanoids, phospholipids and their derivatives, sphingolipids, and isoprenoids41 (Figure 2). Mounting studies have reported that endothelial cell lipid metabolism affects angiogenesis, and the regulatory effects vary due to various lipid categories and structures42–49 (Table 1). A new investigation into membrane phospholipids, in particular lysolipid G protein-coupled receptors (GPCRs), Lysophosphatidic Acid (LPA), and Sphingosine 1-Phosphate (S1P), has expanded our understanding of cellular regulation in endothelial cells during the processes of angiogenesis and vasculogenesis43,50. Understanding the dynamics of endothelial cells and vascular maturation will not only enhance our knowledge of angiogenesis but also lead to the development of therapeutic interventions targeting vascular-related diseases and tissue engineering strategies. The remainder of this section will outline the role that membrane phospholipids play in the regulation of angiogenesis and vascular proliferation. This will further expand on a noticeable dearth in the literature surrounding the role of lipid molecules in the regulation of vascular development.
Figure 2:

Lipids and their metabolites act as signaling molecules and are thus known as ‘bioactive lipids’. Figure demonstrating the variety of compounds that constitute bioactive lipids. (A) ω‐6 (arachidonic acid; AA)‐derived bioactive lipids or eicosanoids. Activation of phospholipase (PLA2) releases AA from the cell membrane phospholipids. AA is metabolized by cyclooxygenases (COX) to prostaglandins (PGs), by cytochrome P-450 (CYP450) to epoxyeicosatetraenoic acids (EETs) and by 5-lipoxygenase (LO) to hydroxyeicosatetraenoic acids (HETEs) (5, 12, 15‐HETEs), leukotrienes and lipoxins. (B) ω‐3‐derived bioactive lipids. PLA2 releases the ω‐3 polyunsaturated acids, Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) from the cell membrane phospholipids. DHA and EPA are converted by CYP450 to 19, 20‐EDP and 17, 18‐EEQ, by COX to PGE3, PGI3, TXA3 and LTB5 and by lipoxygenases to lipoxins, resolvins, neuroprotectins and LTB5. The following original report is credited: Elmasry et al.181
Table 1:
Table enlisting lipid molecules involved in vasculogenesis and angiogenesis in physiology and pathology.
| Lipid Molecules Studied | Physiological/Developmental Condition | Main Findings | Reference |
|---|---|---|---|
| - S1P (SPHINGOSINE 1-PHOSPHPATE)LYSOPHOSPHATIDIC ACID | - S1P & LPA are responsible for activating small GTPases from the Rho family - These GTPases are responsible for vascular stabilization and prevention of hyper-sprouting - LPAs are responsible for nonperfused vascular sprouts; LPA signaling in endothelial cells (ECs) is present in ECs which are in migration or proliferation stages S1P is perfusion dependent and is expressed; commonly expressed in ECs in the integration stage with the endothelium, maturation, and stability |
SP1/LPA interactions with specific G-protein-coupled receptors have effects in the regulation of vascular development, physiology, and cardiovascular diseases | Engelbrecht et al.43 |
| - S1P (SPHINGOSINE 1-PHOSPHPATE) | - S1pr1 suppression led to defects in vascular development, particularly pericardial edema - Excessive filopodial sprouting was observed in zebrafish embryos with S1pr1 knockout - With S1pr1 and S1pr2 double knock-out, the formation of unstable vascular networks was created |
Suppression of S1pr1 and S1pr2 in zebrafish embryos showed significantly reduced vascular vessel development | Mendelson et al.44 |
| - PGI1 - PGE2 |
- Responsible for pro-inflammatory and anti-inflammatory responses - Mediators for LTB4 which attract neutrophils to sites that have inflammatory conditions - Early signalers to procure resolver mediators - Advanced dermal wound healing along with re-epithelization of skin wounds - Rvd1 & Rvd2 can mediate migration of neutrophils which can balance excessive neutrophil actions |
SPMs are resolvers in promoting return to homeostasis. SPMs are responsible for anti-inflammatory mechanisms, and acting as benefactors in microbial clearance | Serhan et al.45 |
| - RESOLVINS: RVD1, RVD2, RVD3, RVD5 | - RvD1, RvD3, and RvD5 can activate GPR 32, GPR32 assists in resolving inflammatory conditions. | Serhan et al.45 | |
| - 13R, 14S-DIHYDROXY-DOCOSAHEXAENOIC ACID (DHA) | - Lipid mediators in human macrophages - Displays anti-inflammatory and pro-resolving actions - Formed by 12-LOX from DHA |
Serhan et al.45 | |
| - LDL - OXIDIZED LDL |
- In reference to T2DM - Oxidized LDL has a high retention rate to attach to the subendothelial layer causing the formation of plaques - Ox-LDL promotes VCAM and ICAM, both of which are cell adhesion molecules which induce plaque formation - Uptake of Ox-LDL by macrophages creates foam cells, which also play a part in plaque formation. |
Diabetic conditions create environments for a higher likelihood of microvascular and macrovascular complications. Exploring the intricate roles of pathological mechanisms in these conditions can lead to therapeutic outcomes | Serhan et al.45 |
| - LDL
- OXIDIZED LDL |
- Hyperglycemia and OxLDL increase TLR4 expression, which contributes to a higher rate of Schwann cell apoptosis. - Caspase 3 dependent apoptosis was increased by oxLDL - TLR4’s hyperactivation leads to Schwann cell injury and neuronal dysfunction |
High LDL levels increased Schwann cell death under hyperglycemic conditions vs, normoglycemic conditions. | Nihei, et al.46 |
| - RESOLVINS: RVD1, RVD2, RVD3, RVD5 | - Involved in signaling reduction of pro-inflammatory cytokines and increase in anti-inflammatory cytokines when nerve damage was treated with lipopolysaccharides - Alleviated mechanical and thermal pain responses - Chemotherapy-induced peripheral neuropathy: In mice found to have reduced M1 macrophage activity and increased IL-10 expression (anti-inflammatory cytokine) - Spinal Nerve Ligation Rat model: Treatment with AT-RvD1 enhanced mechanical/thermal hyperalgesia. Increased activity of microglia and other pro-inflammatory cytokine expression |
Resolvins are key in the reduction of inflammatory and neuropathic pain models by reducing hypersensitivity and regulating inflammatory molecules | Park et al.47 |
| - RVD1 - RVD2 - RVD3 |
- RvD1 helps resolve pain in mice with peripheral inflammation-induced neuropathic pain (PINP), both by direct injection and by modulating immune cells. - RvD1 increases the anti-inflammatory cytokine IL-10 in macrophages and DRGs, which is essential for its pain-relieving effects. Blocking IL-10 or the FPR2 receptor prevents these benefits |
Effects of paclitaxel-induced neuropathic pain (PINP) as a side effect. Evaluation of the effects of RvD1 as a promotor of the resolution of inflammation and chronic pain on PINP and the biological mechanisms in mice |
Su et al.48 |
| - PGE2 | - In neuropathy, PGe2 production is decreased, and this causes immune system dysregulation and increased inflammation. - Acts on peripheral neurons and CNS which guide pain sensation |
How eicosanoids are involved in the inflammatory processes linked to DM. Breakdown of lipid mediators and their functions in diabetic complications | Tessaro et al.49 |
Endothelial S1P is a lysophospholipid that controls cell migration, angiogenesis, and vascular barrier function44. The plasma pool of S1P plays important physiological role by regulating lymphocyte trafficking, endothelial barrier function, vascular tone, and limiting the disruption of vascular endothelial monolayers51,52. S1P binds to and activates endothelial S1PR1 during embryonic vascular development and maturation53. In human umbilical vein endothelial cells (HUVECs), S1P activates Ras-related protein 1 (Rap1) similarly to VEGF-A to control Akt/eNOS phosphorylation induced angiogenic pathway50 (Figure 3). Experiments in transgenic animal models have identified that the endothelial expression and signaling of S1P/S1PR1 is perfusion-dependent54. Endothelial S1PR1 actively participates in embryonic vessel maturation to prevent excessive sprouting. Conditional depletion of S1PR1 in endothelial cells has demonstrated its fundamental role in vascular maturation in the murine embryo via incomplete covering of vessels by vascular smooth muscle cells55. Additionally, the limbs of S1PR1-null mouse embryos display severe vessel malformation and excessive budding, and genetic knockout of S1PR1 results in severe hemorrhaging and intrauterine death of mouse embryos between E12.5 to E14.553. S1PR2 on the other hand functions as a supportive and not as an essential receptor during vasculature development44.
Figure 3.
Angiogenic growth induced by VEGF-A and S1P in vascular endothelial cells. VEGF-A and S1P activate the Rap1 through a Rap1-GEF C3G by binding to their cognate receptors. Activated Rap1 interacts with afadin, an adaptor protein, and recruits afadin and PI3 kinase to the plasma membrane of the leading edge, where VEGF receptor-2 associates with these molecules. Akt and eNOS are then activated, enhancing the angiogenic growth of endothelial cells. PIP2: phosphatidylinositol (4,5)-bisphosphate. Adapted from Shimizu et al.50, under a Creative Commons Attribution (CC BY) license.
The process of vascular specialization is heavily regulated and largely dependent on the nature of the tissues and organs as well as the formation of a mature vessel lumen42. Within this realm, a new investigation into the role of S1P has been elucidated. Previous literature confirms the role of platelet-derived growth factor B (PDGFB) in recruiting smooth muscle precursors to differentiate into smooth muscle to help form a mature vascular lumen56. Differentiated smooth muscle cells migrate along the vessel to establish a mature vasculature. This migration process is intimately intertwined with the S1P. Using a zebrafish model, it has been shown that S1P directly and indirectly acts on smooth muscle migration via the EDG-5 like receptor Miles Apart (Mil)57. This S1P-mediated Mil activation induces further chemoattractant to create an environment for migration of smooth muscle precursors to form mature vascular lumen57.
Lipids are important constituent of the neuronal soma (up to 37% of dry weight) with the largest abundance of phospholipids (57.1%) followed by cholesterol, sphingolipids and other lipid species58,59. Myelinated nerves are composed of Schwann cells that encircle axons and are arranged as multiple layers of compacted membranes forming the myelin sheath around the axons. Lipids also serves as the major constituent (70–85%) of the myelin sheath, which insulates and propagates the action potential58. In the skin, phospholipid molecules such as phosphatidylcholine and phosphatidylethanolamine, alongside significant amounts of cholesterol and galactolipids such as cerebrosides and sulfatides remain primarily concentrated within the myelin sheath60. In addition to serving as an energy source, lipids perform diverse functions ranging from synaptogenesis, impulse conduction and providing structural integrity to the cells60. Cholesterol is an essential structural lipid and provides stability to myelin by regulating both fluidity and permeability of the membrane and is required for myelin development, compaction and homeostasis60. In the peripheral nervous system (PNS), cholesterol is required for shuttling the myelin structural protein, P0, from the endoplasmic reticulum (ER) to the myelin membrane61. In an experimental model, using electron microscopy, it has been shown that the lack of cholesterol biosynthesis in Schwann cells remains associated with severe hypomyelination with stretches of uncompacted myelin62.
Summary box 2:
S1P drives angiogenesis by guiding endothelial cell migration, vessel maturation, and smooth muscle recruitment through the Akt/eNOS pathway
Cholesterol and lipids are essential building blocks in myelin structure, and promote nerve insulation and protein transport in Schwann cells
4.1. Lipid Mediators in Diabetic Vasculopathy
Lipid mediators are crucial in driving general inflammation and specifically vascular inflammation in diabetic vasculopathy. The significance of lipid mediators in acute, self-limited inflammation has evolved given that the perception of inflammation resolution has changed as a more advanced understanding of this process has developed. In the traditional model of inflammation, the migration of neutrophils to the site of inflammation is due to chemoattraction such as via LTB4 and the role of lipids such as PGI2 and PGE2 is known to form an “exudate”, the resolution of which was previously understood to be due to chemoattractant dilution63. However, the process of resolution is now known to be an active construct due to the role of identified “specialized pro-resolving mediators (SPMs)” including maresins, lipoxins, resolvins, and others that fit into the broader class of lipid mediators64. In particular, these SPMs have associated actions including the halting of neutrophil infiltration, the production of stop signals to prevent additional neutrophil action and tissue damage, and the potentiation of macrophage phagocytosis of apoptotic neutrophils and cellular debris64. Such SPM “pro-resolving” action may be applicable in the nervous, reproductive, and other systems and thus demonstrates the broad role of SPMs in inflammation resolution64. As mentioned, examples of SPM include maresins, which are products of macrophages that are formed from docosahexaenoic acid (DHA), resolvins, including RvD1, RvD2, RvD3, and others which have demonstrated action in dermal, renal, pain, wound healing, and other disease states, and 13R,14S-dihydroxy-docosahexaenoic acid, which was shown by metabololipidomics to be produced by macrophages from DHA through the use of the human 12-lipoxygenase (12-LOX) and soluble epoxide hydrolase (sEH)64. The 13,14-dihydroxy-docosahexaenoic acid has also been shown to decrease neutrophil infiltration in mouse peritonitis as well as to augment human macrophage phagocytosis of zymosan, thus demonstrating its pro-resolving actions45.
