Abstract
Background:
Modern treatment strategies have markedly improved the chances of survival for patients with cancer. As the population ages, cancer is becoming more common, as is chronic kidney disease (CKD). CKD increases the risk of cancer; conversely, cancer treatments can cause CKD.
Methods:
This review is based on publications retrieved by a selective literature search concerning the epidemiology and comorbidities of cancer and kidney diseases, the renal side effects of new anticancer drugs, and the need to consider renal function in cancer treatment.
Results:
The prevalence of severe CKD in Germany is 2.3%. Persons who have CKD, are on dialysis, or have undergone kidney transplantation are 1.2 to 3.5 times more likely to develop cancer than the general population. For patients who have CKD or are dialysis-dependent, the doses of approximately 67% of anticancer drugs need to be adjusted on the basis of their glomerular filtration rate and the renally excreted fraction of the drug. The optimal efficacy of therapeutic drugs, as well as of those used for diagnostic purposes, and the minimization of side effects, depend critically on adapted dosing and on proper timing of administration before or after dialysis. Modern anticancer drugs can also cause acute kidney damage (incidence with checkpoint inhibitors: 2–16%).
Conclusion:
Patients who have CKD, are on dialysis, or have undergone kidney transplantation make up a considerable fraction of persons being treated for cancer, and they need interdisciplinary treatment.
CME plus+
This article has been recognized by the Medical Association North Rhine for the Medical Association‘s CME certificate. The CME questions on this article can be found at http://daebl.de/RY95. The deadline for submission is 28 November 2025.
Participation is possible at cme.aerzteblatt.de
Increasing life expectancy, rising incidences of cancer and the development of new anticancer treatments have led to extended patient survival. As a result, there are more and more intersections between oncology and nephrology. Key aspects of onconephrology include measurement of kidney function, epidemiological aspects of cancer in patients with chronic kidney disease (CKD), renal side effects, and the dosing of drugs in patients with impaired kidney function and on dialysis. The aim of this review is to provide an overview of important topics in the field of onconephrology.
Methods
This review is based on publications retrieved by a selective literature search in the PubMed and WebofScience databases. The search terms included “onconephrology”, “cancer“ (title) or “chemotherapy“ (title) AND “chronic kidney disease“, “glomerular filtration rate“, “hemodialysis“, and “dialysis“. The search period covered the years 2000–2023, the search date was in 2023. Peer-reviewed articles in German or English were included in our review. The guidelines of Kidney Disease Improving Global Outcome (KDIGO), the European Society of Medical Oncology (ESMO), and the American Society of Clinical Oncology (ASCO), as well as the classification according to Common Terminology Criteria for Adverse Events (CTCAE) were also taken into account.In addition, the manufacturers’ summaries of product characteristics of anticancer drugs were considered. Given the small patient population with diverse relevant comorbidities and heterogeneous tumor types, no randomized or non-randomized prospective clinical trials are available
Epidemiological aspects of kidney disease and cancer
Chronic kidney disease (CKD) is defined as abnormalities in kidney structure or function of at least 3 months’ duration, with associated health implications (1). CKD is divided into in five stages (G1-G5), based on the glomerular filtration rate (GFR). CKD is classified as mildly to moderately decreased (G3a) if the GFR decreases below 60 mL/min/1.73 m2, as moderately to severely decreased (G3b) if it decreases below 45 mL/min/1.73 m2 and as severely decreased (G4) if it decreases below 30 mL/min/1.73 m2. A GFR <15 mL/min/1.73 m2 (G5) indicates renal failure, often necessitating the initiation of renal replacement therapy (RRT). As an alternative or in addition, albuminuria, electrolyte disorders, histological/structural changes, and other factors may be present and lead to the diagnosis of CKD even in patients without impairment of GFR (1).
The global prevalence of CKD is 9.1%. In Germany, the prevalence of CKD is 2.3%, if only GFR < G3 is taken into account, and 12.7%, if albuminuria is also included. This means that almost two million people in Germany live with CKD (1, 2). The risk of developing CKD increases in the presence of comorbidities, such as diabetes (prevalence ratio: 2.25) or arterial hypertension (prevalence ratio: 3.46) (2). From age 60 onwards, the prevalence of CKD rises significantly.
In 2020, approximately 14 million people were newly diagnosed with cancer, of these approximately 500 000 in Germany (3, 4). By 2045, a worldwide increase in new cancer cases to 30.2 million and a mortality rate of 16.9 million people is expected (5). For Germany, an increase of 20.9% to 716,000 new cases is predicted (excluding non-melanoma skin cancer) (6).
Cancer as a comorbidity in CKD patients pre- and post-transplantation
Cancer is the second most common cause of death in people with chronic kidney disease, only exceeded by cardiovascular disease. However, in patients with a GFR of <60 mL/min/1.73 m2 and proteinuria, cancer mortality is higher than cardiovascular mortality (7). The cumulative cancer frequency is higher with a GFR of <60 mL/min/1.73 m2 compared to a GFR >60 mL/min/1.73 m2 and increases disproportionately over a period of 12 years (5). The causes underlying this increased occurrence are not fully understood. In men, an eGFR <40 mL/min/1.73 m2 is associated with the occurrence of colorectal and lung cancers as well as urogenital tumors, and this regardless of other risk factors, such as age and smoking status. In contrast, prostate cancer is not more common in men with advanced chronic kidney disease (8). No association with cancer incidence was found for women.
Among patients who are on dialysis, the standardized risk of developing cancer is increased compared to the normal population (standardized incidence ratio [SIR] 1.2–1.8). This applies to patients who are on hemodialysis or peritoneal dialysis, and mainly to cancers of the urogenital tract, such as renal cell carcinoma or cervical cancer (SIR up to 9.0). Conversely, the risk of developing breast cancer or prostate cancer is not increased in Europe and North America (9).
