Patients with aspirin-exacerbated respiratory disease (AERD), the triad of asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and respiratory reactions to cyclooxygenase-1 inhibitors, often have difficult-to-treat upper and lower airway symptoms. AERD is characterized by overproduction of type 2 cytokines, blood and tissue eosinophilia, and dysregulated cysteinyl leukotriene production.1, 2 Patients with AERD are at high risk of nasal polyp recurrence following endoscopic sinus surgery (ESS).3, 4 Aspirin therapy after desensitization (ATAD) is an established treatment for patients with AERD that has been shown to improve nasal congestion, improve sense of smell, and delay nasal polyp recurrence following ESS.1 However, the clinical response to ATAD is variable and some patients do not tolerate ATAD.5 Currently, there are no well-established clinical or biomarker predictors of response to ATAD to guide clinical decision-making.
Biologic therapy is efficacious for both asthma and CRSwNP in patients with AERD.6 Dupilumab, a monoclonal antibody targeting IL-4Rα, improves sinonasal symptoms and asthma and reduces need for oral corticosteroids and ESS in patients with AERD.7, 8 Clinical evidence regarding the simultaneous use of ATAD and dupilumab in patients with AERD is limited, though mechanistic evidence suggests synergy of dupilumab and ATAD in reducing sinonasal inflammation versus dupilumab alone.9 Here we evaluate response to dupilumab in patients who had inadequate response to ATAD plus standard of care management of asthma and CRSwNP; we also compare baseline clinical characteristics of patients who require treatment with dupilumab and ATAD to those who do well on ATAD monotherapy plus standard of care.
We conducted a retrospective chart review of participants with physician-diagnosed AERD treated at Mass General Brigham (MGB) between January 2013 and July 2024 who were enrolled in the Brigham and Women’s Hospital AERD patient registry. The study was approved by the MGB Institutional Review Board and participants provided informed consent. We identified participants who underwent aspirin desensitization followed by ATAD (initial maintenance dose of 650 mg to 1300 mg daily) and continued to follow-up in clinic and a subset who later had dupilumab added to their treatment regimen. Electronic medical records (Epic Systems, Verona, WI) were reviewed for demographics, medications, clinical characteristics, AERD history, Sino-Nasal Outcome Test-22 (SNOT-22) and Asthma Control Test (ACT) scores, spirometry results, and laboratory data at baseline and post-treatment (ATAD monotherapy and ATAD and dupilumab). Data were analyzed using repeated measures ANOVA with post hoc Tukey’s test, paired t-test, unpaired t-test or Fisher’s exact test as appropriate, with GraphPad Prism v10.0.3 (GraphPad, La Jolla, CA).
We identified 58 participants with AERD treated with ATAD who later added dupilumab and 120 participants who continued treatment with ATAD monotherapy. At baseline, there were no significant differences in sex, race, smoking history, lifetime number of ESSs, rate of patient-reported post-ESS nasal polyp recurrence, FEV1% predicted, eosinophil counts, or baseline prednisone use (Table E1). Participants in the ATAD and dupilumab cohort were significantly older than those on ATAD monotherapy (57.8±12.4 versus 53.0±11.7 years, p=0.01). Participants in the ATAD and dupilumab cohort also had significantly higher baseline IgE levels than those on ATAD monotherapy, (mean±SD of 494±702 IU/mL versus 155±355 IU/mL, p=0.002).
Participants who remained on ATAD monotherapy had a greater reduction in total SNOT-22 scores after starting ATAD compared to those who eventually required add-on dupilumab (mean change from baseline of −22.8±22.0 versus −7.97±19.2, p <0.001, Figure 1), suggesting that a less robust response to ATAD was associated with the subsequent need for biologic therapy. Similarly, those in the ATAD monotherapy cohort had a significant improvement in ACT scores after starting ATAD compared to those in the ATAD and dupilumab cohort (mean change of 2.22±5.9 versus −0.41±5.4, p < 0.01, Figure 1). For the participants with inadequate response to ATAD who later started dupilumab, there was no significant ATAD-induced improvement in SNOT-22 and ACT scores from their pre-ATAD baseline to post-ATAD treatment. In contrast, total SNOT-22 scores, ACT scores, and days of prednisone per year significantly improved following the addition of dupilumab to their treatment regimen, compared to both their pre-ATAD baseline and their post-ATAD treatment timepoint (Figure 2).
Figure 1. Change in SNOT-22 and ACT from baseline to after ATAD start.