In contrast, lipids are pro-inflammatory and play an important part in the promotion of vascular disease in diabetes and, consequently, of cardiovascular disease in the same context. Specifically, dyslipidemia and atherosclerosis are key mediators of diabetic vasculopathy. In T2DM, high levels of both postprandial and fasting triglycerides and LDL, low HDL, and small dense LDL particles are the defining lipid parameters. The small dense LDL particles are especially “atherogenic” and thus are key contributors to vascular disease in T2DM. In the vasculature, inflammation is produced via the augmentation of the inflammatory response as a result of a chronic state of hyperglycemia and insulin resistance. Dyslipidemia in this environment allows for the creation of atherosclerotic plaques and subsequent vasculopathy. Additionally, inflammation and oxidative stress are promoted by higher levels of both triglycerides and LDL-C in this setting, which in turn increases the instability of plaques and raises the associated risk of cardiovascular events10. This process is likely linked to the release of hallmark inflammatory markers such as tumor necrosis factor alpha (TNFα) from EC cells65. This release is largely due to certain environmental factors such as chronic ischemia, endothelial damage or vascular leakage. Thus, although lipid mediators in acute inflammation play a significant anti-inflammatory role, lipids in diabetic vasculopathy serve as flagbearers of vascular inflammation and ultimately contribute to the state of disease and resultant pathology such as cardiovascular disease.
Summary box 3:
SPMs like resolvins, lipoxins, and maresins resolve vascular inflammation
LTB4, PGE2, and PGI2 are pro-inflammatory lipids that initiate neutrophil recruitment and promote vascular inflammation, contributing to vasculopathy
Dyslipidemia in T2DM activates endothelial TNFα pathways, stimulating atherosclerosis and plaque instability
4.2. Lipid Mediators in Diabetic Neuropathy
Lipid metabolism plays a crucial role in the pathogenesis of diabetic neuropathy, as demonstrated by various clinical and animal publications. Stemming from dysregulations in lipid homeostasis driven largely by impaired insulin activity, the elevations of certain lipid molecules, such as ceramides, diacylglycerols, and saturated fatty acids, have been implicated in neuronal injury and dysfunction66. These lipids promote mitochondrial stress, oxidative damage, and cellular inflammation67, all hallmark processes that characterize the pathophysiology of diabetic neuropathy.
Among the lipid species implicated in diabetic neuropathy, LDL and its oxidized form ox-LDL have received significant attention. It has been well studied that patients with diabetes exhibit increased levels of LDL and ox-LDL68. Along with its role in atherosclerosis, ox-LDL has also been linked to the development of neuropathy through various biochemical signaling pathways46. Lectin-like oxidized low-density lipoprotein receptor (LOX-1) is a transmembrane receptor for ox-LDL expressed on a range of cell types, including endothelial cells, immune cells, and notably neurons within the dorsal root ganglion (DRG)69. Activation of LOX-1 triggers a cascade of deleterious effects, including inflammation, oxidative cellular injury, and apoptosis. These processes severely compromise cellular and homeostatic functioning70.
The pathogenic role of LOX-1 in diabetic neuropathy is supported by Vincent et al. in their well-cited study investigating the impact of high-fat diets on the development of polyneuropathy in an animal model71. The authors hypothesized that dietary-induced elevation of ox-LDL in mice would result in the activation of LOX-1 on DRG neurons, accelerating the onset and severity of polyneuropathy71. Consistent with their hypothesis, mice subjected to a high-fat diet developed significant impairments in nerve conduction velocities and pure tactile sensation compared to controls71. These findings support the role of ox-LDL dysregulation in promoting oxidative stress and neuronal dysfunction through LOX-1 activation, providing insight into a lipid mediated mechanism of diabetic neuropathy71. Recent studies have highlighted additional lipid mediators, particularly resolvins such as RvD1, as key regulators in the pathogenesis of neuropathy47. RvD1 is known to exert its anti-inflammatory properties through intricate signaling pathways involving the upregulation of miRNAs and downstream target genes specific for inflammation modulation47. In a study using mouse models, Su et al. elucidated the role of the IL-10/Nrf2/HO-1 pathway in DRG neurons, showing that its activation by RvD1 was seen to attenuate oxidative damage and promote anti-inflammatory responses in mice with progressive neuropathy48. Although not specifically associated with diabetic induced neuropathy, the shared mechanisms of neuronal injury in this study suggest a significant role of resolvins like RvD1 in mediating oxidative and inflammatory damage in neurons47.
Dysregulation of S1P in diabetics has been shown to play a significant role in nerve damage, particularly through its influence on endothelial dysfunction72,73. Unbalanced S1P levels can compromise vascular integrity, reducing blood supply to peripheral nerves and worsening ischemic injury, thereby accelerating neuropathy progression74. In addition to its vascular effects, S1P plays a critical role in regulating Schwann cell function, which is essential for myelin maintenance and nerve propagation75. Appropriate S1P signaling can lead to Schwann cell repair and re-myelination, potentially improving nerve conduction and neural regeneration75. Thus, inadequate S1P signaling can impact pathways involved in myelination related to axonal connectivity75. Moreover, S1P actively modulates neuroinflammatory pathways by interacting with pro-inflammatory cytokines such as TNF-α and IL-1β, driving chronic inflammation76. This inflammatory state intensifies oxidative stress and mitochondrial dysfunction, exacerbating axonal degeneration and sensory deficits characteristic of diabetic neuropathy77. Along with S1P, other lipid mediators linked to nerve damage in diabetics include prostaglandins (PGI2, PGD2) and cysteinyl leukotrienes (LTB4, LTC4, LTD4)49. Collectively, these mediators drive local inflammation, microvascular dysfunction, and neural injury by promoting cytokine release, leading to edema, immune cell recruitment, and nerve compression49. Continuous production and activation of these lipid substrates drive oxidative stress and neurodegeneration in diabetic neuropathy, just as chronic ischemic damage produces progressive neural dysfunction49.
Summary box 4:
Lipid dysregulation contributes to diabetic neuropathy by promoting oxidative stress, inflammation, and neuronal dysfunction
Elevated ox-LDL activates LOX-1 receptors on sensory ganglion neurons, triggering apoptosis and sensate loss
Disrupted S1P signaling impairs vascular integrity, Schwann cell function, and myelination, further damaging peripheral nerves.Therapeutic strategies targeting lipid pathways offer promising avenues for reducing neural damage through attenuation of the inflammatory response
5.0. Lipids in Wound Healing
Lipids play essential roles across all phases of wound healing, from inflammation modulation to tissue remodeling78. Prostaglandins, leukotrienes, and thromboxanes, all lipid molecules derived from arachidonic acid, are key hormones capable of regulating the inflammatory process79. Through changes in blood flow, permeability, and chemical signaling, these lipid molecules influence the recruitment of immune cells, like neutrophils, macrophages, and lymphocytes, to the site of injury, allowing for as-needed healing79. The local release of cytokines and chemokines, signaled by various lipids, further accelerates the activation and migration of these immune cells, enhancing the inflammatory process80.
Lipids are also heavily involved in the proliferative and remodeling phases of wound healing81. Concluding inflammation, lipid mediators such as sphingolipids and ceramides influence the mobilization of keratinocytes and fibroblasts to the wound site, promoting collagen synthesis and extracellular matrix formation82. Additionally, sphingolipids and ceramides signal for keratinocyte differentiation and adhesive synthesis mechanisms to revitalize the skin barrier83,84. These functions support the final stages of wound remodeling, allowing for proper wound tensile strength and progression of tissue scarring83. Lastly, apart from signaling properties, sphingolipids and ceramides also serve as structural components of the cellular plasma membrane. Due to their biochemical composition, sphingolipids and ceramides promote membrane integrity and fluidity85. Their unique structure also facilitates membrane trafficking and intercellular transport85, adding to the role of lipid signaling in wound healing.
The diverse functions of lipids, from membrane support to biochemical communication, mark their importance in wound healing. Through precise signaling and maintenance of structural integrity, lipids enable the optimization of tissue repair to restore the skin’s natural protective barrier86. A coordinated lipid-mediated response across all stages of wound healing is essential to ensure proper wound regeneration and closure. Notably, evidence has been shown to also support the role of lipid signaling pathways in angiogenesis, a crucial aspect of the inflammatory cascade87. This potential lipid response adds to the complexity of wound healing, as explored further in this review.
5.1. Lipid Mediators in Physiologic Wound Healing
Before investigating lipid signaling within diabetic and chronic non-healing wounds, we explore the physiological role of different lipid signaling cascades on wound angiogenesis and healing. The increased cellular heterogeneity at the wound-edge due to injury brings a repertoire of lipid molecules which act in an autocrine and paracrine way to orchestrate the different phases of wound healing78 (Table 2). One such signaling cascade comes from a lipid derivative of a long-chain fatty acid N-acyl- lautetnicotinamide (NATs), which are created via an enzyme called fatty acid amide hydrolase (FAAH)88. Studies done by Sasso et al. (2016) in murine wounds showed a novel pathway where FAAH disruption resulted in increased stimulation of fibroblasts as well as accelerated wound healing88.
Table 2:
The different lipid molecules and their role in different phases of wound healing process78,182,183.
| Symbol | Name | Wound Healing Phase | Downstream effects in Wound Healing |
|---|---|---|---|
| TXA2 | Thromaboxane A2 | Hemostasis/Inflammation | Increases platelet aggregation |
| PGE2 | Prostaglandin E2 | Proliferation | Promotes angiogenesis |
| PGI2 | Prostaclyclin | Proliferation | Increases fibrinolysis, angiogenesis, and fibroblast migration |
| LTB4 | Leukotriene B4 | Hemostasis/Inflammation | Increases Inflammatory response |
| 12=HHT | 12-Hydroxyheptadeca-5Z,8E,10E-trienoic acid | Proliferation | Increases keratinocyte migration |
| S1P | Sphingosine-1-Phosphate | Hemostasis/Inflammation, Proliferation | Increases angiogenesis, Fibroblast proliferation |
| LPA | Lysophosphatidic acid | Proliferation | Increased keratinocyte migration |
Cells with disrupted plasma membranes were in fact shown to have disruptions in different phospholipids, which line the membrane bilayer. Researchers specifically targeted one phospholipid, diacylglycerol (DAG), and found that DAG formation is required for downstream healing89. This literature showcases the first lipid with a specific role in the wound response via Rho and Cdc42 signaling and downstream membrane fusion. More so, this same research group showed that other lipids have implications in wound repair, albeit less delineated than DAG. Similarly, studies focusing on senescent cells have emphasized the involvement of lipids in the process of wound healing. With aging cellular mechanisms are disrupted and there is an escalation found in certain senescent cell populations at the wound-edge. Previous literature has shown that clearance of these cells at the wound edge has delayed wound healing78. Notably, dysregulation in the formation of phospholipids, eicosanoids, and other lipids has been linked to the senescent cell phenotype. Thus, there is an interesting link between lipid signaling, the senescent cell type, and wound healing under physiological conditions.
Lipids have also been implicated in dysregulated inflammatory states and chronic wound formation90. Saturated fats have been shown to drive continuous activation of the inflammatory response, ultimately impairing wound healing91. In their animal model study, Milanski et al. explored specific inflammatory receptors targeted by saturated fats92. By administering a high saturated-fat diet, the authors revealed a significant increase in the activation of toll-like receptor 4 in the hypothalamus, an area well-known for regulating inflammation92. Toll-like receptor 4 signaling has been linked to local cytokine release, which induces an inflammation response93. Heightened toll-like receptor 4 signaling maintains the body in a state of inflammatory dysregulation, leading to impaired wound healing and elevating the risk of chronic wound formation, especially in patients with comorbid inflammatory conditions like obesity and diabetes94,95. Fatty acid synthesis remains an important but unexplored role in the formation of endothelial cells. Literature has shown that Fatty Acid Synthase (FASN) knockout cells have impeded angiogenesis in vivo96. Such a signaling cascade involves post-transcriptional modification via malonylation96. Similarly, the process of angiogenesis is also regulated by lipoproteins, in particular LDL. Bogachov et al. (2020) showed that mice with a deficiency in apolipoprotein E displayed delayed wound closure97. This delay was attributed to a decrease in the expression of platelet endothelial cell adhesion molecule (PECAM) and subsequent anti-angiogenic effects97. This last piece of literature is exceptionally exciting as it offers a detailed mechanism for how hypercholesterolemia can be antiangiogenic via increases in LDL. Hence, under physiological conditions, lipids play an important role ranging from irregular cell clearance to decreased angiogenesis.