Even after kidney transplantation, a 3.5-fold increase in cancer risk remains (9). The most common malignancies include non-melanoma skin cancers, tumors of the urogenital tract and post-transplantation lymphoma. For example, 30–45% of all transplant recipients develop non-melanoma skin cancers (NMSC) within ten years of transplantation (10). The clinical course of the disease is the same as for patients without immunosuppression (11, 12). In addition to the cancer screening programs for the general population, KDIGO recommends for transplant recipients further special checks, such as annual skin checks by experienced doctors, regular UV protection, annual ultrasound examinations of the patients’ own kidneys, and EBV viral load measurement in high-risk patients (R -/D +) (13).
Determining renal function in cancer patients
Renal filtration, secretion and reabsorption are the three mechanisms underlying renal excretory capacity. Renal excretory capacity is described by GFR and synonymous with renal function. The gold standard of GFR measurement is inulin or iohexol clearance; however, this method is not used for reasons of practicality. Thus, GFR is estimated by measuring the serum levels of creatinine, which are dependent on a person‘s muscle mass; then, the eGFR is calculated based on these values. The Cockcroft-Gault formula allows the determination of creatinine clearance, the Modification of Diet in Renal Disease (MDRD) or CKD-EPI equation allows the determination of eGFR. These formulas take into account factors such as sex, weight, age, and ethnicity, as well as correction factors. All formulas to estimate GFR take the decline in renal function with age into account, which amounts to 1 mL/min per year. Each of these formulas has a certain accuracy range; for example, the MDRD formula significantly underestimates the true GFR in the upper GFR range. Here, the CKD-EPI formula provides more precise values, with fewer false positive results in the upper GFR range. In a wide intermediate range, however, the CKD-EPI formula estimates the GFR higher than the MDRD, resulting in a tendency to classify patients in higher stages of CKD. Alternatively, cystatin C can be used to determine the eGFR. It was shown for patients with solid tumors that the highest accuracy is achieved when the eGFR is determined using a combination of creatinine and cystatin C measurements (14). There is no guideline that specifies measurement intervals. It is advisable to determine the baseline levels of creatinine/cystatin C prior to the initiation of treatment. In patients undergoing treatment, regular monitoring should be performed according to the treatment protocol and the manufacturer‘s instructions/summary of product characteristics: weekly in patients receiving chemotherapy treatment and every two to four weeks in patients receiving immunotherapy treatment. If deviations occur and a renal cause is suspected, a nephrologist’s assessment should be obtained as part of the work-up (consensus of the authors).
No clear evidence exists as to which method of measurement or formula should be used to determine the GFR in patients receiving specific medications. Clearance should be determined based on a 24-hour urine sample (creatinine) or the measurement of cystatin C, if the therapeutic window of a drug is very narrow, in patients with cachexia or after amputation of a limb, as this is associated with decreased creatine levels and the clearance is overestimated by the Cockcroft-Gault formula (15). Therefore, the KDIGO guideline also recommends the use of a combined measurement method, the eGFRcr-cys, to determine the eGFR in patients with solid tumors (1). Given the limitations of the various formulas and of the molecule used for measurement, it is recommended that oncologists and nephrologists consult with each other.
Dosing of medications in patients with CKD and need for dialysis
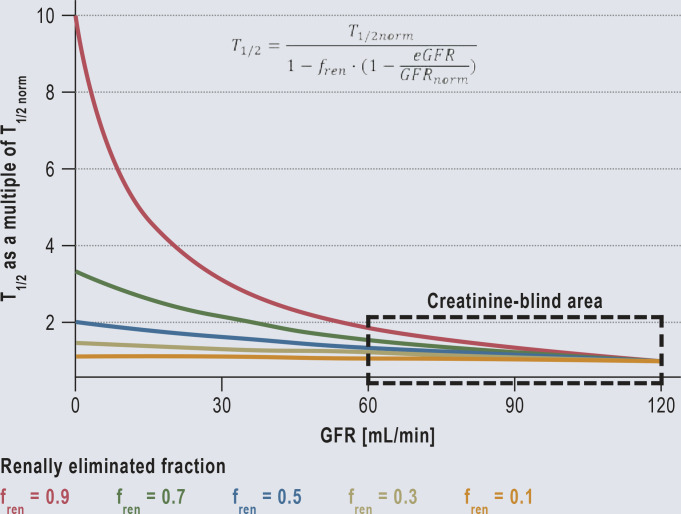
For the majority of drugs, toxic blood levels are more than twice as high as therapeutic levels. This means that dose adjustment is only necessary if the GFR falls below the no-effect boundary of 60 mL/min/1.73m2 (Figure 1 and eMethods a) for an example) (17).
Figure 1.
Dose reduction and the effects: Dose reduction and the effects: The half-life (T1/2) increases with decreasing renal function (GFR).
Even for a drug that is 90% excreted via the kidneys, the half-life will at most double if the GFR decreases to 60 mL/min. Thus, in the creatinine-blind area (GFR 60–120 mL/min) it is not yet necessary to adjust the dose, as with most substances even blood levels twice as high are not yet toxic. Consequently, even in the absence of renal function (GFR = 0), a dose adjustment is only necessary for substances that are excreted via the kidneys to more than 50% (fren >0.5).
The formula allows the half-life (T1/2) to be calculated for each stage of renal function impairment (eGFR). This is because the normal half-life (T1/2norm) and the renally eliminated fraction (fren) are part of the drug approval process and must therefore be known. Other authors also consider a dose adjustment to be necessary only from a GFR <60 mL/min) (17).
fren, renally eliminated fraction; GFR, glomerular filtration rate; T1/2, half-life
eMethods.
a) Example of carboplatin dose adjustment to kidney function
The renally excreted fraction of carboplatin is high (fren= 0.70) and, as with all medicines, the dosage must have been determined prior to approval. Thus, this information is available. Thus, the eGFR, determined by the laboratory (for example 30 mL/min) is sufficient to individually adjust the dose from the standard dose (Dnorm= 400 mg/m2) to the level of renal function. The derivation of the formula can be found at b).
