Abstract
Purpose
The cystine/glutamate antiporter (xCT) mediates glutamate export and cyst(e)ine import. In the retina, we previously demonstrated that xCT is important in glutamate/glutamine cycling between photoreceptor and Müller cells. This study investigates the contribution of xCT to cyst(e)ine import and glutathione homeostasis and its impact on mitochondrial function.
Methods
C57BL/6J wild type (WT) and xCT knockout (KO) retinas were analyzed at six weeks and nine months. Mass spectrometry and silver-intensified immunogold labeling were used to measure cysteine (CSH) and glutathione (GSH) in the retinal layers, whereas high-resolution respirometry measured mitochondrial activity and reactive oxygen species (ROS) levels.
Results
While CSH and GSH were similar between WT and KO whole retinas at both ages, localized reduction of CSH and GSH were evident in the photoreceptors. ROS levels increased in six-week KO compared to WT retinas, and these levels were sustained at nine months. At six weeks, but not nine months, loss of xCT resulted in increased mitochondrial complex I activity and reduced mitochondrial ROS levels.
Conclusions
As early as six weeks of age, the loss of xCT resulted in localized changes in CSH and GSH levels, suggesting that xCT plays a role in GSH homeostasis. An increase in overall ROS levels was detected, but this was not attributed to the mitochondria. Changes detected in six-week KO retinas were comparable to those seen in nine-month WT retinas, suggesting that loss of xCT may accelerate changes associated with aging. The lack of differences between WT and xCT KO retinas at nine months indicate adaptations to these early changes over time.
Keywords: cystine/glutamate antiporter, retina, glutamate, glutathione, mitochondria
The cystine/glutamate antiporter (also called “system xc-”) is an Na+-independent antiporter consisting of a heavy chain subunit (4F2hc) and a light chain subunit cystine/glutamate antiporter (xCT).1–3 The light chain subunit is the functional subunit of system xc- and is involved in the exchange of extracellular cystine (CSSC) for intracellular glutamate. The xCT has been shown to facilitate the uptake of cysteine, which is rapidly reduced to cysteine for synthesis of the antioxidant glutathione (GSH),4–8 maintaining extracellular cysteine/cystine redox balance,6 and mediating the release of glutamate for signaling in neuronal tissues.9–11 As such, xCT has been studied for its role in a number of physiological processes, including maintenance of redox balance, extrasynaptic signaling, excitotoxicity, and aging.2,9,12–14
The xCT has been localized to a number of tissue types throughout the central nervous system,2 and also to a number of ocular tissues including the cornea,15 lens,16 and retina.12 In the retina, xCT has been localized to the photoreceptor ribbon synapse in the retinal outer plexiform layer in the rat, cow, chicken, and monkey,17 and shown to mediate glutamate export and the opening of post-synaptic bipolar cell glutamate receptors.17 To further investigate the role of xCT in retinal glutamate homeostasis, we mapped the localization of glutamate and glutamine in different retinal cell types of six-week and nine-month C57BL WT and xCT KO retinas using silver intensified immunogold labeling. We found that the loss of xCT disrupts glutamate and glutamine homeostasis in the retina as early as six weeks of age, with accumulation of glutamate within the photoreceptor inner segments and outer plexiform layer and a decrease in glutamine levels in Müller cells.18 These findings indicate that xCT is a key exporter of glutamate and is important in maintaining the glutamate-glutamine cycle between the photoreceptor and Müller cells.
The accumulation of glutamate in the inner segment of photoreceptors corresponds to areas of high mitochondrial content. Since changes in glutamate concentrations have been shown to directly affect mitochondrial function in retinal cell cultures,19 it is possible that the loss of xCT may lead to altered mitochondrial function. Alterations in mitochondrial function could also contribute to oxidative stress. The mitochondria are a primary site of reactive oxygen species (ROS) production via reverse electron transport (RET), in which electrons flow backward from complex II to complex I generating superoxide and subsequently hydrogen peroxide.20 Therefore altering mitochondrial activity through glutamate use in the xCT KO retina may disrupt both energetic and redox homeostasis in the retina. Moreover, in addition to glutamate export, xCT is known to import cystine, for the rapid reduction to cysteine and the synthesis of the antioxidant GSH. Hence, loss of xCT function, may reduce cysteine levels, the rate limiting amino acid required for GSH synthesis and subsequently GSH levels exacerbating redox imbalance within the retina.
The xCT KO mice from two age groups, six weeks (young) and nine months (middle age), were used in biochemical assays to measure cysteine and GSH levels, whereas silver intensified immunogold labeling localized cysteine and GSH in the different retinal layers. Additionally, high-resolution respirometry to measure overall ROS levels, mitochondrial activity, and mitochondrial ROS levels investigated the contribution of xCT to redox balance and mitochondrial function in the retina and how this may alter with age.