Summary box 5:
phospholipids and fatty acid derivatives activate signaling cascades like Rho and Cdc42 to promote fibroblast activity, membrane repair, and angiogenesis during wound healing
Pathological TLR4 signaling due to saturated fats, and reduced PECAM expression from excess LDL, drives chronic inflammation and delayed wound healing
5.2. Role of S100 Family Proteins in Lipid Signaling
The S100 family of protein members plays an essential role in various cellular and physiological processes, including lipid metabolism, angiogenesis, and wound healing, especially in the context of metabolic disorders such as obesity and diabetes98–100. These small, secreted protein members are characterized by their EF-hand calcium-binding motifs, which allow them to interact with different types of target proteins that regulate lipid signaling, angiogenesis, and wound healing98–100. S100 family of proteins are involved in the regulation of acute and chronic inflammation, cell proliferation, and differentiation, making them potential players in the pathophysiology of metabolic disorders. Among the S100 family members, S100A7, also well known as psoriasin, stands out due to its crucial role in regulating inflammatory responses and contributing to the pathogenesis of various diseases, including cancer and inflammatory disorders101–103.
S100A7 is an essential protein of the S100 family of proteins that play a potential role in the modulation of acute and chronic inflammatory responses101–103 that may potentially regulate lipid signaling, angiogenesis, and wound healing mechanisms. Recent studies also highlighted the high expression of S100A7 in diabetic foot ulcers104,105. Moreover, S100A7 has been reported to interact with epidermal fatty acid binding protein (E-FABP) in keratinocytes and regulate the formation of focal adhesion-like structures106. Interestingly, E-FABP, a well-known protein that regulates lipid transport and metabolism in the epidermis, is overexpressed alongside S100A7 in psoriasis107. A large number of prospective clinical studies also indicate that metabolic disorders such as obesity are a strong risk factor for psoriasis108 and psoriasis severity is linked with an increased risk of diabetes109. Thus, the overexpression of S100A7 and its signaling partners may also lead to abnormal lipid metabolism, increasing the overall risk of obesity and delayed wound healing107. Moreover, the direct interaction of S100A7 with its receptor, RAGE (receptor for advanced glycation end products), activates various inflammatory pathways102 that have also been reported to exacerbate the chronic inflammation often observed in obesity-associated diabetic wounds110,111.
In addition, S100A7-mediated downstream signaling influence the process of angiogenesis in response to different stimuli such as stress by modulating the expression of different angiogenic factors such as VEGF112–114. In diabetic and obese individuals, impaired angiogenesis is a crucial factor contributing to delayed wound-healing outcomes115 and therefore, the pro-angiogenic ability of S100A7 could be potentially leveraged to develop novel therapeutic strategies aimed at improving the vascularization of chronic wounds associated with these metabolic disorders. However, the S100A7-linked chronic inflammatory milieu in these conditions may counteract the beneficial effects of S100A7-induced angiogenesis, indicating a complex interplay between the potential role of S100A7 in inflammation, lipid signaling, and vascularization in wound healing which warrants further research investigations.
6.0. Angiogenesis and Neuropathy in Diabetic Wounds
Prior to injury, the body functions in a steady state where nutrient delivery is met by demand and there is a basal level of angiogenic factors. This basal level is regulated by an appropriate ratio of pro-angiogenic factors (e.g. VEGF and FGF) and anti-angiogenic factors (Ang-1 and pigment epithelial-derived factor (PEDF) resulting in net zero proliferation116,117. Under a traumatic assault, this homeostasis is interrupted leading to injury to endothelial cells and the creation of a hypoxic environment. After the initial influx of neutrophils and edema, the proliferation of new blood vessels begins. VEGF stimulates the formation of capillaries along with increased expression of other cofactors such as Hypoxia Inducible Factor-1 (HIF-1) which in turn upregulates angiogenic genes. Thus, vascular growth begins in areas of high blood supply and proceed toward the area of injury. Malformed capillaries with blind end loops form resulting in an increased number of capillaries and vessels in the injured area118. Soon after vessel refinement occurs, and the antiangiogenic factors promote apoptosis of unnecessary vessels and only competent vessels remain119. As the wound heals, the existing high level of oxygen demand diminishes, and the capillaries slowly shrink in size. This retreat of the new vessels is followed by a remodeling stage in which the appropriate ratio of pro-angiogenic and anti-angiogenic signals is established.
6.1. Neuropathy in Diabetic Wounds
Diabetic wounds, particularly DFUs, commonly form in the foot in part due to the multifactorial influence of neuropathy120. As briefly outlined earlier, diabetic neuropathy follows a characteristic stocking-glove pattern, affecting the upper and lower extremities progressively. Neuropathy in diabetes can be further classified into sensory, motor, and autonomic subtypes, all of which directly contribute to DFU development through distinct mechanisms120.
The loss of sensation plays a critical role in the formation of DFU. In early sensory neuropathy, symptoms of burning pain, loss of vibratory sense, and reduced superficial sensitivity are predominantly experienced in patients16. Over time, chronic neuropathy ensues, causing complete sensory loss in the extremities, leaving patients unaware of traumatic and injurious stimuli16. This lack of sensitivity can result in continuous, repetitive, and prolonged pressure on specific areas in the foot, resulting in localized ischemia, inflammation, and ultimately necrosis. This unrelieved pressure is especially harmful to already inflamed tissue in the foot, causing abrupt autolysis and subsequent ulceration. The presence of motor neuropathy compounds this pressure damage16. As muscle atrophy in the foot progresses, an unequal foot load prompts a compensating walking gait, resulting in the formation of abnormal pressure points16. This combinations of factors contributes to the well-recognized anatomical sites associated with DFUs: the metatarsal heads and the heel areas120.
Autonomic neuropathy in diabetes also contributes to DFU pathogenesis. Due to impairments in sudomotor function and vasodilatation, sweat production becomes inefficient, resulting in dry, fissured skin that compromises barrier integrity16. This causes diminished external protection, increasing the risk for pathogen invasion and ultimately skin infection121. Additionally, autonomic dysfunction has also been linked to the formation of arteriovenous shunts throughout the lower extremities122. These arteriovenous shunts divert blood away from the essential capillaries in the foot, impeding the supply of oxygen, nutrients, and regenerative cells to injured peripheral tissue122. As a result, impaired thermoregulation and reduced immune cell recruitment further compromise the skin's ability to heal, increasing the risk of chronic wounds and infection123. At a clinical level, this development of neuropathy is a morbid change in the daily life of affected individuals. Symptomatic control is paramount, as is increasing conditions that promote appropriate wound healing, such as glucose control and removal of infected tissue.
The triad of sensory, autonomic, and motor neuropathy are significant risk factors for the development of DFUs. Understanding the mechanisms behind neuropathy allows for a comprehensive outlook into potential therapeutic interventions aimed at the prevention and management of DFUs in diabetic populations.
6.2. Diabetic Peripheral Nerve Entrapment Neuropathy
A lesser known but significant contributor to neuropathy and DFUs is peripheral nerve entrapment. Even in the absence of clinically apparent polyneuropathy, entrapment neuropathy can serve as an early and relatively subtle manifestation of diabetes-related nerve dysfunction22. Resulting from impaired metabolic processes, chronic hyperglycemia triggers widespread anatomical and functional damage to peripheral nerves, stemming from inflammatory driven connective tissue deposition, perineural edema, and microvascular compromise22. These changes compromise nerve mobility and heighten susceptibility to mechanical compression at anatomical entrapment sites, most notably at the tarsal tunnel and the common peroneal nerve at the fibular head22. Over time, persistent compression exacerbates nerve ischemia and dysfunction, ultimately contributing to sympathetic and autonomic nerve failure24. As previously discussed, the loss of autonomic functioning impairs vasomotor control, resulting in arteriovenous shunting and capillary microcirculation collapse that deprives nutrient delivery to the dermal layer, compromising skin integrity124. Due to these significant complications, addressing nerve entrapment through surgical decompression has gained increasing attention as a potential therapeutic strategy to complement the standard of care.
Emerging evidence supports the role of anatomical decompression in alleviating neuropathic symptoms and improving clinical outcomes. A recent double-blinded randomized controlled trial by Rozen et al. demonstrated significant pain reduction following surgical decompression of fibro-osseous tunnels in the lower extremities27. Additionally, studies have observed immediate improvements in EMG amplitudes postoperatively, suggesting neurophysiological recovery of peripheral nerves28. Most notably, Nickerson et al. provided evidence supporting the hypothesis that epineurolytic decompression, compared to standard of care, was associated with lower DFU recurrence rates, though this association remains poorly understood26.
Among surgical techniques, “Dellon Decompression,” involving tarsal tunnel release, has gained recognition as a promising intervention for restoring nerve function125. Utilizing this technique, Pejkova et al. demonstrated significant improvements in blood flow, sensory function, nutrient delivery, and overall wound healing in patients with DFUs125. These findings underscore the potential for transformative approaches to DFU management, as conventional wound care practices remain limited125. While continued investigation into peripheral nerve decompression is warranted, the preliminary findings are encouraging, suggesting that it may complement or even enhance the current standard of care.
6.3. Management of Diabetic Foot Ulcers
Symptomatic control of diabetic foot ulcers primarily involves promoting an optimal environment for wound healing. The current clinical paradigm for DFU management includes a combination of strict glucose control, antibiotics, local wound care, and ultimately surgical management. Surgical management typically involves removal of nonviable tissue, and in cases of compromised blood flow, revascularization. Through this multifactorial approach, clinicians can address DFU before systemic infection or further loss of limb occurs.
Two aspects of clinical DFU management are particularly noteworthy. First, local wound care and topical treatment of these wounds remain a robust area of research. A variety of non-invasive topical treatments have emerged, including negative therapy pressure, hydrogel dressings126, offloading, and protective footwear. Second, from a surgical perspective, patients with DFUs and concurrent arterial disease benefit from arterial revascularization127. This is achieved through endovascular therapy such as balloon angioplasty and stenting, but can also involve surgical bypass or endarterectomy128.
Together, this multifaceted approach to DFUs remains the gold standard for treatment. However, an evolving body of literature continues to introduce novel treatment options. Emerging surgical techniques such as deep venous arterialization (DVA) and advancements in endovascular therapies are pushing the boundaries within the realm of DFU care, improving patient outcomes129.
6.4. Role of Lipids in Diabetic Vasculopathy and Neuropathy
Large multicenter studies have identified lipids as a risk factor for reducing diabetic vascular complications such as myocardial infarction. A large multicenter hospital-based study by Agrawal et al. (2006) showed a distinct influence of increased lipid levels in diabetes and diseases such as coronary artery disease and PAD130. More recent literature has delineated the exact role that lipids have in angiogenesis. Lipids in the human body are simply organic fats with a primary role in supporting and maintaining the cell membrane. Another role of lipids is the transfer of lipophilic molecules due to the lipophilic nature of carbon-carbon bonds that the fat contains. In this realm, biomolecules such as chylomicrons, triglycerides, VLDL, LDL, and HDL arise with their unique cargo molecule131. In the realm of diabetes, the exact mechanism of lipids has been limited to its deposition of cholesterol and other fatty acids along with the subsequent role in microvascular and macrovascular injury. In fact, serum triglycerides have been shown to have even a stronger predictive value for atherosclerotic disease than HbA1c measurements132. Furthermore, hyperlipidemia works in a multifactorial way to promote glucose abnormalities133. Most evidently there is an increase in atherosclerotic plaque burden in those with hyperlipidemia. The subsequent plaque burden creates an environment of endothelial damage as well as increase in lipid accumulation in the fibroathermatous cap134,135. The most elucidated mechanism is the increase in triglycerides due to compensatory hyperinsulinemia which is itself brought on by loss of insulin sensitivity. This creates the perfect environment for lipid synthesis to run unmitigated.
6.5. Infection and Chronic Inflammation in Diabetic Vasculopathy and Neuropathy
Both in compromised skin and in wounds, associated inflammation and microbial pathogens are recognized as key drivers in the pathogenesis of diabetic vasculopathy and related neuropathy121. In the context of diabetic neuropathy, dysfunction of the blood-nerve barrier (BNB) represents a hallmark contributor to nerve injury121. Stemming from chronic inflammation, the accumulation of exudative edema and immune cells surrounding the endoneurial vascular endothelium and the perineurium of the neural fascicle disrupts the transport of essential blood-borne molecules required for repair121. This interference with the BNB facilitates unchecked inflammation and injury, leading to progressive neural damage and the clinical manifestations associated with diabetic neuropathy121.
Inflammation has also been recognized as a significant contributor to vasculopathy in patients with diabetes. Particularly in those with DFUs, the exposed wound environment increases susceptibility to vasculitis, a process defined as direct inflammation of the vessel wall, culminating in ischemic injury121. Vasculitis poses a significant detriment to all vessels associated with DFU. Notably, vasculitis has also been linked to the development of neuropathy, as vasculitis not only affects the vessels of the wound bed, but also the blood vessels directly supplying peripheral nerves121. The resulting cycle of ischemia, inflammation, and impaired healing highlights the multifaceted role of vasculopathy in DFU development. Additionally, the loss of skin barrier integrity seen in diabetics predisposes patients to an increased susceptibility to external pathologies121. Highlighted often is infection, as the deceased barrier protection invites invasion of innate skin surface bacteria such as the Staphylococcus aureus and Streptococcus species136. Compromised skin and inflammatory mechanisms allow these bacteria to induce a chronic, sometimes subclinical, infection137. This persistent infection further exacerbates vasculopathy and neuropathy in diabetics due to the stimulation of inflammation and oxidative stress from bacteria121.