Explanation of the Hill coefficient
According to the Hill equation, the greater the maximum effect (Emax) of a drug, the stronger its effect (Erev); however, the effect cannot exceed the maximum effect.

A high concentration (C) produces a strong effect (Erev), but this effect weakens as the concentration decreases. A drug of high potency achieves the half-maximum effect (1/2 Emax) already at a low concentration (CE50). Drugs with high Hill coefficients (H >1.0) have an S-shaped (sigmoid) effect-concentration relationship. This means that there is a threshold concentration (CE05) below which no effect is achieved (eFigure).
b) Derivation of drug dose adjustment
There is a consensus that the dose should be adjusted in proportion to drug clearance. The half-life changes almost inversely proportional to the clearance. Consequently, the dose must be reduced inversely proportionally if the half-life is prolonged. (D/Dnorm= T1/2norm/T1/2). As shown in the Figure 1, there is a hyperbolic dependence of the half-life on the renally eliminated fraction of a drug (fren) and the glomerular filtration rate (GFR) (16). Both statements together result in an equation that permits to adjust the dose to the individual renal function (eGFR) based on two known variables (the normal dose [Dnorm] and the renally excreted fraction [fren]):
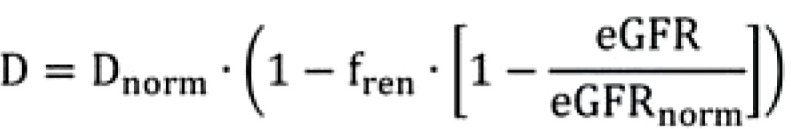
For the majority of drugs, toxic blood levels are more than twice as high as therapeutic levels. This means that dose adjustment is only necessary if the GFR falls below the no-effect boundary of 60 mL/min/1.73m2 (Figure 1 and eMethods a) for an example) (17).
The adjusted dose should achieve the same effect (E) as with normal renal function; thus, not only pharmacokinetics but also pharmacodynamics must be taken into account. The effects are usually mediated by receptors and can be described using the Hill equation (eFigure and eMethods “Explanation of the Hill coefficient“). Such reversible effects are large, when the maximum effect (Emax) is large, stronger at higher concentrations (C), but weaker, if a high concentration is needed to achieve the half-maximum effect (CE50). The higher the Hill coefficient (H), the more sigmoid the effect-concentration relationship (eFigure).
eFigure.
Pharmacodynamics: Threshold concentration (CE05) and ceiling concentration (CE95); for a Hill coefficient of 1.0, the effect-concentration relationship corresponds to the Michaelis-Menten equation. However, the higher the Hill coefficient (H >> 1.0), the more sigmoid the effect-concentration relationship.
Approximately 67% of all anticancer drugs require dose adjustment for renal function. In addition, several anticancer drugs are eliminated by hemodialysis and thus require the administration of an adjusted dose before and after dialysis. However, for more than 50% of the anticancer drugs, no information on this aspect is available (Table 1 and eTable) (18–20).
Table 1. Dose adjustment of anticancer drugs in chronic kidney disease (CKD) and need for dose adjustment (according to [19] and the summaries of product characteristics of the various drugs).
| Need for dose adjustment in chronic kidney disease (CKD) | Substance |
| No | 5-fluoruracil, abemaciclib, abiraterone acetate, aflibercept, anagrelide, anastrozole, azacitidine, bendamustine, bevacizumab, bicalutamide, binimetinib, bortezomib, cabazitaxel, carfilzomib, cetuximab, daratumumab, decitabine, docetaxel, dostarlimab, doxorubicin, elotuzumab, enasidenib, encorafenib, enfortumab vedotin, epirubicin, erlotinib, everolimus, gefitinib, ibrutinib, inotuzumab ozogamicin, larotrectinib, letrozole, nab-paclitaxel, necitumumab, nivolumab, obinutuzumab, octreotide, osimertinib, paclitaxel, palbociclib, panobinostat, panitumumab, pazopanib, pembrolizumab, rituximab, sunitinib, tamoxifen, trabectedin, vemurafenib, venetoclax, vinblastine, vincristine, vismodegib |
| No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated* | Avelumab, axitinib, belantamab-mafodotin, belzutifan dabrafenib, durvalumab, gemtuzumab ozogamicin, glasdegib, idealisib, ipilimumab, ramucirumab, regorafenib, relugolix, selinexor, selpercatinib siltuximab, tivozanib, trametinib, zanubrutinib |
| No (GFR ≥ 21 mL/min), GFR <21 ml/min: not stated* | Cemiplimab |
| No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated* | 177Lu-PSMA-617, acalabrutinib, alectinib, amivantamab, apalutamide, arsenic trioxide, atezolizumab, blinatumomab, cabozantinib, capmatinib, ceritinib cobimetinib, dacomitinib, entrectinib, enzalutamide, fruquintinib, gilteritinib, ivosidenib, luspatercept, midostaurin, moxetumomab pasudotox, nintedanib, niraparib, olaratumab, pertuzumab, ponatinib, radium 223, rucaparib, sacituzumab govitecan, sonidegib, sotorasib, tebentafusp, trastuzumab, trastuzumab deruxtecan, trastuzumab emtansine, tucatinib |
| No (GFR ≥ 30 mL/min), GFR<30 ml/min: not recommended* | Brentuximab Vedotin, Mitomycin C |
| Yes | Lenvatinib |
| Yes (if GFR <10 ml/min)* | Cyclophosphamide |
| Yes (if GFR <30 ml/min)* | Afatinib brigatinib, crizotinib, dacarbazine, darolutamide, gemcitabine, ixazomib, lorlatinib, oxaliplatin, pemigatinib, ribociclib, trifluridine tirapicil |
| Yes (if GFR <40 ml/min)* | Sorafenib, topotecan |
| Yes (if GFR <45 ml/min)* | Pemetrexed, pomalidomide |
| Yes (if GFR <50 ml/min)* | Bleomycin, bosutinib capecitabine, chlorambucil, cladribine, daunorubicin, eribulin, etoposide, ifosfamide, irinotecan (consider), methotrexate, olaparib, vandetanib |
| Yes (if GFR <60 ml/min)* | Carboplatin, carmustine (not recommended if GFR <30 ml/min), cisplatin (not recommended if gfr <40 ml/min), cytarabine (not necessary if low-dose protocol), lenalidomide, melphalan, pentostatin, ruxolitinib, talazoparib, vinflunine |
| Yes (if GFR <70 ml/min)* | Fludarabine |
| Not recommended in patients with severe chronic kidney disease* | Mitotane, treosulfan |
| Not stated* | Axicabtagene ciloleucel, brexucabtagene autoleucel, ciltacabtagene autoleucel, lisocabtagene maraleucel, tisagenlecleucel |
* For a patient-specific treatment decision, additional searches by the treating physicians are required.