Methods
Animals
All animals used in this study were treated according to protocols approved by the University of Auckland Animal Ethics Committee (Ethics application number R001413), and the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. The xCT KO mice used in this study were those described in Martis et al.12 and Knight et al.18 and were descendants of the global xCT KO strain developed by Sato et al.6 All xCT KO mice exhibited normal, healthy appearances, were fertile, and had comparable lifespans to WT.6 C57BL/6J mice (WT) were obtained from the Vernon Jansen Unit at the University of Auckland. Animals of either sex were used and studied at six weeks (young) and nine months (middle age) of age. The nine-month-old animals were selected for this study, because it was previously shown that at this age there was an increased prevalence of subretinal deposits in xCT KO retinas compared with WT retinas.12 These deposits are not drusen and do not affect global retinal function, but appear to accumulate naturally with age. Mice were housed at 22°C with as desired access to food and water under a 12-hour light/dark cycle. Because of the high susceptibility of photoreceptors to light-induced oxidative stress, all animals were houses under identical conditions, ensuring that cages were not placed in the top racks close to overhead lights and all tissues were processed at the same time of day under constant lighting conditions. It is acknowledged that variation in light intensity could affect redox related phenotypes especially over a nine-month experiment. However, our protocols are controlled for light exposure as much as possible within standard housing conditions.
Genotyping
Genotyping was performed as described by Sato and colleagues6 and verified by PCR of DNA extracted from the mouse's tail, as previously stated.21 PCR and Western blotting was used to confirm the absence of xCT mRNA or protein expression in xCT KO mice.21 Additionally, the absence of the rd8 mutation, which leads to the presence of retinal lesions was verified through PCR analysis using specific primers for the WT and rd8 alleles as previously described.22
Retina Collection and Tissue Preparation
Tissue collection and processing was performed at the same time of day under constant lighting conditions to minimize any variability introduced via light exposure. Mice were euthanized with CO2 asphyxiation followed by cervical dislocation before enucleation of the eyes. Eyes were immediately dissected in ice-cold PBS to prevent metabolite degradation, and the posterior eyecup quickly separated from the anterior segment. For respirometry experiments, the sclera, choroid, and retinal pigment epithelium (RPE) were removed, and the two retinas per animal placed in 700 µL (mitochondrial activity) or 1000 µL (ROS production) Mitochondrial Respiration Medium 05 Buffer (Miro5, described below). Retinal wet weight was measured, and the retinas were briefly homogenized at 5000 rpm using a Bio-Gen PRO200 homogenizer (Pro Scientific, Inc., Oxford, CT, USA). For silver-intensified immunogold labeling, each eyecup was placed in 1% paraformaldehyde with 2.5% glutaraldehyde for 60 minutes, washed in 0.1 M PBS, successively dehydrated in 70%, 80%, 90%, and 100% ethanol, and then embedded in eponate resin according to established protocols.23 For liquid chromatography tandem mass spectrometry (LC-MS/MS) experiments and oxidative stress marker assays, extracted retinas were placed in prechilled 50 mM EDTA, homogenized, and prepared as previously described.16
LC-MS/MS
LC-MS/MS was used to quantify cysteine and glutathione levels as per the methods outlined in Martis et al.21 Briefly, both reduced cysteine (CSH), total cysteine (CSH + CSSC), reduced glutathione (GSH), and total glutathione (GSH + GSSG) were measured. Cystine (CSSC) and oxidized glutathione (GSSG) concentrations were quantified by subtraction of reduced CSH or GSH from total CSH or GSH. To determine total CSH and total GSH levels, samples were first diluted in 50% acetonitrile. Half of the sample preparation was incubated with 1 mM Tris (2-carboxyethyl) phosphine (TCEP) for 20 minutes to reduce disulphide bonds and then incubated with 2 mM monobromobimane (MBrB) to form thiol-bimane conjugates. To measure reduced CSH and reduced GSH, the other half of the sample preparations was incubated with 2 mM MBrB. To determine concentration, 4.92 µM of heavy CSH (13C) and GSH (13C) were added to the treated samples as internal standards. Samples with internal standards were then added to individual Oasis HLB 30 µm solid phase extraction cartridge (Waters Corporation, Milford, MA, USA) and eluted with 10% acetonitrile before being vacuum concentrated until approximately 50–100 µL remained and transferred to a vial for LC-MS/MS.
For LC MS/MS, a 10 µL injection was made of each sample onto a ZORBAX SB-C18 3.5 µm 150 × 0.3 µm column (Agilent Technologies, Winooski, VT, USA). The sample was directed into the electrospray ionisation source of a QSTAR XL Quadrupole-Time-of-Flight mass spectrometer (SCIEX, Framingham, MA, USA). All fragment ions were detected by Multiple Reaction Monitoring using the parent ion mass-to-charge ratios and product ions and previously tabled.16,21 Data was analyzed using MultiQuant software (SCIEX) to determine the area under the curve for each of the compound fragment ions and final molar concentration of the analytes calculated as previously described.21
Silver-Intensified Immunogold Labeling
Silver-intensified immunogold labeling was performed as previously described.18,23–28 In brief, eye cups were fixed on ice using a low concentration of glutaraldehyde combined with paraformaldehyde (1%) to strike a balance between tissue preservation and retention of diffusible molecules. Fixation was also carried out on ice to further reduce diffusion, and tissue was promptly embedded in resin avoiding the use of organic solvents during dehydration which can extract small polar compounds including amino acids.29 Fixed samples were then sectioned at 500 nm using a Leica Ultracut UCT ultramicrotome (Lecia Microsystems, Wetzlar, Germany). Retinal sections were then labeled with primary rabbit polyclonal anti-glutathione (1:200; ab9443; Abcam, Cambridge, UK), which detects free glutathione with no distinction between oxidized or reduced GSH; or rabbit polyclonal anti-cysteine (1:50; Signature Immunologics, Salt Lake City, UT, USA) antibodies, before secondary labeling with a 1.4 nm Nanogold conjugated, IgG, goat anti-rabbit secondary antibody (GαR-gold; 1:100, no. 2003; Nanoprobes, Yaphank, NY, USA). Parallel control sections were also prepared in which no primary antibody was added. Labeling was then amplified through immersion in a silver intensification solution comprised of 60% distilled water, 0.02 M citrate buffer, 0.15% silver nitrate, and 0.03 M hydroquinone.30 In this solution, silver ions are deposited onto the nanogold particles conjugated to the secondary antibody, causing positive labeling to become visibly gray. WT and KO retinal sections were collected, labeled, and developed simultaneously in the same immunolabeling solutions to ensure minimal differences in processing and to allow comparison of amino acid levels between samples.