Patients who went on to start dupilumab in addition to ATAD (green) compared to patients who continued ATAD alone (blue). Scores obtained prior to initiation of any biologic. Comparisons with Mann-Whitney U Test; **p<0.01, ***p<0.001. SNOT-22: 22 question Sino-Nasal Outcome test; ACT: Asthma Control Test. Median duration of ATAD was 35.5 months in the ATAD and dupilumab cohort and 23 months in the ATAD monotherapy cohort.
Figure 2. Participants with inadequately controlled symptoms on ATAD alone had improvements in SNOT-22 and ACT scores, and reduction in OCS use on ATAD and dupilumab co-treatment, (N = 58).
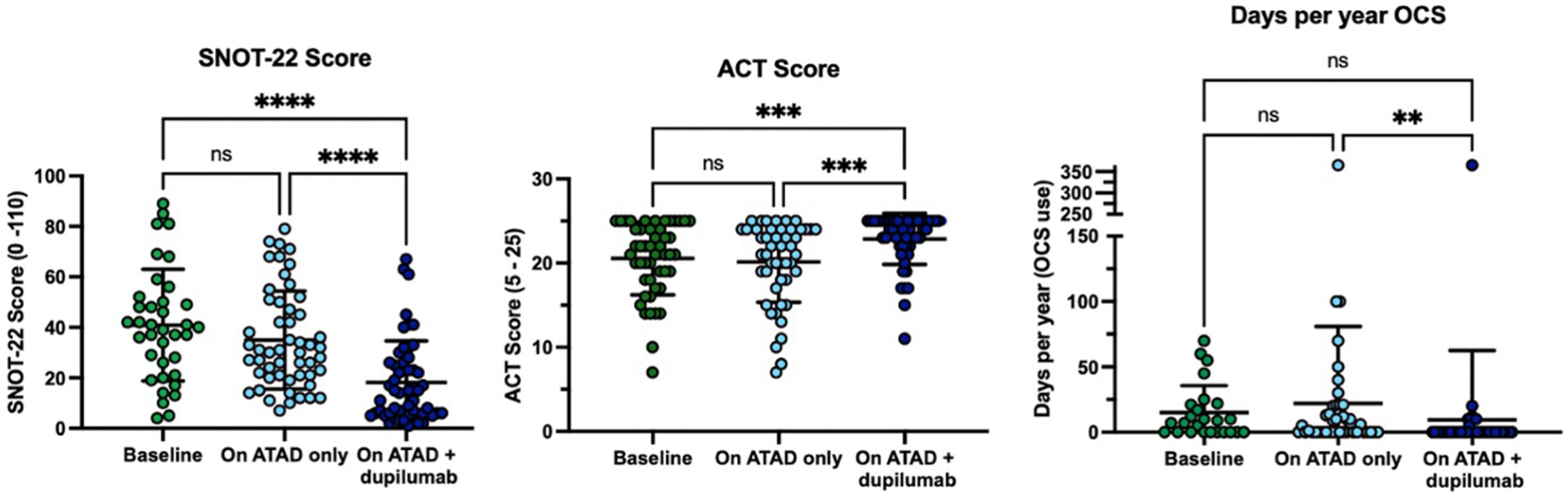
Comparisons with analysis of variance, with post hoc Tukey’s multiple comparison test; **p<0.01, ***p<0.001, ****p<0.0001. SNOT-22: 22 question Sino-Nasal Outcome test; ACT: Asthma Control Test; OCS: oral corticosteroids. Median duration of ATAD and dupilumab was 37 months.
The participants in the ATAD and dupilumab cohort were on ATAD for an average duration of 41.2±33.7 months prior to starting dupilumab, then utilized co-treatment with ATAD and dupilumab for an average duration of 41.6±16.7 months of clinical follow-up. Of the participants on ATAD and dupilumab, 35 continued the same ATAD regimen, 15 reduced their ATAD dose, and 8 stopped ATAD completely. Participants who reduced or stopped ATAD cited reasons including sufficient benefit from dupilumab (n=15), ATAD side effects (n=6), lack of ATAD efficacy (n=1), and peri-procedural discontinuation of ATAD (n=1). The participants in the ATAD monotherapy cohort (n=120) were followed on ATAD for an average duration of 40.6±39.2 months; 95 patients continued ATAD, 21 stopped ATAD, and 4 were unknown/lost to follow up.