177Lu-PSMA-617, lutetium (177Lu) vipivotide tetraxetan; GFR, glomerular filtration rate
eTable. Dose adjustment, need of dose adjustment and dialyzability of anticancer drugs in chronic kidney failure and dialysis dependence (according to 18–20).
| Substance* | Need for dose reduction in chronic kidney disease | Need for dose adjustment after dialysis/standard dose before hemodialysis |
| 177Lu-PSMA-617 | No (GFR 30 mL/min), GFR < 30 ml/min: not stated | Not stated |
| 5-Fluoruracil | No | No, if necessary, infusion during HD |
| Abemaciclib | No | No |
| Abiraterone acetate | No | No |
| Acalabrutinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Afatinib | Yes ifi GFR <30 ml/min) | Yes, if necessary, dose prior to HD |
| Aflibercept | No | No |
| Alectinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Amivantamab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Amsacrine | Yes (if crea >2 mg/dL) | Not stated |
| Anagrelide | No | No |
| Anastrozol | No | No |
| Apalutamide | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Arsenic trioxide | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Asciminib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Atezolizumab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Avelumab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Axicabtagene ciloleucel | Not stated | Not stated |
| Axitinib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Azacitidine | No | No |
| Belantamab mafodotin | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Belzutifan | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Bendamustine | No | No |
| Bevacizumab | No | No |
| Bicalutamide | No | No |
| Binimetinib | No | No |
| Bleomycin | Yes (if GFR <50 ml/min) | Yes, or dose prior to HD |
| Blinatumomab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Bortezomib | No | No |
| Bosutinib | Yes (if GFR <50 ml/min) | Not stated |
| Brentuximab vedotin | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not recommended | Not stated |
| Brexucabtagene autoleucel | Not stated | Not stated |
| Brigatinib | Yes (if GFR <30 ml/min) | Not stated |
| Cabazitaxel | No | Not stated |
| Cabozantinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Capecitabine | Yes (if GFR <50 ml/min) | Not recommended, or dose prior to HD |
| Capmatinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Carboplatin | Yes (if GFR <60 ml/min) | Yes, or dose prior to HD |
| Carfilzomib | No | No |
| Carmustine | Yes (if GFR <60 ml/min), gfr <30 ml/min: not recommended | Not recommended |
| CCNU (lomustine) | Yes (if GFR <60 ml/min), gfr <30 ml/min: not recommended | Not recommended |
| Cemiplimab | No (GFR ≥ 21 mL/min), GFR <21 ml/min: not stated | Not stated |
| Ceritinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Cetuximab | No | No |
| Chlorambucil | Yes (if GFR <50 ml/min) | Yes |
| Ciltacabtagene autoleucel | Not stated | Not stated |
| Cisplatin | Yes (if GFR <60 ml/min), gfr <40 ml/min: not recommended | Yes, or dose prior to HD (palliative use not recommended) |
| Cladribine | Yes (if GFR <50 ml/min) | Yes |
| CPX-351 | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Cobimetinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Crizotinib | Yes (if GFR <30 ml/min) | Not stated |
| Cyclophosphamide | Yes (if GFR <10 ml/min) | Yes, or dose prior to HD |
| Cytarabine | Yes (if GFR <60 ml/min, not required with low-dose protocol) | Yes, or dose after HD |
| Dabrafenib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Dacarbazin | Yes (if GFR <30 ml/min) | Yes |
| Dacomitinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Daratumumab | No | No |
| Darolutamide | Yes (if GFR <30 ml/min) | Not stated |
| Daunorubicin | Yes (if GFR <50 ml/min) | Yes |
| Decitabine | No | Not stated |
| Docetaxel | No | No, or dose prior to HD |
| Dostarlimab | No | No |
| Doxorubicin | No | No, if necessary, consider low initial dose |
| Durvalumab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Elotuzumab | No | No |
| Enasidenib | No | No |
| Encorafenib | No | No |
| Enfortumab vedotin | No | Not stated |
| Entrectinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Enzalutamide | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Epcoritamab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Epirubicin | No | No |
| Eribulin | Yes (if GFR <50 ml/min) | Yes |
| Erlotinib | No | If necessary, dose prior to HD |
| Etoposide | Yes (if GFR <50 ml/min) | Yes (administration before or after dialysis) |
| Everolimus | No | No |
| Fludarabine | Yes (if GFR <70 ml/min) | Yes, or dose prior to HD |
| Fruquintinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Gefitinib | No | No |
| Gemcitabine | Yes (if GFR <30 ml/min) | No, or dose prior to HD |
| Gemtuzumab ozogamicin | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Gilteritinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Glasdegib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Glofitamab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Ibrutinib | No | No |
| Idelalisib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Ifosfamid | Yes (if GFR <50 ml/min) | Not recommended, or dose prior to HD |
| Inotuzumab ozogamicin | No | No |
| Ipilimumab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Irinotecan | Yes (consider if GFR <50 ml/min) | Yes, or dose prior to HD |
| Ivosidenib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Ixazomib | Yes (if GFR <30 ml/min) | Yes |
| Iodine-131 | Yes | Yes, or dose prior to HD |
| Larotrectinib | No | No |
| Lenalidomide | Yes (if GFR <60 ml/min) | Yes, or dose prior to HD |
| Lenvatinib | Yes | Not stated |
| Letrozole | No | No |
| Lisocabtagene maraleucel | Not stated | Not stated |
| Loncastuximab tesirine | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Lorlatinib | Yes (if GFR <30 ml/min) | Not stated |
| Luspatercept | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Melphalan | Yes (if GFR <60 ml/min) | Yes, or dose prior to HD |