Of particular interest is the role of biofilms in wounds. Biofilms are a collection of polymicrobial communities structured and encased in microbial extracellular matrix138. This complex ecosystem acts as a shield for bacteria, preventing immune clearance, reducing antimicrobial efficacy, and fostering a microenvironment of persistent inflammation138. It has been demonstrated that a vast majority of DFUs contain biofilms121. Their presence not only impairs wound healing but also perpetuates vascular and neural damage, highlighting the intertwined nature of infection, inflammation, vasculopathy, and neuropathy in diabetic wounds121.
7.0. Application of New Lipidomic Technologies in Wound Healing Research
Lipidomics, the large-scale study of lipid compositions in biological systems, has emerged as an intriguing research field, calling the attention of researchers and clinicians alike for its potential to provide valuable insight into disease mechanisms and therapeutic interventions139. As the name implies, lipidomics involves the comprehensive identification and profiling of lipid species within a biological sample utilizing diverse imaging techniques. Through Mass Spectrometry Imaging (MSI) and layered chromatography, both powerful tools used for tissue analysis, researchers can study and monitor single-cell and single-organelle interactions and distributions at an unprecedented level140. By combining spatial and molecular findings, MSI and chromatography lipidomics enable the analysis of a vast array of data regarding lipid architecture, signaling, gene expression, and metabolism in the human body.
7.1. Skin Spatial Multiomics
Along with lipid-based signaling pathways in vascularized tissue, recent advances in skin lipidomics have given researchers deeper insight into the spatial and temporal dynamics of lipids and their role throughout the wound healing process141. Essentially, spatial and temporal lipidomics can be described as dynamic molecular map of a lipid atmosphere through time. While skin lipids may have some mediating function in healing, they primarily serve to maintain and protect the skin’s external barrier142. Ceramides, the primary lipid precursor in the epidermal layer, are essential for regulating moisture and preserving skin integrity143. Diabetes notably affects the production and functioning of ceramides, impairing the lipid barrier and contributing to skin breakdown143. This disruption in skin lipid composition further exacerbates ulcer susceptibility and complicates the wound healing process, highlighting the importance of spatial lipidomic analysis in understanding the role of skin lipid atmospheres in wound pathology141.
In their recent review article, Sochorová et al. highlighted the feasibility of skin lipidomics research through the systemic integration of both sampling techniques, such as sebum collection, and various imaging technologies, including MSI and thin-layer chromatography (TLC)141. The authors provide a comprehensive framework for future researchers, detailing lipidomic methodologies that can effectively produce high-resolution views into the lipid systems in both pathologic and healthy skin141. A report by Kendall et al. also adds to the instructional framework of skin lipidomics by detailing the appropriate methods required to successfully obtain lipid sampling, extraction, and analysis based on the researcher’s interest144. Specific skin compartments, lipid composition, and type of data needed may alter the experimental approach required. For instance, in cases where researchers aim to study sebum or stratum corneum, a tape strip can be used to sample the epidermal lipids. To obtain deeper dermal components, a full-thickness skin biopsy is preferred144. For imaging and analysis, a custom strategy can be adopted based on the research hypothesis being tested. Chromatography can be utilized as an untargeted approach to visualize the lipidomic profile, while MSI utilizes a more targeted approach aimed at differentiating between lipid classes and mediators in biochemical pathways, such as eicosanoids and ceramides144. A full breakdown of experimental approach guidelines for Skin lipid analysis has been extensively reviewed by Kendall et al144. (Figure 4).
Figure 4.
Schematic representation of skin lipidomics workflow. Several mass spectrometry-based lipidomics approaches are available for the investigation of the skin lipidome. A combination of these platforms can reveal the most information about skin lipids. 3Q, triple quadrupole; DESI, desorption electrospray ionisation; ESI, electrospray ionisation; IS, internal standards; MALDI, matrix-assisted laser desorption ionisation; RPC, reverse-phase chromatography; UHPSFC, supercritical fluid chromatography. The following original report is credited: Kendall et al.,144.
Following these methodological developments, various studies have looked at specific skin conditions through the application of lipidomic analysis. For instance, a research article by Zhou et. al studied lipid distribution and associated gene expression in patients with acne145. Using liquid chromatography and tissue sampling, the authors were able to conduct detailed lipidomic analysis in patients with mild to severe acne. The authors noted that in those with severe acne, the fatty acid class and length compared to mild patients varied significantly145. Severe patients were found to have shorter fatty acid chain length and an increase in unsaturated fatty acid content in relation to mild patients, hinting towards a disruption in cellular membrane integrity145. The authors also pointed out the abundance of phytosphingosine and sphinganine in patients with mild acne, potentially serving as mitigators of severe acne formation145.
Summary box 6:
Skin lipidomics provide an insight into the spatial and temporal roles of lipids during wound healing
Unbiased lipidomics reinforced the impact of dysregulated ceramide in diabetic ulcer
Skin lipidomics techniques like sebum sampling, MSI, and TLC provide important information about lipid distributions in health and disease
7.2. Skin Spatial Lipidomics
Regarding wound healing, lipidomics research has emboldened the role of lipid signaling in the repair and regeneration of healthy tissue. Through an extensive review, Pils et al. identify the key lipid mediators involved in the various wound healing pathways78. Eicosanoids (unsaturated fatty acids) for example serve as key coordinators in the Cox enzymatic pathways, enhancing the pro- and anti-inflammatory phases of wound healing through the signaling mechanisms of leukotrienes and prostaglandins78. While traditional research methods have provided valuable insights into the molecular mechanisms of diabetic wound healing, they often lack the spatial resolution necessary to fully comprehend the intricate cellular interactions and signaling events occurring within the wound microenvironment146.
The use of spatial lipidomics enables researchers to analyze the differing compositions of lipid mediators at times of inflammatory stress. By mapping the spatial landscapes of lipids in the four phases of tissue repair and regeneration, researchers can gain a deeper understanding of the exact roles of lipids and how they contribute to wound healing outcomes.
7.3. Spatial Transcriptomics and Identification of Lipid Mediators in Wound Healing
Spatial transcriptomics (ST) represents a groundbreaking approach to investigating the complexities of wound healing. This technique allows researchers to analyze gene expression patterns while maintaining essential spatial context, thereby transforming our understanding of wound biology and paving the way for targeted therapies. ST enables the precise mapping of various cell types within the wound, such as fibroblasts, keratinocytes, immune cells, and endothelial cells (Figure 5). By examining the gene expression profiles of these cells in their spatial context, researchers can gain deeper insights into their interactions and contributions to different stages of wound healing. This spatial information is particularly crucial for studying diabetic wounds, where healing processes are frequently disrupted and cellular composition can significantly vary among patients147. Below are the key areas where ST is advancing diabetic wound healing research:
Figure 5.
Applications of ST in wound healing. Schematic representation of the spatial transcriptomics workflow, illustrating the collection of tissue samples, spatially resolved RNA sequencing, and subsequent data analysis. Representative figure from a diabetic wound biopsy, illustrating the complex cellular environment. The image highlights the presence of various cell types involved in the wound healing process.
7.3.1. Identifying Fibroblast Subtypes:
Fibroblasts play a vital role in wound healing by producing extracellular matrix components and regulating inflammation148,149. ST has identified distinct fibroblast subpopulations with specialized functions in wound repair. For instance, a recent study has highlighted a fibroblast subtype associated with healing that is localized to the wound bed of diabetic foot ulcers150. By analyzing ST data across different time points during wound healing, the authors tracked the movement and differentiation of fibroblast subpopulations150. The authors observed that fibroblasts migrate from the outer wound edge inward and undergo differentiation as they proliferate, supporting a spatially informed differentiation process. Furthermore, ST data can be combined with single-cell chromatin accessibility data (scATAC-seq) to impute spatial epigenomic properties150. This integration allows mapping of changes in chromatin accessibility across the wound, providing insights into the regulatory mechanisms driving fibroblast behavior150. Such an understanding of the unique gene expression profiles and roles of these fibroblast subtypes could lead to targeted therapies aimed at enhancing wound closure.
7.3.2. Mapping the Immune Landscape:
The immune response is critical for effective wound healing; however, it can become dysregulated in diabetic wounds, contributing to chronicity151. ST facilitates detailed mapping of immune cells within the wound, offering insights into their spatial organization and interactions. This capability is essential for understanding how the immune system influences either wound resolution or persistent inflammation. It has been shown that healing diabetic ulcers contain a higher abundance of pro-inflammatory M1 macrophages compared to non-healing ulcers, which predominantly feature anti-inflammatory M2 macrophages. ST allows the identification and comparison of regions of interest (ROI) from a similar depth of healing and non-healing diabetic ulcers. They found that healing-specific ROIs were similar in transcript profile compared to non-healing-specific ROIs, which were more dissimilar. Such findings could inform immunomodulatory therapies that target specific immune cell populations or signaling pathways to restore a balanced inflammatory response152,153.
7.3.3. Unveiling the Spatial Organization of Re-epithelialization:
ST is being used to visualize the spatial arrangement of different keratinocyte subpopulations involved in re-epithelialization154,155. This has revealed a distinct separation between proliferating and migrating keratinocytes in human wounds, a feature not observed in murine models. A novel wound margin structure was thus identified which is characterized by a non-proliferative migrating front surrounded by a highly proliferative hub, showcasing the unique aspects of human wound healing compared to mouse models. The spatial information obtained from ST can be used to identify the proximity and potential interactions between different cell types within the wound microenvironment. For example, Liu et al. used ST data to demonstrate close associations between migrating keratinocytes and pro-inflammatory macrophages at the wound edge, suggesting paracrine signaling pathways through the release of epidermal growth factor (EGF) ligands like EREG (epiregulin), which binds to EGF receptors (EGFR) on keratinocytes154. In chronic wounds, a significant reduction in migrating keratinocytes and pro-inflammatory macrophages, along with a lack of proliferating fibroblasts, were observed. This was found to be associated with insufficient recruitment and activation of keratinocytes and macrophages leading to failure of re-epithelization. In addition, spatial variations in expression were identified for genes related to key signaling pathways, such as EGF and HGF, which are crucial for re-epithelialization154. Such spatial context can help to investigate cell-to-cell communication networks that drive wound healing, providing a deeper understanding of the coordinated cellular responses during repair. A recent report from our laboratory has identified 2 distinct keratinocyte clusters that were identified as Kera1 (KRT14+KRT1+) and Kera2 (KRT19+KRT7+) using scRNA seq and ST data155. ST analysis also revealed that compared to Kera1, which accounts for almost all the keratinocytes in human chronic WE tissue, Kera2 cells were enriched in genes related to cellular metabolism and glycolysis, and directly implicated in the process of epithelial-to-mesenchymal transition (EMT)155.
7.3.4. Spatial Transcriptomics Identifies Changes in Skin Lipid Metabolism:
ST is now being utilized in the investigation of lipid metabolism-related gene expression in non-lesional human skin along with different inflammatory skin diseases such as psoriasis, atopic dermatitis (AD) and other related diseases147,156. In both AD and psoriasis, the sebaceous glands (SG) express genes encoding for lipid metabolism and transport such as ALOX15B, APOC1, FABP7, FADS1, FADS2, FASN, PPARG, and RARRES1 at high levels156. Analysis of differentially expressed genes (DEGs) in SGs in non-lesional skin and AD skin revealed the enrichment of the synthesis of very long chain fatty acyl-CoAs, SREBP-regulated cholesterol biosynthesis, glycerophospholipid biosynthesis, and biotin transport in AD. Additionally, spatial localization of characteristic gene clusters that determine sebaceous gland function, such as peroxisomal lipid, steroid, fatty acid, cholesterol, and linoleic acid metabolism continued to be expressed in AD and psoriasis. As diabetic wounds suffer from a chronic inflammatory state, a review of findings from the aforementioned inflammatory skin diseases might give a clue for the deregulation of lipid metabolism157.
In summary, by generating high-resolution spatial maps of gene expression, ST uncovers new insights into the complex cellular and molecular mechanisms driving diabetic wound healing. These insights are vital for identifying new therapeutic targets and developing personalized treatment approaches. Although ST holds great promise for advancing our understanding of diabetic wound healing, it is essential to recognize that this technology is still emerging. As ST platforms continue to evolve and become more accessible, we can expect rapid advancements in research that will enhance our understanding of wound biology and lead to innovative therapies addressing the global burden of diabetic foot ulcers.
7.4. Lipidomics in Targeted Therapeutics for Pathologic Conditions
To date, much of the literature surrounding lipidomics has focused on refining methodologies and analytical techniques to best interpret lipid landscapes. While studies specifically addressing pharmacological interventions in chronic and ischemic wounds remain limited, emerging research has suggested that lipidomics could play an essential role in developing targeted therapies for diabetic foot ulcers (DFUs). Insights from related pathologic conditions, such as atherosclerosis —where lipid dysregulation is a key pathological driver—provide a valuable framework for exploration, given the shared mechanisms between atherosclerosis and diabetic complications.