Silver-intensified immunogold labeling was imaged with a LEICA DMR upright bright field microscope (Leica Microsystems) equipped with a Leica DFC495 camera (Leica Microsystems) attachment. The Leica Application Suite software (version 4.8; Leica Microsystems) was used to capture images at 300 dpi under a 40x magnification oil immersion objective lens in grayscale with a fixed exposure time of 65.8 ms and a gain of 5.4. At least six images from the central retina, less than 5 mm from the optic nerve, were taken for each label, age, and genotype.
CSH and GSH were quantified using the ImageJ software (Version 1.50i; National Institutes of Health, Bethesda, MD, USA) as previously described.18,24,25,27,29,31–33 In brief, the average pixel intensity (ranging from 0 [black] to 255 [white]) of a fixed (97-pixel) circular area of interest was measured using the histogram tool. Regions of interest were captured from across the whole width of each retinal layer, and intracellular areas were measured near the middle of the somata to reduce potential bias toward areas of greater or lesser staining. Pixel intensity was normalized against 10 samples of average pixel intensity outside the retinal area (background intensity) and intensity values were plotted as a ratio of KO/WT. Ten to 30 samples of average pixel intensity were obtained from the ganglion cell layer (GC), Müller cell endfeet (MCef), inner plexiform layer (IPL), amacrine cells (AC), Müller cell bodies (MC), bipolar cells (BP), the outer plexiform layer (OPL), the outer nuclear layer (ONL), photoreceptor inner segments (IS), and photoreceptor outer segments (OS). Because of the larger width of the inner plexiform layer, 30 sample regions of interest were measured per retina. GCs were identified as large cell bodies in the innermost retinal layer, whereas the MCef was measured as the tissue in-between GCs. The IPL/OPL were distinguished by their low nuclear density and fibrillar appearance. ACs were rounded cells in close proximity to the IPL, whereas bipolar cells were elongated, teardrop-shaped cells located proximal to the OPL within the inner nuclear layer. MC bodies were identified by their rhomboidal shape and location in the middle of the inner nuclear layer. The ONL was identified by the dense packing of photoreceptor nuclei, whereas the IS and OS were identified by their characteristic elongated appearance and location adjacent to the photoreceptors and RPE respectively.
Darker staining corresponds to higher levels of silver ion deposition reflecting greater immunoreactivity, whereas lighter staining reflects lower levels of immunoreactivity, allowing for quantitative analysis of changes in the neurochemical profiles. Differences in immunoreactivity were presented as the fold-change in pixel intensity in KO retinas relative to WT retinas (whereby a result of 1.0 represents a 100% increase in labeling intensity in KO retinas compared to WT retinas,31 whereas a result of 0.0 represents equal labeling intensity in KO retinas compared to WT retinas), assuming pixel values in WT are a direct measure of amino acid concentrations within the retina.24
High-Resolution Respirometry
High-resolution respirometry (Oroboros Oxygraph 2K; Oroboros Instruments, Innsbruck, Austria) was performed to determine mitochondrial electron transport system (ETS) complex activity and hydrogen peroxide (H2O2) levels during rest and during mitochondrial activation. To measure mitochondrial activity, each oxygraph chamber was filled with 1.2 mL Miro5 buffer (0.5 mM EGTA, 3 mM MgCl2·6H2O, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 1 g/L BSA essentially fatty-acid free, pH 7.1). The oxygraph was calibrated at 37°C and to atmospheric oxygen concentrations (O2 background a° = −7.8662 pmol/s.ml; b° = 0.0414).34 For ROS measurements, 1.3 mL of Miro5 buffer were used and the Oroboros O2k-Fluorometers were inserted into the front two windows of the chambers to measure fluorescence. Superoxide dismutase (2.5 mU), Amplex UltraRed (3.75 µL), and horseradish peroxidase (2.5 mU) were added to each chamber, and the fluorescence signal was calibrated with the addition of H2O2 (up to 330 nM).35,36 All recordings were taken with DatLab software (Oroboros Instruments).