| Methotrexate | Yes (if GFR <50 ml/min) | Avoid use, or dose prior to HD |
| Midostaurin | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Mitomycin C | No (not recommended if GFR <30 ml/min) | Not recommended |
| Mitotane | Not recommended with severe chronic kidney disease | Not stated |
| Moxetumomab pasudotox | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not recommended | Not stated |
| Nab-Paclitaxel | No | No |
| Necitumumab | No | No |
| Nintedanib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Niraparib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Nivolumab | No | No |
| Obinutuzumab | No | No |
| Octreotide | No | No |
| Olaratumab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Olaparib | Yes (if GFR <50 ml/min) | Not stated |
| Osimertinib | No | No |
| Oxaliplatin | Yes (if GFR <30 ml/min) | Avoid use, or dose prior to HD |
| Paclitaxel | No | No |
| Palbociclib | No | No |
| Panobinostat | No | No |
| Panitumumab | No | No |
| Pazopanib | No | No |
| Pembrolizumab | No | No |
| Pemetrexed | No (not recommended if GFR <45 ml/min) | Avoid use, or dose prior to HD |
| Pemigatinib | Yes (if GFR <30 ml/min) | No |
| Pentostatin | Yes (if GFR <60 ml/min) | Not stated |
| Pertuzumab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Pomalidomide | Yes (if GFR <45 ml/min) | Yes |
| Ponatinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Radium-223 | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Ramucirumab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Regorafenib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Relugolix | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Ribociclib | Yes (if GFR <30 ml/min) | Not stated |
| Rituximab | No | No |
| Rucaparib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Ruxolitinib | Yes (if GFR <60 ml/min) | Yes |
| Sacituzumab govitecan | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Selinexor | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Selpercatinib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Siltuximab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Sonidegib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | No |
| Sorafenib | Yes (if GFR <40 ml/min) | Yes |
| Sotorasib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Streptozotocin | Yes (if GFR <50 ml/min) | Not stated |
| Sunitinib | No | No, or dose prior to HD |
| Talazoparib | Yes (if GFR <60 ml/min) | Not stated |
| Talquetamab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Tamoxifen | No | No |
| Tebentafusp | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Teclistamab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Tegafur uracil | Yes (if GFR <50 ml/min) | No, or dose prior to HD |
| Temozolomide | No (GFR ≥ 36 mL/min), GFR <36 ml/min: not stated | Not stated |
| Tisagenlecleucel | keine Angabe | Not stated |
| Tivozanib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Topotecan | Yes (if GFR <40 ml/min) | Avoid use, or dose prior to HD |
| Trabectedin | No | No |
| Trametinib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Trastuzumab | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Trastuzumab deruxtecan | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Trastuzumab emtansin | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Tremelimumab | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
| Treosulfan | No, not recommended with severe chronic kidney disease | Not stated |
| Trifluridine tirapicil | Yes (if GFR <30 ml/min) | Use not recommended |
| Tucatinib | No (GFR ≥ 30 mL/min), GFR <30 ml/min: not stated | Not stated |
| Vandetanib | Yes (if GFR <50 ml/min) | Not stated |
| Vemurafenib | No | No |
| Venetoclax | No | No |
| Vinblastine | No | No |
| Vincristine | No | No |
| Vinflunine | Yes (if GFR <60 ml/min) | Not stated |
| Vismodegib | No | No |
| Zanubrutinib | No (GFR ≥ 15 mL/min), GFR <15 ml/min: not stated | Not stated |
* For a patient-specific treatment decision, additional searches by the treating physicians are required. 177Lu-PSMA-617, lutetium (177Lu) vipivotide tetraxetan; GFR, glomerular filtration rate; HD, hemodialysis; crea, creatinine
Schematic dose reduction in patients with impaired renal function may cause the anticancer effect to be missed (Figure 2 und eMethods b) and can be one reason why survival rates are significantly poorer in cancer patients with a GFR <60 mL/min/1.73 m2, as has been shown retrospectively (21). Studies found better survival rates in patients with NSCLC and mildly impaired GRF (<90 mL/min/1.73 m2), if the cisplatin/pemetrexed treatment was not adapted (higher cumulated dose) (22).There were no fatal side effects, which means that a higher dosage appears at least feasible in patients with CKD. However, this does not allow to draw general conclusions for all medications and unselected cohorts of patients with CKD. Here, randomized trials could help to arrive at clear recommendations. With dose adjustment, the risk of side effects must be weighed against the chance of successful treatment.
Figure 2.
Anticancer drugs with chronic kidney disease—Dose reduction and its consequences: Proportional dose reduction can lead to a disproportionate loss of effect.
Intuitively, the dose is reduced by half when the half-life doubles from 6 to 12 hours. The blood levels (black line) are getting lower, but are decreasing more slowly. However, the effect (red lines) is almost completely lost. Even though peak levels are reduced by half, the area under the concentration-time curve (AUC) remains constant, because after 24 hours levels after twice as high and the area is extrapolated to infinity to the right. In contrast, the effect at the beginning (Eo) decreases to one fifth and the area under the effect-time curve (AUEC) even to one seventh, since the peak level (1/2 Cpeak) is clearly below the concentration of the half-maximum effect. (1/2 Cpeak < CE50 = 100).