One such study, conducted by Wei et al., utilized lipidomics to investigate the anti-atherosclerotic effects of SUB885C, a multi-component herbal preparation, in ApoE*3Leiden.CETP mice158. Through comprehensive lipidomic analysis, the study identified significant molecular anti-atherogenic changes in response to therapeutic treatment, highlighting the potential of lipidomics in uncovering previously unrecognized therapeutic effects at the molecular level158. Although this study’s primary focus is on atherosclerosis, its findings highlight the broader applicability of lipidomic profiling in disease modulation, particularly in conditions with similar pathophysiological mechanisms, such as diabetes158. By enabling the identification of lipid alterations in response to therapeutic intervention, the study reinforces how lipidomic analysis provides a powerful tool for uncovering molecularly driven treatment effects that were previously difficult to detect or quantify, especially in complex diseases158. This approach could serve as a foundation for future research applying lipidomic analysis to chronic wounds and ischemic skin in diabetics, where lipid-targeted therapies may offer novel and transformative strategies to enhance healing outcomes.
8.0. Targeting Lipid Signaling in Rescue of Perfusion in Diabetic Ischemic Tissue
The concept of ischemic tissue rescue stems from the ability to address hypoxic, poorly vascularized diabetic wounds and prevent the progressive loss of limbs. One of the most feared downstream complications is amputation. The mortality surrounding amputation and 1 year and 5-year post-operation is dismal. Thus, there is an emphasis on preventing amputation and ‘rescuing’ the limb. Perhaps the most common comorbidity of an ischemic limb is the nonhealing wound found commonly with chronic uncontrolled diabetes. Preceding literature has explored the basis of angiogenesis, as mentioned earlier, with a focus on modulating the angiogenic/anti-angiogenic relationship through markers such as VEGF. VEGF has been subject of many studies ranging from reduction therapy in the realm of cancer159 to ophthalmology160. However, in these instances, there has been a focus on inhibiting the VEGF pathway. Hypoxic environments such as in non-healing diabetic wounds create an environment for an increase in VEGF. To this extent, pro-VEGF pathways can function as an ischemic limb rescue therapy. Studies by Zhu et al. (2019) have done much to explore this activation archetype161. The group used a novel drug Roxadustat (HIF prolyl-4-hydroxylase inhibitor) to study its therapeutic effect on cutaneous wound healing in diabetic rats by modulating the angiogenesis process161. They found an increase in cutaneous wound healing via activation of the HIF-1α/VEGF/VEGFR2 pathway in diabetic rats161. This cutaneous wound healing offers a clinical endpoint in comparison to amputation and limb loss. Confirming these studies, Jang et al. (2021) found that VEGF-containing peptides increase cell viability while simultaneously promoting the proliferation and tubular formation of new vascular beds162. Current literature presents compelling evidence recognizing the critical role of active lipid mediators such as eicosanoids in enabling VEGF-dependent angiogenesis163,164. In addition, VEGF signaling in turn activates Notch signaling by increasing the expression of classical ligands such as Dll4165. Upregulation of Notch ligands and their binding to neighboring Notch receptors leads to diminished VEGFR2 expression. Thus, Notch signaling may assist in pruning and patterning vascular networks by locally regulating endothelial cell responsiveness to global pro-angiogenic stimuli, particularly VEGF165. Thus, there is this unique intersection that can be sought out by finding the details surrounding VEGF expression from the point of view of lipid signaling. In fact, in endothelial cells, Notch signaling is blocked by oxidized lipids such as 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylc holine (Ox-PAPC). Ox-PAPC also triggers downregulation of JAG1 and target genes HES1 and HEYL leading to a emergence of pro-inflammatory microenvironment166. In this, there is an opportunity to offer a novel therapeutic solution by building upon an existing framework. There is no doubt about the angiogenic nature of VEGF nor is there any doubt of the potential therapies that VEGF modulation can have in the treatment of diabetic ischemic tissue167. However, through the exploration of VEGF regulation from the perspective of lipid signaling, one can offer a new therapeutic approach.
The lipid pathways in non-physiologic states begin from the basis of promoting angiogenesis in the setting of decreased pro-angiogenic factors as mentioned before. Thus, the ability to manipulate angiogenic factors is a large underlying theme in diabetic ischemic tissue rescue. Modulation of lipid pathways aligns with the previous dogma where manipulation of angiogenic factors rescues diabetic ischemic tissue rescue. However, through the lens of lipid signaling, we can explore new and exciting pathways that are not conventionally studied. An integral aspect of this review explored this fundamental relationship by inducing incisional wounds in genetically diabetic mice. This work by Altavilla et al. (2001) explored wound edge tissues on different days and analyzed the magnitude of conjugated dienes (CDs)168. CDs in this context function as a measurement of lipid peroxidation and wound-breaking strength. It was found that wounds of diabetic mice had an increased level of CDs and were associated with a delay in angiogenesis. Additionally, the usual induction of VEGF mRNA seen in non-diabetic animals was not seen in in diabetic mice168. Importantly, it was also found that the inhibition of lipid peroxidation rescued the loss of VEGF expression in diabetic mice during the wound-healing process and normalized the defect. Hence controlling VEGF expression through lipid pathways presents as an additional level of manipulation towards rescuing diabetic ischemic tissue. Recent research in our laboratory has explored the role of phospholipase C-gamma 2 (PLCγ2), an enzyme that cleaves membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), resulting in increased VEGF signaling. These studies demonstrated that effective endothelial cell-specific delivery and expression of PLCγ2 via tissue nanotransfection (TNT) technology during VEGF therapy improved the outcomes11,167. TNT is a minimally invasive approach that uses electric pulses to deliver specific genes/plasmids to tissues, reprogramming their function11,167,169–174. This delivery method of endothelial PLCγ2 was shown to be effective in diabetic ischemic tissue rescue via increasing the abundance of VWF+/CD31+ and VWF+/SMA+ vascular elements11. However, different sets of research have implicated different mechanisms of wound healing apart from VEGF and angiogenesis. Corneal injury as seen in settings of diabetes with neovascularization and hyperglycemic conditions also has the inability to properly regenerate the functions of injured tissues with symptoms ranging from dry eyes to ulceration. To this end, the exploration of lipoxygenase (LOX) derivatives was seen to act as second messengers for epidermal proliferation and repair175. Stimulation and synthesis of LOX derivatives were shown to regenerate injured corneal nerves. This is an entirely new signaling pathway in pathologic conditions that is distinct from the VEGF angiogenic pathways.
As highlighted earlier, IWGDF guidelines for standard of care in DFU treatment involve sharp debridement, offloading devices, topicals like sucrose-octasulfate impregnated dressings, and adjunctive therapies like hyperbaric oxygen29,30. However, current IWGDF guidelines do not incorporate lipid-based diagnostics or therapeutics into DFU standard of care. Integrating spatial lipidomics could complement these standards by identifying patient-specific lipid imbalances, like ceramide deficiency or pro-inflammatory mediator abundances, and guiding personalized interventions, such as targeted topical therapies or lipid modulators. This approach aligns with the outlines set forth by the American Diabetes Association (ADA) to prioritize precision-based medicine in managing diabetes and its complications176.
9.0. Lipid Therapy in Ischemic Diseases
Adjacent to the topic of impaired wound healing is the slow development of atherosclerotic burden prior to the formation of wounds. This clinical picture presents itself in the form of peripheral arterial disease (PAD). When atherosclerosis occurs in the peripheral arterial tree (outside of the coronary vasculature), there is an exponential increase in loss of life and limb. For instance, nearly half of patients with DFU also have PAD177. In this population, the risk of amputation was nearly four times higher than the national average for amputation risk factors178. Even in the absence of PAD or overt ischemia, a DFU alone markedly raises the likelihood of amputation and death178,179. While the formation of nonhealing wounds is the penultimate progression of this disease, there are intricate lipid pathways that occur prior. The same lipid molecules as outlined above have been implicated in the formation and progression of atherosclerosis as it relates to the clinical pathology of PAD. Thus, the target for many patients with PAD stems from the ability to target lipids and affect their molecular pathways180. At the forefront of therapy targeting lipids is the inhibition of the 3-Beta-Hydroxy B methylglutaryl CoA reductase pathway via statins. This enzyme is responsible for creating mevalonic acid, which is a rate-limiting step in the formation of cholesterol. Downstream mechanisms of this de-novo cholesterol formation have been linked to higher atherogenic burden180. Inhibition of this pathway via statins has been shown to promote plaque stability and even some level of plaque regression. These results were verified as early as the 1990s in the Scandinavian Simvastatin Survival Study and the EUCLID (Examining Use of Ticagrelor in Peripheral Artery Disease) trial. Newer mechanisms of lipid therapies have also been identified. PSCK9 is a serine protease that is intimately involved in the degradation of LDL receptors on hepatocytes180. PSCK9 inhibitors (e.g. evolocumab, alirocumab) bind to the protein and inhibit the ability of the receptors to uptake, lowering serum LDL levels. In double-blind randomized trials, this was shown to decrease acute coronary and vascular events. In a similar vein, an ester derivative of eicosatetraenoic acid called icosapent ethyl (IPE) has had similar results via its mechanism of hepatic LDL and triglyceride synthesis. Current molecular therapies are evolving targeting the lipid cascade with clinical results showing all cause reduction in mortality and morbidity. These therapies include sRNA inhibitors (Inclisiran) and other monoclonal antibodies targeting LDL and apoprotein receptors180. Therefore, further research studies are required to fully elucidate the mechanism of these therapies as well as their clinical viability.
Clinical Implications Box:
- Potential lipid biomarkers for elevated DFU risk:
- Increased ceramide levels, altered fatty acid profiles, dysregulated lipid mediators like S100A7, S1Ps, sphingolipids, all may serve as predictive biomarkers for DFU formation and progression
- Lipid-modulating therapies for wound management
- Studied therapeutics including statins, PSK-9 inhibitors, and icosapent ethyl improve vascular health and atherosclerotic burden to combat PAD and wound development
- Evolving agents like resolvins, sphingosine-1-phosphate (S1P) analogs, and sRNA inhibitors offer inventive anti-inflammatory and lipid-regulating strategies relevant to wound healing and PAD prevention
- Role of spatial lipidomics in personalized wound care
- Through mapping of lipid environments across skin layers and wound beds, spatial lipidomics holds the potential to tailor therapies based on individual lipid signatures, especially in patients with diabetes or PAD
10.0. Discussion
This review highlights the intricate interplay between diabetic neuropathy, vasculopathy and the development of non-healing diabetic wounds. On its own, DFUs significantly impact the patient’s quality of life, leading to adverse complications like chronic wounds, progressive infection, and limb amputation. Addressing these complications requires added comprehension of the complex mechanisms driving angiogenesis, vascular injury, and inflammation in diabetics, with lipid signaling emerging as a potential target for therapeutic intervention. By potentially addressing these multifactorial variables, clinical management of DFUs becomes feasible. Currently, the body of literature revolves around targeting cholesterol synthesis, preventing atheromatous plaque formation, and reducing chronic vascular injury. However, we outline advances that expand beyond the current dogma by targeting lipid therapy, VEGF, and other upstream factors that have not been addressed by conventional medical therapy.
Angiogenesis, vascular injury, and chronic inflammation all contribute to the formation and sentience of diabetic wounds. Previous research has long isolated these complications to impaired glycemic control, however, inspiring recent evidence has illustrated the importance of lipid mediation in diabetic pathophysiology. Lipid mediators, such as sphingolipids, prostaglandins, and ox-LDLs have been shown to impact endothelial function, nerve survival, and immune function, featuring the dual impact that lipids have on vascular and neural injury in diabetes. These advances also offer further impact outside of just the realm of diabetes. Chronic non-healing ulcers with the aforementioned derangements are seen in a plethora of individuals. Targeting lipid modulators to aid chronic wound healing offers a therapeutic option even for those non-afflicted by diabetes. Thus, there is application of these techniques towards vasculitis, trauma, chronic kidney disease, and other auto-immune or immunomodulating disorders. Spatial lipidomics, as highlighted in this review, provides researchers and clinicians with a transformative opportunity in identifying key lipid molecules involved in diabetic wounds. High-resolution mapping of lipid molecules in various tissues and skin pathologies improves the analysis of molecular signaling and allows for direct insight into cell-specific functions. This precise technology offers unique targets for future pharmacological interventions, serving to complement the current landscape of diabetes management with applications that can be added to other debilitating diseases.
11.0. Summary
The central dogma regarding the role of lipids in vasculopathy has for the most part been focused on the development of atherosclerotic plaque. To that end, lipids are a prime cause of coronary artery disease and peripheral vascular disease through the development of atheromas and foam cells. The review has outlined multiple mechanisms by which the lipid chemical cascade affects the vascular and nervous systems. Most importantly, diabetic ischemic limbs and nonhealing wounds exhibit several lipid-induced derangements of vascular proliferation. This derangement is largely through the loss of angiogenic balance between proangiogenic factors and anti-angiogenic factors. Research in the lipid cascade has shown that chemicals in the lipid biosynthesis and peroxidation pathways can affect this gentle balance of angiogenic factors. Modulating angiogenic factors such as VEGF and HIF-1 create pro-angiogenic conditions and vascular proliferation under hyperglycemic conditions where normal proliferation is impeded. Furthermore, the upstream role of lipid biosynthesis has also been implicated.