Mitochondrial Activity
Mitochondrial function was evaluated with a protocol adapted from Han et al.,37 presented in Supplementary Figure 1. Three major respiratory states were measured: LEAK respiration, which represents the non-phosphorylated state in the absence of ADP;38 OXPHOS respiration, which is a measure of the maximal oxidative respiratory ability when ADP is present;36,38,39 and the residual oxygen consumption (ROX), which, although not a “coupling” state like LEAK or OXPHOS, represents the consumption of O2 by processes unrelated to proton translocation and ADP phosphorylation.38
To measure mitochondrial respiration, 400 µL of retinal homogenate in Miro5 buffer were added to each chamber, and then the following substrates and inhibitors were added (final chamber concentration): pyruvate (5 mM), malate (5 mM), ADP (1.25 mM), cytochrome c (10 µM), succinate (10 mM), rotenone (1 µM), antimycin A (5 µM), ascorbate (2 mM), tetramethylphenylenediamine (0.5 mM), and sodium azide (100 mM) µM. Pyruvate and malate were added before the homogenate, and the chambers were closed and sealed immediately after homogenate addition. Mitochondrial respiration was corrected for oxygen flux (change in oxygen concentration over time) and ROX values after inhibition of complex I (CI) with rotenone, complex III (CII) with antimycin A, and complex IV (CIV) through addition of sodium azide.37
Values were normalized against citrate synthase activity (E.c. 4.1.3.7) as per Eigentler et al.40 Citrate synthase standards or homogenates in Miro5 were added to assay buffer consisting of acetyl CoA (0.1 mM) and 5,5′-Disthiobis(2-bitrobenzoic acid) (0.2 mM) in 0.5 mM Tris-HCl. The reaction was started by addition of oxaloacetate (0.5 mM) to sample wells, with absorbance at 412 nm measured for five minutes at 31-second intervals that included intercalibration time of one second40 (Biotek Synergy 2 multi-mode microplate reader; Millennium Science, Victoria, Australia).
For detection of changes in mitochondrial ETS complex activity, respiration was expressed as flux control ratios (FCR) for CI, CII, and CIV normalized to the oxygen flux of the individual complex against that of simultaneous Complex I and II activation (CI + II). To evaluate mitochondrial health, the coupling control ratio (CCR), which is a measure of coupling between the ETS and phosphorylating systems, was measured. This was calculated as the O2 flux of the LEAK state normalized to that of CI + CII convergent OXPHOS where 0.0 represents completely coupled and 1.0 represents completely uncoupled.37
ROS Production
ROS production during rest and active mitochondrial function were measured with a protocol adapted from Makrecka-Kuka et al.,36 presented in Supplementary Figure S2. In this protocol, in addition to the LEAK, OXPHOS, and ROX respiratory states discussed above, the ETS state, which represents maximal electron flow through the ETS and maximal O2 flux in the absence of the rate-limiting complex V (CV),36,38 was also measured. After temperature, oxygen, and H2O2 calibration, 300 µL of homogenate in Miro5 buffer was added to each chamber. Pyruvate and malate (5 mM and 0.5 mM respectively; Supplementary Figure S2A) or succinate (10 mM) without (Supplementary Figure S2B) or with rotenone (Supplementary Figure 2C) were added to determine H2O2 flux during CI or CII-mediated LEAK respiration. ADP was added (1.25 mM) to obtain OXPHOS H2O2 flux, after which CI (pyruvate and malate) or CII (succinate) substrates were then added to stimulate convergent CI and CII-linked respiration. Repeated titrations of 0.5 µM trifluoromethoxy phenylhydrazone were used to uncouple mitochondria41 and determine the maximal ETS capacity. Rotenone (1 µM, if not added as part of the initial substrate) and antimycin A (5 µM) were used to determine H2O2 production during ROX.36 Due to the appearance of negative fluxes when H2O2 production values were normalized to background H2O2 levels in the absence of homogenate, data was instead normalized to the lowest H2O2 flux observed as suggested by Makrecka-Kuka et al.36
Statistical Analysis
Statistical comparison was performed using two-way ANOVA followed by Tukey's multiple comparisons test. For variables that did not meet these criteria, age-matched differences were analyzed with a Mann-Whitney U test. Values reported in this study represent mean ± SEM unless stated otherwise. Statistical analysis was performed using GraphPad Prism 8, and P < 0.05 was considered statistically significant. Major increments of statistical significance are displayed as *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001.
Results
Loss of xCT Function Does Not Alter CSH and GSH Concentrations in the Whole Retina
Given that in other tissues, xCT mediates the uptake of cystine where it is then reduced to cysteine for GSH synthesis, we first investigated the effect of loss of xCT on antioxidant balance in the retina. Whole tissue cysteine/cystine (CSH/CSSC) and glutathione/oxidized glutathione (GSH/GSSG) concentrations were measured in WT and xCT KO retinas at six weeks and nine months of age. Between WT and xCT KO retinas, there were no significant differences in intracellular levels of CSH (Fig. 1A), GSH (Fig. 1B), CSSC (Fig. 1C), or GSSG (Fig. 1D) for any age group, nor were there any significant differences in concentrations between age groups. Although there appeared to be a difference in CSH and CSSC levels in nine-month KO versus WT retinas, these differences were not statistically significant (CSH: P = 0.6676; CSSC: P = 0.5594), suggesting that loss of xCT function does not affect intracellular cysteine or GSH levels in the whole retina.
Figure 1.
Intracellular CSH, GSH, CSSC, and GSSG levels in the retina. Supernatants from retinal homogenates were incubated with or without TCEP and derivatized with MBrB to quantify total CSH (CSH + CSSC) and GSH (GSH + GSSG), and reduced CSH/GSH using LC-MS/MS. Intracellular CSH (A) and GSH (B) concentrations were measured for six-week and nine-month WT (gray) and xCT KO (black) mice, whereas intracellular CSSC (C) and GSSG (D) concentrations were calculated via subtraction of reduced CSH/GSH concentrations from total CSH/GSH concentrations. Six-week n = 10 WT and xCT KO, nine-month n = 7 WT and xCT KO.