AUC, area under the curve; AUEC, area under the effect curve; CE50, Concentration at half-maximum effect; Cpeak, Speak concentration; Emax, maximum effect; Eo, effect at the time; H, Hill coefficient, showing S-shape of the curve; h, hours; T, time
Contrast medium administration
Imaging with contrast medium (CM) is part of the diagnosis and aftercare of patients with and after cancer. The renal clearance rate of iodine-containing and iodine-substituted contrast media is 90%. Approximately 2.3–11% of patients develop acute, usually reversible, nonoliguric renal failure within 1–7 days after contrast medium administration—a contrast-induced nephropathy. Approximately 90% of cases were patients with pre-existing CKD. Only 0.4% of patients with contrast-induced nephropathy require permanent dialysis (23). Risk factors are listed in Table 2 (23). The causes are not yet fully understood. Underlying mechanisms include direct cytotoxic effects, autocrine und paracrine factors as well as changed rheological properties affecting renal hemodynamics and tubulodynamics, and regional hypoxia (24).
Table 2. Risk factors that predispose to contrast-induced nephropathy.
| Patient-associated | Non-patient-associated |
| Chronic kidney disease (GFR < 60 ml/min/1.73 m2) | High-osmolarity contrast medium |
| Diabetes mellitus | Ionic contrast medium |
| Congenital heart defect | High contrast medium volume |
| Hypertension/hypotension | |
| LVEF <40% | |
| Age (>65) |
GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction
The preventative effect of hydration, acetylcysteine, sodium bicarbonate, vasodilators, hemodialysis, and hemofiltration is controversial and appears to be beneficial only in high-risk persons (eGFR <45 mL/min). Thus, the KDIGO guideline recommends to choose the lowest possible contrast medium dose, to use low- or iso-osmolar contrast media, to administer fluid in the form of isotonic saline solution or sodium bicarbonate solution intravenously (but not only oral fluid replacement), and oral administration of acetylcysteine together with isotonic saline intravenously. The use of theophylline, fenoldopam and prophylactic hemodialysis or hemofiltration is not recommended (25). Whenever possible, imaging modalities should be used where no or non-nephrotoxic contrast media are used, such as magnetic resonance imaging and ultrasonography.
Kidney transplantation after cancer
Patients who had cancer and wish to undergo kidney transplantation are not necessarily ineligible for receiving a kidney graft. However, there is difficulty in determining the right timing. Given the ever improving treatment outcomes, fixed waiting times with the longest possible recurrence-free interval do not seem to be appropriate any longer. Waiting times should instead be determined on a prognosis-oriented and interdisciplinary basis. This is important particularly with regard to living kidney donations, long waiting times and late, i.e. not long before the transplantation, diagnosed cancer, as it means that the waiting time is extended once again. For common malignancies, such as renal cell carcinoma, the specialist societies recommend waiting times of 0–2 years for localized tumors and five years for advanced cancer (26). Recommendations are not available for all cancer entities; interdisciplinary discussions (“cancer–transplantation boards“) can be helpful in this situation (26). While survival rates in patients with cancer prior to transplantation are lower compared to those of patients without cancer, this is not associated with age, sex and cancer per se (27).
Anticancer treatment and its effect on the kidneys
The excretion of many anticancer drugs is dependent on renal function. In addition, many substances are nephrotoxic, and both aspects can add up (carboplatin). These effects can be addressed with dose adjustment. The recommend adaption of a carboplatin-containing chemotherapy leads to halving the normal dose from a GFR <60 mL/min/1.73 m2; however, this approach entails the risk that the treatment becomes ineffective due to underdosing. It is frequently recommended that patients who are on dialysis should receive anticancer drugs after dialysis (if the substances are dialyzable) and in reduced dose (19). Relevant factors influencing dialyzability include molecular weight, protein binding and volume of distribution of the substance. In deviation from this, hemodialysis can be used as a pharmacokinetic tool. With this approach, the standard dose is administered; 1–2 hours after infusion stop, a 6-hour hemodialysis is performed and then repeated daily. In this way, a drop in blood levels, similar to that seen with normal kidney function, can be achieved. This may help to minimize the loss of effectiveness of chemotherapy (Figure 3).
Figure 3.
Hemodialysis as a pharmacokinetic tool: The drop in concentration of a chemotherapeutic agent depends on kidney function.
The drop in concentration of a chemotherapeutic agent after stop of its infusion is shown. The blue line shows the drop in concentration with normal renal function, the red line the drop with end-stage renal disease requiring dialysis. The black line shows the decrease in dialysis-dependent renal failure where hemodialysis was performed twice at 18-hour intervals. It is apparent that a similar drop in concentration can be achieved with hemodialysis as with normal kidney function (28).
HD, hemodialysis
Only a few reviews on the use anticancer substances in dialysis-dependent patients have been published so far. Examples include the studies of Hann et al. on pancreatic cancer and Zhang et al. on gynecological malignancies (28, 29).
Examples of modern anticancer drugs and their effects on kidney function
IIn 2011, ipilimumab was the first immune checkpoint inhibitor (ICI) to receive approval. ICIs are generally considered to be well-tolerated treatments. Higher-grade side effects (CTCAE grade ≥3) are reported for 20% of the treated patients. The incidence of acute kidney damage is in the range of 2% und 16% (30). The rate of immune-related adverse events (irAEs) is reported to be <5%. Acute interstitial nephritis is most commonly detected in kidney biopsy specimens (80–90%). Acute tubular damage and glomerular disorders are rarer. Median occurrence of renal irAEs is approximately three months after treatment initiation. However, IrAEs can be observed with significant delay (several months) after end of treatment. These side effects are treated empirically with corticosteroids (31).