Lipid dysregulation not only impacts angiogenesis but also contributes to the development of neuropathy in diabetic ischemic tissue. Lipid peroxidation products, particularly ox-LDL, have been shown to impact nerve function, promoting oxidative stress and injury to sensory, motor, and autonomic neurons. The compromised release of key neuropeptides further compounds this damage, accelerating the dysfunction of healing mechanisms and the risk for ulceration. The interplay between lipid-induced neuropathy and vasculopathy dysregulation highlights the dual-pathway approach towards understanding ischemic injury and the resultant wound impact.
With recent advancements in spatial lipidomics, an even deeper understanding of the exact signaling pathways for lipids regarding angiogenesis, neuropathy, skin barrier functioning, and wound healing can be uncovered, potentiating the specialization of therapeutics in skin lipid distributions. Future implications of restoring blood flow range from preventing ischemic limb and amputation to even regeneration of nonhealing wound. The role of lipids is an exciting aspect of investigating diabetic ischemic tissue. The ability to use a multifactorial approach toward restoring blood flow and nerve function creates an exciting new world to explore the role of lipids in an area where their role has yet to be fully elucidated. Such an approach will open avenues for future clinical trials based on lipid-targeted therapies such as statins, fibrates, and lipid-based nanocarriers aimed at underlying factors such as dyslipidemia, inflammation, and impaired wound healing to improve outcomes for patients with diabetic foot ulcers.
Figure 1: Summary Figure.
This review summarizes how in diabetic subjects, persistent hyperglycemia affects lipid mediators and downstream signaling pathways that directly affect vascular integrity and nerve health leading to wound healing impairment and amputation.
SCOPE AND SIGNIFICANCE.
Vasculopathy and neuropathy remain pivotal areas of research in diabetic patients, given their profound consequences on disease progression and patient outcomes. This review focuses on the role of lipid signaling in the vascular and nervous systems, and their influence on wound healing (Figure 1). It highlights the interplay between these domains in diabetes, as previously these pathologies have largely been studied in isolation. This review emphasizes how key lipids function as mediators in neuropathy, vascular inflammation, and angiogenesis, with an emphasis on the affected signaling pathways in wound healing processes. Skin lipids, notably relevant to the pathophysiology of diabetic wounds, will also be explored in this review. By combining the discussion of vasculopathy and neuropathy, this review hopes to bridge the gap for understanding the framework behind diabetic wound pathology.
CLINICAL RELEVANCE.
Given the significant health burden associated with diabetic foot ulcers (DFUs), including the risk for amputation and the related socioeconomic effects, this review capitalizes on the need for continued research into targeted therapeutic interventions. The emerging field of lipidomics, particularly spatial lipidomics, highlighted in this review, allows for directed insight into promising strategies that both researchers and clinicians can utilize. Through visualization of lipid atmospheres and lipid-mediated processes at a molecular level, lipidomics holds the potential for revolutionizing future approaches into the therapeutic management of angiogenesis, nerve regeneration, and wound healing in diabetic tissues. By identifying lipid mediators as prospective interventional targets, this review not only expands on the current understanding of diabetic pathology but also introduces novel approaches for clinical advancement beyond standard glycemic control.
TRANSLATIONAL RELEVANCE.
Translating the potential for lipid-based interventions into the optimization of blood flow, nerve function, and tissue regeneration is of notable importance in diabetic wound management. The integration of lipidomic technology allows for targeted analysis into precise, cell-tracking of lipid dynamics, which can motivate the development of lipid-based medications for the treatment and prevention of diabetic vasculopathy and neuropathy. These advancements can undoubtedly serve to improve the quality of life for diabetic patients. Reducing the incidence of amputation, a significant contributor to diabetic-related morbidity and mortality, is of critical importance. Ultimately, through lipid-based strategies, this review hopes to inspire development and utilization of individualized, precision-based approaches to manage diabetic vasculopathy and neuropathy.
TAKE-HOME MESSAGES.
Lipid signaling plays a pivotal role in the pathogenesis of diabetic vasculopathy, neuropathy, and wound healing. Targeted interventions can influence processes like angiogenesis, inflammation, repair, and nerve function.
The interplay between diabetic vasculopathy and neuropathy is significant. Lipid dysregulation in both conditions drives oxidative stress, endothelial damage, and nerve injury. Addressing these conditions may inspire remarkable therapeutic innovations in wound healing.
Advancements in spatial lipidomics could provide a transformative approach into lipid analysis at a cellular level, allowing for potential personalized care in diabetics.
Targeting lipid mediators like sphingolipids, resolvins, and oxidized LDLs may help restore angiogenesis, inspire nerve regeneration, and enhance tissue repair. Utilization of lipid-mediated pathways and lipidomic analysis, the future of diabetic wound management may shift towards targeted treatment strategies beyond glycemic control in addressing nervous and vascular pathologies.
Acknowledgements
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant DK136814 to K.S. This work was also supported in part by U.S. Department of Defense grants W81XWH-22-1-0146 and HT9425-24-1-0131 to K.S. This work was also supported in part by NIDDK grants DK141513 and DK135447 to C.K.S. Research programs led by K.S. and C.K.S. were also supported by Commonwealth of Pennsylvania (BT McGowan) research grants.
Abbreviations Used
- DFU
Diabetic Foot Ulcer
- VEGF
Vascular Endothelial Growth Factor
- HIF-1
Hypoxia-Inducible Factor 1
- S1P
Sphingosine-1-Phosphate
- GPCR
G Protein-Coupled Receptor
- LOX
Lipoxygenase
- DAG
Diacylglycerol
- SPM
Specialized Pro-Resolving Mediators
- Ox-LDL
Oxidized Low-Density Lipoprotein
- LOX-1
Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1
- CGRP
Calcitonin Gene-Related Peptide
- SP
Substance P
- BNB
Blood-Nerve Barrier
- PLCγ2
Phospholipase C-gamma 2
- IP3
Inositol 1,4,5-trisphosphate
- CD
Conjugated Dienes
- PECAM
Platelet Endothelial Cell Adhesion Molecule
- TNT
Tissue Nanotransfection
- FASN
Fatty Acid Synthase
- RAGE
Receptor for Advanced Glycation Endproducts
Footnotes
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors state no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Theja Bhamidipati, DO is an Integrated Vascular Surgery Resident at Jefferson Einstein Health. John Hajj, BS is a fourth-year medical student at Indiana University School of Medicine. Nehal I Ghoneim, MS, is a student in the Biotechnology program, School of Science and Engineering, The American University in Cairo, Egypt. Arhat M Pradhan, BS is a research technician at the McGowan Institute of Regenerative Medicine (MIRM), University of Pittsburgh. Sanjay Mishra, PhD, is a Research Assistant Professor in the Department of Pathology at The Ohio State University. Bharat Gummalla, BS is a medical student at Indiana School of Medicine. Ahmed Safwat Abouhashem, PhD, is an Assistant Professor at the MIRM, University of Pittsburgh. Avanish Singh Parmar, PhD, is an Associate Professor, Department of Physics, Indian Institute of Technology (BHU) Varanasi. Savita Khanna, PhD, is a Professor at MIRM, University of Pittsburgh. Sashwati Roy, PhD, is a Professor at MIRM, University of Pittsburgh. Chandan K. Sen, PhD, is a Professor and Director at the McGowan Institute for Regenerative Medicine, University of Pittsburgh. He is Associate Vice Chancellor for Life Science Innovation and Technology Commercialization and Chief Scientific Officer of University of Pittsburgh Medical Center (UPMC) Wound Healing Services. Kanhaiya Singh, PhD, is an Associate Professor at the MIRM, University of Pittsburgh.
Conflicts of Interest
The authors declare no conflict of interest.
Data Availability
No data was used to support this review article.
References:
- 1.Akkus G, Sert M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. Dec 15 2022;13(12):1106–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amelia R, Wahyuni AS, Yunanda Y. Diabetic Neuropathy among Type 2 Diabetes Mellitus Patients at Amplas Primary Health Care in Medan City. Open Access Maced J Med Sci. Oct 30 2019;7(20):3400–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger). Ann Med. Mar 2017;49(2):106–116. [DOI] [PubMed] [Google Scholar]
- 4.McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care. Jan 1 2023;46(1):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puranik AK, Maity S, Meena SP, Santra A, Lodha M, Badkur M. Quality of Life of Patients With Major Amputations in the Tertiary Care Center of Western Rajasthan: A Prospective Observational Study in 2019–2020. Cureus. Dec 2021;13(12):e20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajj J, Sizemore B, Singh K. Impact of Epigenetics, Diet, and Nutrition-Related Pathologies on Wound Healing. Int J Mol Sci. Sep 28 2024;25(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhamidipati T, Kumar M, Verma SS, et al. Epigenetic basis of diabetic vasculopathy. Front Endocrinol (Lausanne). 2022;13:989844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhamidipati T, Sinha M, Sen CK, Singh K. Laser Capture Microdissection in the Spatial Analysis of Epigenetic Modifications in Skin: A Comprehensive Review. Oxid Med Cell Longev. 2022;2022:4127238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh K, Pal D, Sinha M, et al. Epigenetic Modification of MicroRNA-200b Contributes to Diabetic Vasculopathy. Mol Ther. Dec 6 2017;25(12):2689–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G, Bai H, Mather B, Hill MA, Jia G, Sowers JR. Diabetic Vasculopathy: Molecular Mechanisms and Clinical Insights. Int J Mol Sci. Jan 9 2024;25(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rustagi Y, Abouhashem AS, Verma P, et al. Endothelial Phospholipase Cgamma2 Improves Outcomes of Diabetic Ischemic Limb Rescue Following VEGF Therapy. Diabetes. May 1 2022;71(5):1149–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mota RI, Morgan SE, Bahnson EM. Diabetic vasculopathy: macro and microvascular injury. Curr Pathobiol Rep. Mar 2020;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloete L Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand. Jan 5 2022;37(1):61–66. [DOI] [PubMed] [Google Scholar]
- 14.Bodman MA, Dreyer MA, Varacallo M. Diabetic Peripheral Neuropathy. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Mark Dreyer declares no relevant financial relationships with ineligible companies. Disclosure: Matthew Varacallo declares no relevant financial relationships with ineligible companies.2024. [Google Scholar]
- 15.Bodman MA, Dreyer MA, Varacallo MA. Diabetic Peripheral Neuropathy. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Mark Dreyer declares no relevant financial relationships with ineligible companies. Disclosure: Matthew Varacallo declares no relevant financial relationships with ineligible companies.2025. [Google Scholar]
- 16.Volmer-Thole M, Lobmann R. Neuropathy and Diabetic Foot Syndrome. Int J Mol Sci. Jun 10 2016;17(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomatos EL, Dulebohn SC, Rehman A. Sensory Neuropathy. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Scott Dulebohn declares no relevant financial relationships with ineligible companies. Disclosure: Anis Rehman declares no relevant financial relationships with ineligible companies.2024. [Google Scholar]
- 18.Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol (1985). Feb 2009;106(2):566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raja JM, Maturana MA, Kayali S, Khouzam A, Efeovbokhan N. Diabetic foot ulcer: A comprehensive review of pathophysiology and management modalities. World J Clin Cases. Mar 16 2023;11(8):1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. Oct 2009;25(7):604–614. [DOI] [PubMed] [Google Scholar]
- 21.Andersen H Motor neuropathy. Handb Clin Neurol. 2014;126:81–95. [DOI] [PubMed] [Google Scholar]
- 22.Rota E, Morelli N. Entrapment neuropathies in diabetes mellitus. World J Diabetes. Sep 15 2016;7(17):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorneskog G, Fagrell B. Discrepancy in skin capillary circulation between fingers and toes in patients with type 1 diabetes. Int J Microcirc Clin Exp. Nov-Dec 1996;16(6):313–319. [DOI] [PubMed] [Google Scholar]
- 24.Watkins PJ, Edmonds ME. Sympathetic nerve failure in diabetes. Diabetologia. Aug 1983;25(2):73–77. [DOI] [PubMed] [Google Scholar]
- 25.Pejkova S, Georgieva G, Jordanova SP, et al. Dellon decompression of the tarsal tunnel: An effective approach to improving blood flow, promoting ulcer healing and recovery of sensibility in diabetic patients. J Plast Reconstr Aesthet Surg. Apr 2025;103:48–57. [DOI] [PubMed] [Google Scholar]
- 26.Nickerson DS, Yamasaki DS. Objective Evidence That Nerve Decompression Surgery Reduces Neuropathic DFU Recurrence Risk to Less than 5%. Adv Wound Care (New Rochelle). Jul 2024;13(7):363–374. [DOI] [PubMed] [Google Scholar]