While the availability of biologics has broadened treatment options for AERD patients, limited data exists to guide selection of biologic therapy versus ATAD. For the participants with inadequate response to ATAD monotherapy, most derived improvement in upper and lower airway symptoms and reduction in systemic corticosteroids following addition of dupilumab to their treatment regimen. In contrast, participants with adequate initial response to ATAD did not go on to require treatment with a biologic as they likely had sufficient improvement in upper and lower airway symptoms on ATAD alone. Serum IgE level was significantly higher in the ATAD and dupilumab cohort, and IgE may serve as a useful biomarker to help guide selection of ATAD versus biologic therapy in patients with AERD. Our study suggests the possibility of different AERD endotypes, which may be partially driven by type 2 cytokines. Despite known side effects of ATAD, only 17% of patients discontinued treatment, suggesting acceptable real-life adherence to ATAD for appropriately selected patients with ATAD-responsive AERD. While ATAD is a viable treatment option for many patients with AERD, approximately 1/3 of patients may further require the addition of dupilumab. Further, in the ATAD and dupilumab cohort, some participants were able to safely reduce the dose of ATAD or discontinue ATAD after dupilumab initiation without significant decrement in symptom control.
Our study is limited in that it is retrospective and relies largely on patient-reported outcomes in a small number of participants. There was limited long-term follow-up in the ATAD monotherapy cohort. Furthermore, individual length of treatment was variable, which could contribute to attenuation of differences in response rates between cohorts. Patients seeking care at our tertiary care AERD center may reflect a patient population with more severe disease not representative of the general AERD patient population. Future prospective and mechanistic studies assessing co-treatment with aspirin and dupilumab in AERD as it correlates with anatomic and physiologic measures of disease, as well as immunologic biomarkers such as IgE, will further shape our understanding of co-treatment of dupilumab and ATAD. Large, multi-center prospective studies will also be helpful in answering further nuanced questions including costs, long-term benefits, and adverse effects of ATAD alone, dupilumab alone, and ATAD and dupilumab co-treatment.
Supplementary Material
Clinical implications:
This study provides evidence for the addition of dupilumab to aspirin-therapy after desensitization (ATAD) in patients with aspirin-exacerbated respiratory disease who previously had inadequate symptom response to ATAD; many patients can taper or discontinue ATAD after dupilumab initiation.
Funding sources:
This work was supported by the National Institutes of Health grants U19 AI095219, K23AI139352, K24AI180296, R21AI182809 and by generous contributions from the Vinik and Kaye Families.
Conflicts of Interest:
K.M. Buchheit has served on scientific advisory boards for AstraZeneca, Regeneron, Sanofi-Genzyme, Eli Lilly and GlaxoSmithKline, and has received research funding from Regeneron. T. M. Laidlaw has served on scientific advisory boards for GlaxoSmithKline, AstraZeneca, Sanofi-Genzyme, Regeneron, and Eli Lilly, and has received research funding from Sanofi.
References:
- 1.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med. 2018;379(11):1060–70. [DOI] [PubMed] [Google Scholar]
- 2.Laidlaw TM, Boyce JA. Updates on immune mechanisms in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2023;151(2):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter’s triad. Am J Rhinol. 2006;20(6):573–6. [DOI] [PubMed] [Google Scholar]
- 5.Chu DK, Lee DJ, Lee KM, Schunemann HJ, Szczeklik W, Lee JM. Benefits and harms of aspirin desensitization for aspirin-exacerbated respiratory disease: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2019;9(12):1409–19. [DOI] [PubMed] [Google Scholar]
- 6.Buchheit KM, Vandewalle E, Elzinga HBE, Reitsma S, Fokkens W, Geveart P. Efficacy of Biologics in NSAID-ERD: United Airways From the Nose to the Bronchi. J Allergy Clin Immunol Pract. 2024;12(11):2917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullol J, Laidlaw TM, Bachert C, Mannent LP, Canonica GW, Han JK, et al. Efficacy and safety of dupilumab in patients with uncontrolled severe chronic rhinosinusitis with nasal polyps and a clinical diagnosis of NSAID-ERD: Results from two randomized placebo-controlled phase 3 trials. Allergy. 2022;77(4):1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullur J, Maurer R, Ryan T, McGill A, Bensko JC, Laidlaw TM, Buchheit KM. Dupilumab Treatment for Aspirin-Exacerbated Respiratory Disease in a Real-World Setting: Impact on Quality of Life and Healthcare Utilization. Am J Rhinol Allergy. 2024:19458924241298817. [DOI] [PubMed] [Google Scholar]
- 9.Buchheit KM, Hacker J, Maurer R, McGill A, Ryan T, Bensko JC, Laidlaw TM. Co-treatment of non-steroidal anti-inflammatory drug-exacerbated respiratory disease with dupilumab and aspirin therapy after desensitization. Clin Exp Allergy. 2023;53(9):974–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