In recent years, poly (ADP-ribose) polymerase inhibitors (PARPi) have also been used for the treatment of various tumor entities. A special feature of these substances is the drug-induced increase in creatinine, which is caused by impaired tubular creatinine secretion (without influencing glomerular creatinine filtration) and does not require specific therapy. Renal function testing should be supplemented by measuring cystatin C (32). According to the summary of product characteristics, it is not recommended to use PARPi in patients with moderate CKD with dose adjustment and in patients with severe CKD. However, the benefits and risks of treatment must be weighed up on a case by case basis.
Lutetium (177Lu) vipivotide tetraxetan is a radiopharmaceutical medication targeting the prostate-specific membrane antigen (PSMA); it is approved to treat advanced prostate cancer and has increasingly found its way into earlier lines of treatment. The kidneys are dose-limiting organs with a historically described cumulative dose limit of 23 Gy (33). The dosimetry substudy of the VISIONstudy found that five of 30 patients experienced CTCAE grade 1–2 renal toxicities when having undergone up to six cycles (34).
Conclusion
Impaired renal function is associated with an increased risk of cancer. Diagnostic imaging with contrast media as part of the cancer workup can trigger renal failure, for example in patients with diabetes. Anticancer treatment in patient with impaired renal function is challenging and raises questions addressed by onconephrology. Approximately two thirds of the anticancer drugs would require adjustment—in dose and in patients who are on dialysis also with regard to the timing of treatment. These challenges should not prevent patients with chronic kidney disease from receiving treatment.
Questions regarding the article in issue 24/2024:
Onconephrology: The Significance of Renal Function for the Development, Diagnosis, and Treatment of Cancer
The closing date for entries is 28 November 2025. Only one answer is possible per question.
Please select the answer that is most appropriate.
Question no. 1
Chronic kidney disease (CKD) is defined as abnormalities in kidney structure or function with associated health implications.. How long does they have to be present for a diagnosis of CKD to be made?
At least three weeks
At least eight weeks
At least three months
At least six months
At least nine months
Question no. 2
A patient has a glomerular filtration rate of 58 mL/min/1.73 m2. What stage (G) of CKD would this patient be diagnosed with?
G1
G2
G3a
G3b
G4
Question no. 3
In men, a GFR <40 mL/min/1.73 m2 is associated with an increased incidence of certain types of cancer. Which types of cancer do not occur more frequently even in the presence of severe chronic kidney disease?
Prostate cancer
Lung cancer
Rectal cancer
Colonic cancer
Urogenital cancer
Question no. 4
Of all organ recipients after kidney transplantation, what percentage develop non-melanoma skin cancers within ten years of the transplant?
0.5–1.5%
3–4%
5–10%
15–20%
30–45%
Question no. 5
Which of the following parameters is considered the gold standard for determining the GFR?
Serum creatinine
Albumin concentration in the urine
The difference between blood glucose and urine glucose concentrations
Inulin clearance
Cysteine concentration in the urine
Question no. 6
The first checkpoint inhibitor was approved in 2011. What is its name?
Rituximab
Ipilimumab
Pembrolizumab
Panitumumab
Nivolumab
Question no. 7
Which statement about the estimates of GFR using equations and formulas is most appropriate?
The MDRD formula overestimates GFR in the upper GFR range, the CKD-EPI formula overestimates the GFR in the intermediate GFR range.
The MDRD formula underestimates the GFR in the upper GFR range, the CKD-EPI formula overestimates the GFR in the intermediate GFR range.
The MDRD formula underestimates the GFR in the upper GFR range, the CKD-EPI formula underestimates the GFR in the intermediate GFR range.
The MDRD formula overestimates the GFR in the lower GFR range, the CKD-EPI formula overestimates the GFR in the intermediate GFR range..
The MDRD formula overestimates the GFR in the intermediate GFR range, the CKD-EPI formula underestimates the GFR in the upper GFR range.
Question no. 8
What percentage of anticancer drugs need dose adjustment for kidney function?
Approx. 7%
Approx. 15%
Approx. 27%
Approx. 45%
Approx. 67%
Question no. 9
Which of the following factors increase the risk of nephropathy when contrast media is administered?
Non-ionic contrast media and low-osmolar contrast media
Low volume of contrast media and low-osmolar contrast media
Ionic contrast media and high volume of contrast media
Low-protein diet of the patient and iso-osmolar contrast media
Left ventricular ejection fraction >50% and nonionic contrast media
Question no. 10
Which statement regarding dose reduction of drugs in patients with chronic kidney disease is most appropriate?
Most medications require dose adjustment to prevent toxicity, even if renal function is only mildly decreased (GFR ≤ 60 mL/min/1.73 m2).
If the half-life doubles, the dose must be reduced by 2/3 to prevent toxicity.
In general, a dose reduction is only required from a GFR <30 mL/min/1.73 m2.
Toxic blood levels of medications are usually more than twice as high as therapeutic levels.
The dose reduction must be determined empirically and cannot be calculated.
Acknowledgments
Translated from the original German by Ralf Thoene, M.D.
Footnotes
Conflict of interest
SZ received consultation and lecture fees from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Eisai, EUSA, Gilead, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer, Roche, and Sanofi Aventis.
The remaining authors declare no conflict of interest.