- 27.Rozen SM, Wolfe GI, Vernino S, et al. Effect of Lower Extremity Nerve Decompression in Patients With Painful Diabetic Peripheral Neuropathy: The Diabetic Neuropathy Nerve Decompression Randomized, Observation Group and Placebo Surgery-Controlled Clinical Trial. Ann Surg. Jul 1 2024;280(1):35–45. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JC, Nickerson DS, Tracy BL, Paxton RJ, Yamasaki DS. Acute Improvement in Intraoperative EMG Following Common Fibular Nerve Decompression in Patients with Symptomatic Diabetic Sensorimotor Peripheral Neuropathy: 1. EMG Results. J Neurol Surg A Cent Eur Neurosurg. Sep 2017;78(5):419–430. [DOI] [PubMed] [Google Scholar]
- 29.Bus SA, Armstrong DG, Crews RT, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. Mar 2024;40(3):e3647. [DOI] [PubMed] [Google Scholar]
- 30.Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. Mar 2020;36 Suppl 1:e3283. [DOI] [PubMed] [Google Scholar]
- 31.Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. Jul 3 2017;18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. Sep 8 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 33.Lamont P, Franklyn K, Rayman G, Boulton AJ. Update on the diabetic foot 2012: the 14th biennial Malvern Diabetic Foot Conference, May 9–11, 2012. Int J Low Extrem Wounds. Mar 2013;12(1):71–75. [DOI] [PubMed] [Google Scholar]
- 34.Griffioen AW, Dudley AC. Angiogenesis: a year in review. Angiogenesis. May 2021;24(2):195–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yetkin-Arik B, Vogels IMC, Nowak-Sliwinska P, et al. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Sci Rep. Aug 30 2019;9(1):12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. Jun 2011;10(6):417–427. [DOI] [PubMed] [Google Scholar]
- 37.Xu C, Hasan SS, Schmidt I, et al. Arteries are formed by vein-derived endothelial tip cells. Nat Commun. Dec 15 2014;5:5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. Mar 7 2019;176(6):1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. [DOI] [PubMed] [Google Scholar]
- 40.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. Nov 2011;138(21):4569–4583. [DOI] [PubMed] [Google Scholar]
- 41.Sellmayer A, Hrboticky N, Weber PC. Lipids in vascular function. Lipids. 1999;34 Suppl:S13–18. [DOI] [PubMed] [Google Scholar]
- 42.Yan S-f Zhang J-k, Zhang T, Li Y, Li X. Advances in endothelial cell lipid metabolism and tumor angiogenesis. Results in Chemistry. 2024/01/01/ 2024;7:101467. [Google Scholar]
- 43.Engelbrecht E, MacRae CA, Hla T. Lysolipids in Vascular Development, Biology, and Disease. Arterioscler Thromb Vasc Biol. Feb 2021;41(2):564–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendelson K, Zygmunt T, Torres-Vazquez J, Evans T, Hla T. Sphingosine 1-phosphate receptor signaling regulates proper embryonic vascular patterning. J Biol Chem. Jan 25 2013;288(4):2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol.May 2015;27(3):200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nihei W, Kato A, Himeno T, et al. Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4. Neurol Int. Mar 19 2024;16(2):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Roh J, Pan J, Kim YH, Park CK, Jo YY. Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals (Basel). Sep 27 2023;16(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su CJ, Zhang JT, Zhao FL, Xu DL, Pan J, Liu T. Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice. Front Immunol. 2023;14:1091753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tessaro FH, Ayala TS, Martins JO. Lipid mediators are critical in resolving inflammation: a review of the emerging roles of eicosanoids in diabetes mellitus. Biomed Res Int. 2015;2015:568408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu A, Zankov DP, Kurokawa-Seo M, Ogita H. Vascular Endothelial Growth Factor-A Exerts Diverse Cellular Effects via Small G Proteins, Rho and Rap. Int J Mol Sci. Apr 16 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ksiazek M, Chacinska M, Chabowski A, Baranowski M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res. Jul 2015;56(7):1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camerer E, Regard JB, Cornelissen I, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. Jul 2009;119(7):1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. Oct 2000;106(8):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cartier A, Leigh T, Liu CH, Hla T. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc Natl Acad Sci U S A. Feb 11 2020;117(6):3157–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. Nov 15 2003;102(10):3665–3667. [DOI] [PubMed] [Google Scholar]
- 56.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. Jun 1999;126(14):3047–3055. [DOI] [PubMed] [Google Scholar]
- 57.Kai M, Heisenberg CP, Tada M. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development. Sep 2008;135(18):3043–3051. [DOI] [PubMed] [Google Scholar]
- 58.Iqbal Z, Bashir B, Ferdousi M, et al. Lipids and peripheral neuropathy. Curr Opin Lipidol. Aug 1 2021;32(4):249–257. [DOI] [PubMed] [Google Scholar]
- 59.Cermenati G, Mitro N, Audano M, et al. Lipids in the nervous system: from biochemistry and molecular biology to patho-physiology. Biochim Biophys Acta. Jan 2015;1851(1):51–60. [DOI] [PubMed] [Google Scholar]
- 60.Barnes-Velez JA, Aksoy Yasar FB, Hu J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innovation (Camb). Jan 30 2023;4(1):100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saher G, Quintes S, Mobius W, et al. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. May 13 2009;29(19):6094–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathews ES, Mawdsley DJ, Walker M, Hines JH, Pozzoli M, Appel B. Mutation of 3-hydroxy-3-methylglutaryl CoA synthase I reveals requirements for isoprenoid and cholesterol synthesis in oligodendrocyte migration arrest, axon wrapping, and myelin gene expression. J Neurosci. Feb 26 2014;34(9):3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of Inflammation: What Controls Its Onset? Front Immunol. 2016;7:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira I, Falcato F, Bandarra N, Rauter AP. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules. Mar 3 2022;27(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Junaid A, Schoeman J, Yang W, et al. Metabolic response of blood vessels to TNFalpha. Elife. Aug 4 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adibhatla RM, Hatcher JF. Role of Lipids in Brain Injury and Diseases. Future Lipidol. Aug 2007;2(4):403–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. Jul 25 2013;8(21):2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Njajou OT, Kanaya AM, Holvoet P, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. Nov 2009;25(8):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Duan Z, Gao D, Huang S, Yuan H, Niu X. The new role of LOX-1 in hypertension induced neuronal apoptosis. Biochem Biophys Res Commun. Sep 7 2012;425(4):735–740. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. Oct 2011;25(5):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. Oct 2009;58(10):2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung MY, Park SY, Chung JO, Cho DH, Chung DJ. Plasma sphingosine 1-phosphate concentrations and cardiovascular autonomic neuropathy in individuals with type 2 diabetes. Sci Rep. Jul 29 2020;10(1):12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiong Y, Hla T. S1P control of endothelial integrity. Curr Top Microbiol Immunol. 2014;378:85–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lucaciu A, Brunkhorst R, Pfeilschifter JM, Pfeilschifter W, Subburayalu J. The S1P-S1PR Axis in Neurological Disorders-Insights into Current and Future Therapeutic Perspectives. Cells. Jun 22 2020;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schira-Heinen J, Wang L, Akgun S, et al. Modulation of Specific Sphingosine-1-Phosphate Receptors Augments a Repair Mediating Schwann Cell Phenotype. Int J Mol Sci. Sep 7 2022;23(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun G, Wang B, Wu X, et al. How do sphingosine-1-phosphate affect immune cells to resolve inflammation? Front Immunol. 2024;15:1362459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng Y, Chen Y, Li K, et al. How inflammation dictates diabetic peripheral neuropathy: An enlightening review. CNS Neurosci Ther. Apr 2024;30(4):e14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pils V, Terlecki-Zaniewicz L, Schosserer M, Grillari J, Lammermann I. The role of lipid-based signalling in wound healing and senescence. Mech Ageing Dev. Sep 2021;198:111527. [DOI] [PubMed] [Google Scholar]
- 79.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. Feb 2012;91(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silva JR, Burger B, Kuhl CMC, Candreva T, Dos Anjos MBP, Rodrigues HG. Wound Healing and Omega-6 Fatty Acids: From Inflammation to Repair. Mediators Inflamm. 2018;2018:2503950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borodzicz S, Rudnicka L, Mirowska-Guzel D, Cudnoch-Jedrzejewska A. The role of epidermal sphingolipids in dermatologic diseases. Lipids Health Dis. Jan 19 2016;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albeituni S, Stiban J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv Exp Med Biol. 2019;1161:169–191. [DOI] [PubMed] [Google Scholar]
- 84.Sinha M, Ghosh N, Wijesinghe DS, et al. Pseudomonas Aeruginosa Theft Biofilm Require Host Lipids of Cutaneous Wound. Ann Surg. Mar 1 2023;277(3):e634–e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horn A, Jaiswal JK. Structural and signaling role of lipids in plasma membrane repair. Curr Top Membr. 2019;84:67–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. May 2008;58(5):255–262. [DOI] [PubMed] [Google Scholar]
- 87.English D, Brindley DN, Spiegel S, Garcia JG. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim Biophys Acta. May 23 2002;1582(1–3):228–239. [DOI] [PubMed] [Google Scholar]
- 88.Sasso O, Pontis S, Armirotti A, et al. Endogenous N-acyl taurines regulate skin wound healing. Proc Natl Acad Sci U S A. Jul 26 2016;113(30):E4397–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaughan EM, You JS, Elsie Yu HY, et al. Lipid domain-dependent regulation of single-cell wound repair. Mol Biol Cell. Jun 15 2014;25(12):1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choudhary V, Choudhary M, Bollag WB. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int J Mol Sci. Mar 28 2024;25(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosa DF, Sarandy MM, Novaes RD, da Matta SLP, Goncalves RV. Effect of a high-fat diet and alcohol on cutaneous repair: A systematic review of murine experimental models. PLoS One. 2017;12(5):e0176240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. Jan 14 2009;29(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh K, Singh VK, Agrawal NK, Gupta SK, Singh K. Genetic Alterations in Toll-Like Receptor 4 Signaling Pathway and Impairment of Wound Healing in Patients With Type 2 Diabetes. Int J Low Extrem Wounds. Jun 2014;13(2):162–163. [DOI] [PubMed] [Google Scholar]
- 94.Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines (Basel). Oct 4 2017;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol. Jan 2013;133(1):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruning U, Morales-Rodriguez F, Kalucka J, et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. Dec 4 2018;28(6):866–880 e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bogachkov YY, Chen L, Le Master E, et al. LDL induces cholesterol loading and inhibits endothelial proliferation and angiogenesis in Matrigels: correlation with impaired angiogenesis during wound healing. Am J Physiol Cell Physiol. Apr 1 2020;318(4):C762–C776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez LL, Garrie K, Turner MD. Role of S100 proteins in health and disease. Biochim Biophys Acta Mol Cell Res. Jun 2020;1867(6):118677. [DOI] [PubMed] [Google Scholar]
- 99.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. Jul 2004;123(1):23–33. [DOI] [PubMed] [Google Scholar]
- 100.Singh P, Ali SA. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells. Jul 23 2022;11(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saito-Sasaki N, Sawada Y. S100 Proteins in the Pathogenesis of Psoriasis and Atopic Dermatitis. Diagnostics (Basel). Oct 10 2023;13(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolf R, Howard OM, Dong HF, et al. Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. Jul 15 2008;181(2):1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra S, Charan M, Shukla RK, et al. cPLA2 blockade attenuates S100A7-mediated breast tumorigenicity by inhibiting the immunosuppressive tumor microenvironment. J Exp Clin Cancer Res. Feb 8 2022;41(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaorong Z, Xiaodong L, Qiong P, et al. SNHG12/NFYC-AS1 Acted as the Sponge for hsa-miR-199a-5p to Promote the Expression of S100A8/S100A7/XDH and was Involved in the Progression of Diabetic Foot Ulcers. Mol Biotechnol. Dec 2023;65(12):2038–2048. [DOI] [PubMed] [Google Scholar]
- 105.Wu S, Wang Y, Duan J, Teng Y, Wang D, Qi F. Identification of a shared gene signature and biological mechanism between diabetic foot ulcers and cutaneous lupus erythemnatosus by transcriptomic analysis. Front Physiol. 2024;15:1297810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruse M, Broome AM, Eckert RL. S100A7 (psoriasin) interacts with epidermal fatty acid binding protein and localizes in focal adhesion-like structures in cultured keratinocytes. J Invest Dermatol. Jul 2003;121(1):132–141. [DOI] [PubMed] [Google Scholar]
- 107.Gao C, Li J. Exploring the comorbidity mechanisms between psoriasis and obesity based on bioinformatics. Skin Res Technol. Feb 2024;30(2):e13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med. Aug 13–27 2007;167(15):1670–1675. [DOI] [PubMed] [Google Scholar]