Loss of xCT Function Leads to Specific Depletion of Cysteine and GSH in the Photoreceptors
Although whole tissue analysis of cysteine and GSH levels did not reveal any differences at any age group between WT and xCT KO retinas, the specific localization of xCT to the outer plexiform layer could suggest that changes in cysteine or GSH concentration could be compartmentalized to particular cell types rather than affecting the whole retina. This proved to be the case in our previous work,18 where changes in glutamate and glutamine distribution were seen despite the lack of whole tissue changes in glutamate or glutamine concentration. As such, post-embedding silver-intensified immunocytochemistry was used to map and quantify cysteine and GSH levels in the different retinal cells in six-week- and nine-month WT and xCT KO retinas to determine whether localized changes in the distribution of cysteine and GSH could be detected because of loss of xCT.
Figure 2A shows that six-week WT retinas exhibit strong cysteine labeling in all retinal layers and cell types, particularly in photoreceptor cell bodies, AC, and GC. In the six-week xCT KO retinas (Fig. 2C), there appears to be slightly lighter cysteine labeling in neuronal cell bodies, particularly in the photoreceptor somata. Quantitative analysis of cysteine labeling at six weeks between WT and xCT KO retinas revealed a significant depletion of cysteine immunoreactivity in the ONL (−41% ± 22%; P < 0.05) and a significant increase in cysteine immunoreactivity in the photoreceptor outer segments (47% ± 9%; P < 0.05) in the xCT KO retinas compared to WT retinas (Fig. 2E). Analysis of cysteine labeling in nine-month WT and xCT KO retinas (Figs. 2B, 2D) revealed the appearance of darker labeling in the outer segments, inner segments, outer plexiform layer, and inner plexiform layer in the xCT KO compared to the WT. However, quantitative analysis revealed none of these changes to be statistically significant (Fig. 2F).
Figure 2.
Silver-intensified immunogold staining for cystine content in six-week- and nine-month-old WT and xCT KO retinas. Representative images of cysteine immunoreactivity in the six-week WT (A) and xCT KO (C) retinas, and nine-month WT (B) and xCT KO (D) retinas. The dark arrows in (A) and (C) indicate MC, the thinner arrows in (A) are examples of bipolar cells (BC), AC, and GC, and the individual arrowheads identify the location of the MCef. Scale bar: 50 µm. Insets show magnified views of the outer nuclear layer (A′–D′), inner nuclear layer (A″–D″), and ganglion cell layer (A*–D*), respectively. Cell types identified in these views include the photoreceptor cell bodies (PR), BC, MC, AC, and the MCef. (E, F) Quantitative analysis of cysteine in each retinal layer expressed as the percentage change in amino acid immunoreactivity of the six-week xCT KO (C) or nine-month xCT KO (D) retinas in comparison to age-matched WT retinas, whereby a change of 1 represents a 100% change in labeling intensity. GCL, ganglion cell layer; INL, inner nuclear layer. n = 6 retinas. *P < 0.05.
Figure 3A shows that six-week WT retinas exhibit relatively high GSH immunoreactivity in most retinal neurons, with strong labeling in the RPE, photoreceptor IS, ONL, and GCs. In comparison, the six-week xCT KO retina (Fig. 3C) reveals reduced labeling in the photoreceptor cell bodies of the ONL. On the other hand, more intense labeling was observed in the photoreceptor OS and IS. Figure 3B shows the quantitative analysis of GSH labeling of six-week xCT KO retinas compared to the WT and revealed that xCT KO retinas show a significant depletion of GSH in the ONL (−46% ± 19%; P < 0.05) but a significant increase in GSH immunoreactivity in the OPL (78% ± 11%; P < 0.05) and photoreceptor OS (35% ± 8%; P < 0.05), similar to the changes seen in CSH immunoreactivity in the ONL and at the same age.
Figure 3.
Silver-intensified immunogold staining for glutathione content in six-week- and nine-month-old WT and xCT KO retinas. Representative images of glutathione immunoreactivity in the six-week WT (A) and xCT KO (C) retinas, and nine-month WT (B) and xCT KO (D) retinas. The dark arrows in (A) and (C) indicate MC, and the individual arrowheads identify the location of the MCef. Scale bar: 50 µm. Insets show magnified views of the outer nuclear layer (A′–D′), inner nuclear layer (A″–D″), and ganglion cell layer (A*–D*), respectively. Cell types identified in these views include the photoreceptor cell bodies (PR), BC, MC, AC, and the MCef. (E, F) Quantitative analysis of glutathione in each retinal layer expressed as the percentage change in amino acid immunoreactivity of the six-week xCT KO (C) or nine-month xCT KO (D) retinas in comparison to age-matched WT retinas. n = 6 retinas. *P < 0.05, **P < 0.01.
Analysis of GSH labeling in nine-month WT and xCT KO retinas (Figs. 3B, 3D, respectively) revealed similar labeling for GSH in the ONL and slightly darker GSH labeling in the OS and OPL in xCT KO retinas compared to WT. Quantitative analysis, however, revealed a significant decrease in GSH immunoreactivity in the ONL (−28% ± 9%; P < 0.05), and a significant increase in immunoreactivity in the OPL (64% ± 11%; P < 0.01) (Fig. 3F). However, the significant increase in GSH immunoreactivity in the OS seen at 6-weeks of age was not observed in the nine-month xCT KO retina.