References
- 1.Levin A, Ahmed SB, Carrero JJ, et al. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the evaluation and management of chronic kidney disease: known knowns and known unknowns. Kidney Int. 2024;105:684–701. doi: 10.1016/j.kint.2023.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Girndt M, Trocchi P, Scheidt-Nave C, Markau S, Stang A. The prevalence of renal failure. Results from the German health interview and examination survey for adults, 2008-2011 (DEGS1) Dtsch Arztebl Int. 2016;113:85–91. doi: 10.3238/arztebl.2016.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC. Estimated number of prevalent cases (1-year), both sexes, in 2022. https://gco.iarc.fr/today/en/dataviz/pie-prevalence?mode=population&types=2&group_populations=0 (last accessed on 04 May 2024) [Google Scholar]
- 4.RKI. Krebs in Deutschland. 2019/2020. https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2023/kid_2023_c00_97_krebs_gesamt.pdf?__blob=publicationFile (last accessed on 04 May 2024) [Google Scholar]
- 5.IARC. Estimated number of deaths from 2022 to 2045, both sexes, age [0-85+] https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=1&single_unit=500000 (last accessed on 04 May 2024) [Google Scholar]
- 6.IARC. Estimated number of new cases from 2022 to 2045, both sexes, age [0-85+] https://gco.iarc.who.int/tomorrow/en/dataviz/isotype?populations=276&group_cancers=1&multiple_cancers=1&cancers=40_41&mode=population&multiple_populations=0&sexes=0&years=2045&single_unit=50000&types=0 (last accessed on 04 May 2024) [Google Scholar]
- 7.Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–1350. doi: 10.1681/ASN.2008090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients ondialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 10.Granata S, Tessari G, Stallone G, Zaza G. Skin cancer in solid organ transplant recipients: still an open problem. Front Med (Lausanne) 2023;10 doi: 10.3389/fmed.2023.1189680. 1189680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einollahi B, Nemati E, Lessan-Pezeshki M, et al. Skin cancer after renal transplantation: results of a multicenter study in Iran. Ann Transplant. 2010;15:44–50. [PubMed] [Google Scholar]
- 12.Mittal A, Colegio OR. Skin cancers in organ transplant recipients. Am J Transplant. 2017;17:2509–2530. doi: 10.1111/ajt.14382. [DOI] [PubMed] [Google Scholar]
- 13.Kidney disease: improving global outcomes transplant work G: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 14.Costa ESVT, Gil LA, Jr, Inker LA, et al. A prospective cross-sectional study estimated glomerular filtration rate from creatinine and cystatin C in adults with solid tumors. Kidney Int. 2022;101:607–614. doi: 10.1016/j.kint.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Jäger D, Zeier M. Berlin: Springer Nature; 2019. Onko-Nephrologie. [Google Scholar]
- 16.Hartmann B, Czock D, Keller F. Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int. 2010;107:647–655. doi: 10.3238/arztebl.2010.0647. quiz 55-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol. 2018;13:1085–1095. doi: 10.2215/CJN.00340118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janus N, Launay-Vacher V, Thyss A, et al. Management of anticancer treatment in patients under chronic dialysis: results of the multicentric CANDY (CANcer and DialYsis) study. Ann Oncol. 2013;24:501–507. doi: 10.1093/annonc/mds344. [DOI] [PubMed] [Google Scholar]
- 19.Alan SL, Yu GMC, Luyckx V, Marsden PA, Skorecki K, Taal MW. Brenner and Rector‘s the kidney. Elsevier. 2019 [Google Scholar]
- 20.Kluwer W. Uptodate. www.wolterskluwer.com/de-de/solutions/uptodate (last accessed on 16 July 2024) [Google Scholar]
- 21.Launay-Vacher V, Spano JP, Janus N, et al. Renal insufficiency and anticancer drugs in elderly cancer patients: a subgroup analysis of the IRMA study. Crit Rev Oncol Hematol. 2009;70:124–133. doi: 10.1016/j.critrevonc.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Magali L, Pascal F, Serge A, et al. Better survival in impaired renal function patients with metastatic non-small cell lung cancer treated by cisplatin-pemetrexed. Eur J Clin Pharmacol. 2020;76:1573–1580. doi: 10.1007/s00228-020-02935-8. [DOI] [PubMed] [Google Scholar]
- 23.Pannu N, Wiebe N, Tonelli M Alberta Kidney Disease Network. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295:2765–2779. doi: 10.1001/jama.295.23.2765. [DOI] [PubMed] [Google Scholar]
- 24.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. doi: 10.1093/eurheartj/ehr494. [DOI] [PubMed] [Google Scholar]
- 25.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120 doi: 10.1159/000339789. c179-84. [DOI] [PubMed] [Google Scholar]
- 26.Delecluse S. Tumorerkrankungen von Dialysepatienten und vor Nierentransplantation. Der Nephrologe. 2019 1/2019. [Google Scholar]
- 27.Jacoby F, Holland-Letz T, Zeier M, Delecluse S. All-cause and cancer-specific overall survival in kidney transplant recipients with pre-transplant malignancies in a German cohort. Journal of Onco-Nephrology. 2020:4. [Google Scholar]
- 28.Hann A, Nosalski E, Hermann PC, Egger J, Seufferlein T, Keller F. Chemotherapeutic agents eligible for prior dosing in pancreatic cancer patients requiring hemodialysis: a systematic review. Clin Nephrol. 2018;90:125–141. doi: 10.5414/CN109327. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Liu C, Di W. Systemic treatment for gynecological cancer patients undergoing hemodialysis. Onco Targets Ther. 2023;16:545–558. doi: 10.2147/OTT.S419445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiller K, Delecluse S, Zeier M, Zschäbitz S. Renale Nebenwirkungen moderner Tumortherapien. internistische praxis. 2023:521–529. [Google Scholar]
- 31.Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217–1238. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Zibetti Dal Molin G, Westin SN, Msaouel P, Gomes LM, Dickens A, Coleman RL. Discrepancy in calculated and measured glomerular filtration rates in patients treated with PARP inhibitors. Int J Gynecol Cancer. 2020;30:89–93. doi: 10.1136/ijgc-2019-000714. [DOI] [PubMed] [Google Scholar]
- 33.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann K, Rahbar K, Eiber M, et al. Renal and multiorgan safety of (177)Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer in the VISION dosimetry substudy. J Nucl Med. 2024;65:71–78. doi: 10.2967/jnumed.123.265448. [DOI] [PMC free article] [PubMed] [Google Scholar]