- 109.Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J Am Acad Dermatol. Feb 2018;78(2):315–322 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng Z, Du Z, Shu X, et al. Role of RAGE in obesity-induced adipose tissue inflammation and insulin resistance. Cell Death Discov. Oct 22 2021;7(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Q, Zhu G, Cao X, Dong J, Song F, Niu Y. Blocking AGE-RAGE Signaling Improved Functional Disorders of Macrophages in Diabetic Wound. J Diabetes Res. 2017;2017:1428537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shubbar E, Vegfors J, Carlstrom M, Petersson S, Enerback C. Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res Treat. Jul 2012;134(1):71–80. [DOI] [PubMed] [Google Scholar]
- 113.Vegfors J, Ekman AK, Stoll SW, Bivik Eding C, Enerback C. Psoriasin (S100A7) promotes stress-induced angiogenesis. Br J Dermatol. Dec 2016;175(6):1263–1273. [DOI] [PubMed] [Google Scholar]
- 114.Mishra S, Ahirwar DK, Ganju RK. Psoriasin (S100A7): a novel mediator of angiogenesis. Br J Dermatol. Dec 2016;175(6):1141–1142. [DOI] [PubMed] [Google Scholar]
- 115.Cheng R, Ma JX. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord. Mar 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Demidova-Rice TN, Durham JT, Herman IM. Wound Healing Angiogenesis: Innovations and Challenges in Acute and Chronic Wound Healing. Adv Wound Care (New Rochelle). Feb 2012;1(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. Dec 2000;5(1):40–46. [DOI] [PubMed] [Google Scholar]
- 118.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. APMIS. Jul-Aug 2008;116(7–8):695–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. Feb 2007;24(2):258–264. [DOI] [PubMed] [Google Scholar]
- 120.Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. Jul 3 2023;330(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sen CK, Roy S, Khanna S. Diabetic Peripheral Neuropathy Associated with Foot Ulcer: One of a Kind. Antioxid Redox Signal. Jan 25 2023. [DOI] [PubMed] [Google Scholar]
- 122.Uccioli L, Mancini L, Giordano A, et al. Lower limb arterio-venous shunts, autonomic neuropathy and diabetic foot. Diabetes Res Clin Pract. May 1992;16(2):123–130. [DOI] [PubMed] [Google Scholar]
- 123.Scott AR, MacDonald IA, Bennett T, Tattersall RB. Abnormal thermoregulation in diabetic autonomic neuropathy. Diabetes. Jul 1988;37(7):961–968. [DOI] [PubMed] [Google Scholar]
- 124.Horton WB, Barrett EJ. Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease. Endocr Rev. Jan 28 2021;42(1):29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pejkova S, Georgieva G, Jordanova SP, et al. Dellon decompression of the tarsal tunnel: An effective approach to improving blood flow, promoting ulcer healing and recovery of sensibility in diabetic patients. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2025/04/01/ 2025;103:48–57. [DOI] [PubMed] [Google Scholar]
- 126.Bolton L Diabetic foot ulcer: treatment challenges. Wounds. Jun 2022;34(6):175–177. [PubMed] [Google Scholar]
- 127.Dinoto E, Ferlito F, La Marca MA, et al. The Role of Early Revascularization and Biomarkers in the Management of Diabetic Foot Ulcers: A Single Center Experience. Diagnostics (Basel). Feb 19 2022;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee L, Thwaites SE, Rahmatzadeh M, Yii E, Yoong K, Yii M. Proximal Arterial Inflow Revascularization Improves Pedal Arch Quality and Its Impact on Ischemic Diabetic Foot Ulcer Healing. Ann Vasc Surg. Jun 2024;103:23–30. [DOI] [PubMed] [Google Scholar]
- 129.Saab FA, Mustapha JA, Ansari M, et al. Percutaneous Deep Venous Arterialization: Treatment of Patients with End-Stage Plantar Disease. J Soc Cardiovasc Angiogr Interv. Nov-Dec 2022;1(6):100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agrawal RP, Sharma P, Pal M, Kochar A, Kochar DK. Magnitude of dyslipedemia and its association with micro and macro vascular complications in type 2 diabetes: a hospital based study from Bikaner (Northwest India). Diabetes Res Clin Pract. Aug 2006;73(2):211–214. [DOI] [PubMed] [Google Scholar]
- 131.Ahmed S, Shah P, Ahmed O. Biochemistry, Lipids. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Parini Shah declares no relevant financial relationships with ineligible companies. Disclosure: Owais Ahmed declares no relevant financial relationships with ineligible companies.2024. [Google Scholar]
- 132.Hirano T Pathophysiology of Diabetic Dyslipidemia. J Atheroscler Thromb. Sep 1 2018;25(9):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parhofer KG. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab J. Oct 2015;39(5):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dudley AC, Griffioen AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. Aug 2023;26(3):313–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lubrano V, Ndreu R, Balzan S. Classes of Lipid Mediators and Their Effects on Vascular Inflammation in Atherosclerosis. Int J Mol Sci. Jan 13 2023;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thurlow LR, Stephens AC, Hurley KE, Richardson AR. Lack of nutritional immunity in diabetic skin infections promotes Staphylococcus aureus virulence. Sci Adv. Nov 2020;6(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Polk C, Sampson MM, Roshdy D, Davidson LE. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. Infect Dis Clin North Am. Mar 2021;35(1):183–197. [DOI] [PubMed] [Google Scholar]
- 138.Rajpaul K Biofilm in wound care. Br J Community Nurs. Mar 2015;Suppl Wound Care:S6, S8, S10–11. [DOI] [PubMed] [Google Scholar]
- 139.Swinnen JV, Dehairs J. A beginner’s guide to lipidomics. The Biochemist. 2022;44(1):20–24. [Google Scholar]
- 140.Li D, Ouyang Z, Ma X. Mass Spectrometry Imaging for Single-Cell or Subcellular Lipidomics: A Review of Recent Advancements and Future Development. Molecules. Mar 17 2023;28(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sochorova M, Vavrova K, Fedorova M, et al. Research Techniques Made Simple: Lipidomic Analysis in Skin Research. J Invest Dermatol. Jan 2022;142(1):4–11 e11. [DOI] [PubMed] [Google Scholar]
- 142.Berdyshev E Skin Lipid Barrier: Structure, Function and Metabolism. Allergy Asthma Immunol Res. Sep 2024;16(5):445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Sun Z, Zang G, Zhang L, Wang Z. Role of ceramides in diabetic foot ulcers (Review). Int J Mol Med. Mar 2023;51(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kendall AC, Koszyczarek MM, Jones EA, et al. Lipidomics for translational skin research: A primer for the uninitiated. Exp Dermatol. Jul 2018;27(7):721–728. [DOI] [PubMed] [Google Scholar]
- 145.Zhou M, Yang M, Zheng Y, et al. Skin surface lipidomics revealed the correlation between lipidomic profile and grade in adolescent acne. J Cosmet Dermatol. Dec 2020;19(12):3349–3356. [DOI] [PubMed] [Google Scholar]
- 146.Ferreira DT, Shen BQ, Mwirigi JM, et al. Deciphering the molecular landscape of human peripheral nerves: implications for diabetic peripheral neuropathy. bioRxiv. Jun 16 2024. [Google Scholar]
- 147.Cho C, Haddadi N-S, Kidacki M, et al. Spatial Transcriptomics in Inflammatory Skin Diseases Using GeoMx Digital Spatial Profiling: A Practical Guide for Applications in Dermatology. JID Innovations. 2025/01/01/ 2025;5(1):100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pal D, Ghatak S, Singh K, et al. Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat Commun. Feb 28 2023;14(1):1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gharbia FZ, Abouhashem AS, Moqidem YA, et al. Adult skin fibroblast state change in murine wound healing. Sci Rep. Jan 17 2023;13(1):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Foster DS, Januszyk M, Yost KE, et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci U S A. Oct 12 2021;118(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sinha M, Sen CK, Singh K, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. Mar 5 2018;9(1):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin S, Ramos R. Computational exploration of cellular communication in skin from emerging single-cell and spatial transcriptomic data. Biochem Soc Trans. Feb 28 2022;50(1):297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Theocharidis G, Thomas BE, Sarkar D, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. Jan 10 2022;13(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Z, Li D, Bian X, et al. Spatiotemporal Single-Cell Roadmap of Human Skin Wound Healing. bioRxiv. 2024:2024.2008.2030.609923. [DOI] [PubMed] [Google Scholar]
- 155.Singh K, Rustagi Y, Abouhashem AS, et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J Clin Invest. Sep 1 2022;132(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seiringer P, Hillig C, Schabitz A, et al. Spatial transcriptomics reveals altered lipid metabolism and inflammation-related gene expression of sebaceous glands in psoriasis and atopic dermatitis. Front Immunol. 2024;15:1334844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Worsley AL, Lui DH, Ntow-Boahene W, Song W, Good L, Tsui J. The importance of inflammation control for the treatment of chronic diabetic wounds. Int Wound J. Aug 2023;20(6):2346–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wei H, Hu C, Wang M, et al. Lipidomics reveals multiple pathway effects of a multi-components preparation on lipid biochemistry in ApoE*3Leiden.CETP mice. PLoS One. 2012;7(1):e30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int J Mol Sci. Apr 18 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu AL, Wu WC. Anti-VEGF for ROP and Pediatric Retinal Diseases. Asia Pac J Ophthalmol (Phila). May-Jun 2018;7(3):145–151. [DOI] [PubMed] [Google Scholar]
- 161.Zhu Y, Wang Y, Jia Y, Xu J, Chai Y. Roxadustat promotes angiogenesis through HIF-1alpha/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. Jul 2019;27(4):324–334. [DOI] [PubMed] [Google Scholar]
- 162.Jang MJ, Bae SK, Jung YS, et al. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed Mater. Apr 9 2021;16(4). [DOI] [PubMed] [Google Scholar]
- 163.Mezentsev A, Seta F, Dunn MW, Ono N, Falck JR, Laniado-Schwartzman M. Eicosanoid regulation of vascular endothelial growth factor expression and angiogenesis in microvessel endothelial cells. J Biol Chem. May 24 2002;277(21):18670–18676. [DOI] [PubMed] [Google Scholar]
- 164.Nie D, Tang K, Diglio C, Honn KV. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. Apr 1 2000;95(7):2304–2311. [PubMed] [Google Scholar]
- 165.Cao L, Arany PR, Kim J, et al. Modulating Notch signaling to enhance neovascularization and reperfusion in diabetic mice. Biomaterials. Dec 2010;31(34):9048–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Briot A, Bouloumie A, Iruela-Arispe ML. Notch, lipids, and endothelial cells. Curr Opin Lipidol. Oct 2016;27(5):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verma SS, Sen CK, Srivastava R, et al. Tissue nanotransfection-based endothelial PLCgamma2-targeted epigenetic gene editing rescues perfusion and diabetic ischemic wound healing. Mol Ther. Mar 5 2025;33(3):950–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Altavilla D, Saitta A, Cucinotta D, et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. Mar 2001;50(3):667–674. [DOI] [PubMed] [Google Scholar]
- 169.Mohanty SK, Singh K, Kumar M, et al. Vasculogenic skin reprogramming requires TET-mediated gene demethylation in fibroblasts for rescuing impaired perfusion in diabetes. Nat Commun. Nov 27 2024;15(1):10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Srivastava R, Singh K, Abouhashem AS, et al. Human fetal dermal fibroblast-myeloid cell diversity is characterized by dominance of pro-healing Annexin1-FPR1 signaling. iScience. Sep 15 2023;26(9):107533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gordillo GM, Guda PR, Singh K, et al. Tissue nanotransfection causes tumor regression by its effect on nanovesicle cargo that alters microenvironmental macrophage state. Mol Ther. May 3 2023;31(5):1402–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xuan Y, Ghatak S, Clark A, et al. Fabrication and use of silicon hollow-needle arrays to achieve tissue nanotransfection in mouse tissue in vivo. Nat Protoc. Dec 2021;16(12):5707–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou X, Brown BA, Siegel AP, et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano. Oct 27 2020;14(10):12732–12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gallego-Perez D, Pal D, Ghatak S, et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol. Oct 2017;12(10):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kenchegowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. May 2010;51(5):879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chung WK, Erion K, Florez JC, et al. Precision Medicine in Diabetes: A Consensus Report From the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. Jul 2020;43(7):1617–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fitridge R, Chuter V, Mills J, et al. The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes mellitus and a foot ulcer. J Vasc Surg. Nov 2023;78(5):1101–1131. [DOI] [PubMed] [Google Scholar]
- 178.Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. Aug 2020;40(8):1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi JS, Kumar M, Wilson AR, et al. Multistate Model of Chronic Wounds, Amputations, and Mortality: Cohort Study of a State-wide Registry. Ann Surg. May 21 2025. [DOI] [PubMed] [Google Scholar]
- 180.Belur AD, Shah AJ, Virani SS, Vorla M, Kalra DK. Role of Lipid-Lowering Therapy in Peripheral Artery Disease. J Clin Med. Aug 19 2022;11(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Elmasry K, Ibrahim AS, Abdulmoneim S, Al-Shabrawey M. Bioactive lipids and pathological retinal angiogenesis. Br J Pharmacol. Jan 2019;176(1):93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wiley CD, Brumwell AN, Davis SS, et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight. Dec 19 2019;4(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nakajima M, Hashimoto M, Wang F, et al. Aging decreases the production of PGI2 in rat aortic endothelial cells. Exp Gerontol. Nov-Dec 1997;32(6):685–693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used to support this review article.