Although cysteine and GSH immunogold patterns were consistent in six-week-old mice, this was not the case for the nine-month-old mice. Further validation such as additional biological replicates or complementary techniques would help to fully exclude the possibility that the lack of correlation in labeling of CSH and GSH in older retinas was not due to technical noise from immunogold quantification. However, if this variation in nine-month CSH and GSH labeling was due solely to technical noise, this would have also been apparent in the six-week retinas.
Basal Levels of ROS Are Increased in xCT KO Retinas
With the finding that loss of xCT function results in localized changes in GSH levels in the retina, we next investigated whether these changes were associated with changes in overall ROS levels. High-resolution respirometry was used to measure resting or baseline H2O2 flux in six-week and nine-month WT and xCT KO retinas (Fig. 4). WT retinas showed an age-related increase in H2O2 flux from six weeks to nine months (2.03-fold increase; P < 0.05), but there was no change between ages in xCT KO retinas. Between genotypes, at six weeks, H2O2 flux was higher in the xCT KO compared to age-matched WT retinas (1.93-fold increase; P < 0.05). However, at nine months there was no difference in H2O2 flux between WT or xCT KO retinas. There was also a significant increase in H2O2 flux seen in nine-month xCT KO samples compared to six-week WT retinas (1.93-fold increase; P < 0.05). These data indicate that resting ROS levels are high as early as six weeks of age in xCT KO retinas compared to age-matched WT retinas, and that these ROS levels are still maintained at these high levels at nine months in WT and xCT KO retinas.
Figure 4.

Measurement of basal H2O2 levels in retinal homogenates. Baseline levels of H2O2 were measured through high-resolution respirometry in retinal homogenates at rest (before the addition of any substrates) in six-week (6wk) and nine-month (9m) WT (gray) and xCT KO (black) tissues. n = 6, *P < 0.05.
Mitochondrial Activity Is Altered in xCT KO Retinas
To investigate the effects of heightened ROS and localized alterations in GSH and glutamate on mitochondria, we used high-resolution respirometry to examine mitochondrial health (Fig. 5A), content (Fig. 5B), and activity (Fig. 5C) in six-week and nine-month WT and xCT KO retinas. We first measured the CCR, which is a measure of coupling between the electron transport system and phosphorylating systems and is used to evaluate mitochondrial health (Fig. 5A). The lower the CCR the greater the coupling of mitochondrial systems.37,39,42 With age, there was a decrease in the CCR between six-week WT and nine-month WT retinas (1.16-fold decrease; P < 0.05), indicating that the mitochondria may have improved coupling with age. However, this was not apparent with age in the xCT KO retinas. Between genotypes, there were no significant differences in the CCR between WT and xCT KO six-week or nine-month retinas, indicating that there were no differences in mitochondrial health because of loss of xCT function.
Figure 5.
Mitochondrial activity in WT and xCT KO retinas. Retinal tissue homogenates were placed in sealed chambers and the change in oxygen concentration (oxygen flux) was measured as mitochondrial ETS complex substrates and inhibitors were added. (A) The coupling control ratio is calculated as the oxygen flux of the LEAK state divided by the physiological OXPHOS capacity and is an indicator of the degree of coupling between the ETS and phosphorylation systems. A value of 0.0 indicates completely coupled mitochondrial systems, whereas a value of 1.0 represents completely uncoupled mitochondrial systems. (B) Citrate synthase activity for retinal homogenates. Citrate synthase activity is used as a measure of mitochondrial content and was used to normalize oxygen flux data to show the oxygen flux per mitochondrion. (C) Retinal homogenate oxygen flux values normalized against citrate synthase activity for six-week (6wk) and nine-month (9m) WT (gray) and xCT KO (black) retinas. Oxygen flux was measured in tissues in kinetically saturating environments of CI substrates pyruvate and malate but depleted in ADP (LEAK), followed by addition of saturating ADP, stimulating ETS complex I (CI), addition of the CII substrate succinate (CI + II), and inhibition of CI with rotenone (CII). Six-week/nine-month WT/xCT KO n = 6. *P < 0.05, **P < 0.01, or ****P < 0.0001.
Figure 5B shows the activity of the mitochondrial enzyme citrate synthase, a marker of mitochondrial content in a tissue.37,40 Citrate synthase activity increases with age in both the WT (1.43-fold increase; p < 0.05) and KO retinas (1.92-fold increase; p < 0.0001). While there was no change in activity between WT and xCT KO retinas in the 6-week retina, in the 9-month retina there was a significant increase in activity (1.43-fold increase; p < 0.01) between WT and KO retinas.
Using this measure of mitochondrial content, mitochondrial O2 flux values were normalized to citrate synthase activity to determine mitochondrial activity (Fig. 5C). With age, O2 flux during LEAK respiration remained similar between WT and xCT KO retinas. For CI OXPHOS, while it appeared that there was a decrease in O2 flux with age for both WT and xCT KO retinas, this was not statistically significant. For CI + II OXPHOS, there was a significant decrease in O2 flux between 6-week and 9-month xCT KO retinas (1.86-fold decrease, p < 0.01). There were no significant differences in CII OXPHOS O2 flux with age. It should be noted that while CI was measured directly, CI + CII OXPHOS was measured as a combined parameter. The measurement of CII OXPHOS is not a direct assessment of CII activity alone. Instead, it is obtained by stimulating CII while inhibiting CI.
Between genotypes, there were no differences in LEAK respiration between WT and xCT KO retinas at 6-weeks or 9-months of age. While it appeared that there may have been an increase in CI OXPHOS respiration in 6-week xCT KO retinas compared to 6-week WT retinas, and a decrease in CI OXPHOS in 9-month xCT KO retinas compared to 9-month WT retinas, neither of these were statistically significant. There was, however, a significant decrease in CI+II OXPHOS respiration in 9-month xCT KO retinas compared to 6-week WT retinas (1.71-fold decrease, p < 0.01). There were no differences in CII OXPHOS respiration between WT and xCT KO retinas. Finally, CIV OXPHOS respiration was significantly decreased between 6-weeks WT and 9-month xCT KO retinas (2.05-fold decrease, p < 0.0001), and 6-week xCT KO and 9-month WT retinas (1.69-fold decrease, p < 0.0001).
Mitochondrial Electron Transport Through Complex I Is Altered in the 6-Week-Old xCT KO Retina
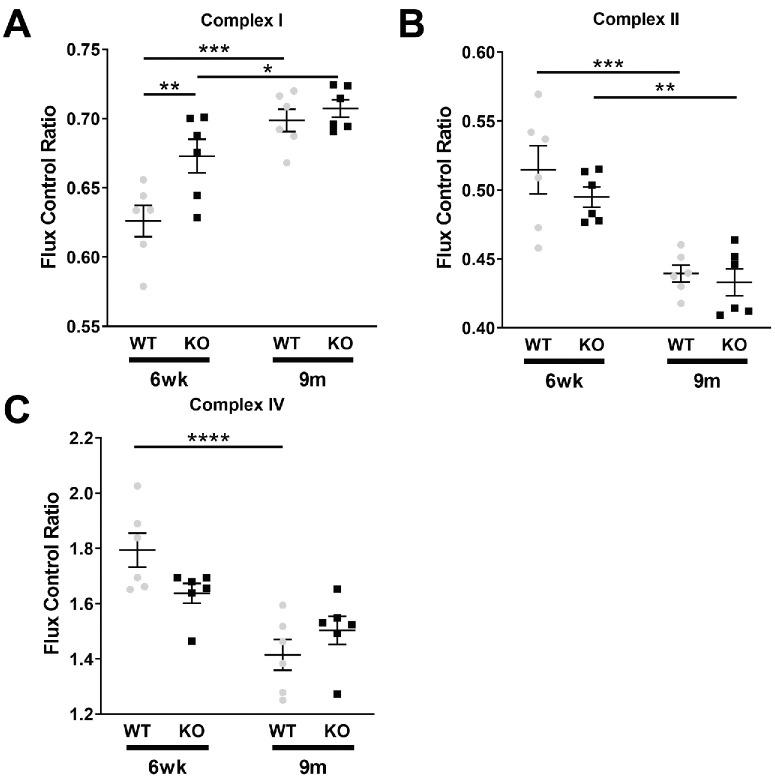
Having shown that mitochondrial activity is altered in xCT KO retinas, we next investigated the specific activities of the individual ETS complexes. To determine the specific activities of the individual ETS complexes or FCR, the ratio of the O2 flux of a particular complex in the OXPHOS state and that of convergent CI + CII OXPHOS activity was calculated. Figure 6 shows the FCRs for CI (A), CII (B), and CIV (C). For CI, there were age-related increases in CI activity from 6-week to 9-month observed in both WT (1.12-fold increase; p < 0.0001) and KO (1.05-fold increase; p = 0.0436) retinas. Between genotypes, at 6-weeks there was a significant increase in the xCT KO relative to WT retina (1.1-fold increase; p = 0.0059), but there were no differences in activity between 9-month WT and xCT KO retinas (Fig. 6A). For CII, there were age-related decreases in activity observed in WT (1.17-fold decrease, p = 0.0002) and xCT KO (1.14-fold decrease, p = 0.0016) retinas. However, there were no differences in activity between genotypes at neither 6-weeks nor 9-month (Fig. 6B). For CIV, a significant age-related decrease in CIV activity was observed between 6-weeks and 9-month in WT retinas (1.27-fold decrease; p = 0.0022), but not in xCT KO retinas (p = 0.0649). Between genotypes, CIV activity in the 6-weeks of age xCT KO retinas appeared lower than that of same age WT retinas (1.27-fold decrease); however, this was not statistically significant. There were no differences at 9-month between WT and xCT KO retinas (Fig. 6C).
Figure 6.
Flux control ratios of mitochondrial ETS complexes. The flux control ratio is a measure of the oxygen flux during the OXPHOS state of a particular ETS complex normalized to that of simultaneous CI and CII stimulation through the NADH and FADH2 pathways, respectively, and is used to determine the specific activity of individual ETS complexes. The FCRs are shown for six-week (6wk) and nine-month (9m) WT (gray) and KO (black) retinas for CI (A), CII (B), and CIV (C). Six-week/nine-month WT/KO. n = 6. *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001.
In summary, through these respirometry experiments, while we observed age related changes in all complex activity at 6-weeks and 9-months of age for WT and xCT KO retinas, it was for complex I that we detected a significant change in activity at 6-weeks between WT and xCT KO retinas. Since Complex I is the primary site of ROS generation in the mitochondria during the RET process,20 we next measured mitochondrial ROS generation to determine if the overall increase in ROS levels in KO retinas were attributed to an increase in mitochondrial ROS.
The xCT KO Retinal Mitochondria Show Reduced Levels of Reverse Electron Transport
Within the mitochondria, electrons that exit the inner membrane electron transport chain can generate superoxide and H2O2. These electrons can either move through the electron transport chain from complexes I and II to leave the chain at complex IV in a process known as ‘forward electron transport’; or they can flow backwards from complex II to complex I and exit the chain in a process known as ‘reverse electron transport’ (RET). To understand the contribution by the WT and xCT KO mitochondria to ROS levels in the retina, we used high-resolution respirometry which can assess reverse and forward electron transport and thus rates of ROS production. H2O2 flux values were measured and compared between 6-week and 9-month WT and xCT KO retinal homogenates and results normalized to the lowest observed H2O2 flux. Although H2O2 flux was measured for all mitochondrial respiratory states (Supplementary Figure 3), we show H2O2 flux during the CII-stimulated LEAK state as this represents ROS production generated through RET20 (Fig. 7).
Figure 7.

Measurement of reverse electron transport during LEAK state in retinal homogenates normalized to the lowest H2O2 signal recorded during experiment. Retinal tissue homogenates were placed in sealed chambers and the change in H2O2 concentration over time (H2O2 flux) was measured as mitochondrial ETS complex substrates and inhibitors were added. H2O2 flux was recorded in six-week (6wk) and nine-month (9m) old WT (gray) and xCT KO (black) retinas over a trio of related experiments, in which pyruvate and malate (PM), succinate (S), or succinate and rotenone (S(rot)) were used as the initial substrates. n = 6, ****P < 0.001.
It can be seen for 6-week WT and KO retinas and 9-month WT and KO retinas that in the LEAK state, following CI stimulation with pyruvate and malate (LEAK-PM), indicative of forward electron transport, little H2O2 flux was observed. However, after stimulation of CII with succinate (LEAK-S), there was a significant increase in H2O2 flux represented by a 24-fold increase in 6-week WT retina (p < 0.0001), a 15.5-fold increase (p < 0.0001) in 6-week xCT KOs, a 4.13-fold increase (p < 0.0001) in 9-month WT, and a 4.75-fold increase (p < 0.0001) in 9-month xCT KO compared to the LEAK-PM state. However, after stimulation with both succinate and rotenone (LEAK-S(rot)), which causes intoxication of CI and, as a result, reduces its ability to accept reverse-flowing electrons from CII and ubiquinol, there was a significant decrease in H2O2 flux compared to the LEAK-S stated represented by a 4.8-fold decrease (p < 0.0001) in 6-week WT, a 3.88-fold decrease (p < 0.0001) in 6-week xCT KO, a 2.54-fold decrease (p < 0.0001) in 9-month WT, and a 2.71-fold decrease (p < 0.0001) in 9-month xCT KO. This indicates that the majority of H2O2 production was via RET.
Given that the greatest H2O2 fluxes were observed in the CII-stimulated LEAK state, H2O2 flux values from this state were measured and compared between 6-week and 9-month WT and xCT KO retinal homogenates (Fig. 8). With age, WT retinas showed a significant decrease in H2O2 flux from 6-weeks to 9-months (2.02-fold decrease, p < 0.05), which was not apparent in xCT KO retinas. Between genotypes, there was a significant reduction in H2O2 flux in 6-week xCT KO retinas compared to 6-week WT retinas (2.2-fold decrease, p < 0.05), which was not apparent in the 9-month retinas.
Figure 8.

Measurement of reverse electron transport in retinal homogenates normalized to the lowest H2O2 signal recorded during experiment. H2O2 fluxes normalized to the lowest H2O2 flux observed in each experiment were recorded during the CII LEAK state for six-week (6wk) and nine-month (9m) WT (gray) and xCT KO (black) retinas and compared. n = 6, *P < 0.05.
Taken together, the majority of mitochondrial H2O2 production occurs via RET since the greatest H2O2 flux was exhibited in the LEAK state after stimulation with the substrate succinate and was significantly reduced in the presence of the CI inhibitor rotenone. In addition, these findings also show that the loss of xCT function at 6-weeks of age, but not 9-months of age, results in a decrease in mitochondrial RET ROS production.
Discussion
Given the important roles of xCT in other tissues, we examined the role of xCT in the retina. Previously we showed that in the retina, xCT is localized to the ribbon complex of the photoreceptors and contributes to glutamate release,17 and more recently that loss of xCT function results in glutamate metabolic disruption through the accumulation of glutamate in photoreceptors and the reduced uptake of glutamate and glutamine production by Müller cells.18 These findings indicate that xCT is a significant exporter of glutamate for maintaining the glutamate-glutamine cycle between the photoreceptor cells and Müller cells. We did not observe accumulation of glutamate in the inner retinal layers or retinal ganglion cells (RGCs), or find any evidence of metabolic impairment indicative of RGC excitotoxicity. Although glutamate accumulates presynaptically in photoreceptors, Müller cell buffering and possible retinal adaptation mechanisms may protect downstream neurons, including RGCs, from excitotoxic damage.
The purpose of this study was to investigate the role of xCT in cyst(e)ine uptake for the synthesis of glutathione and determine how it impacts the tightly regulated balance between ROS generation and antioxidant homeostasis. Moreover, as loss of xCT results in the accumulation of glutamate within the photoreceptor terminals and inner segments, which coincide with areas of high mitochondria content,43 we also examined its impact on mitochondrial function.
The xCT Plays a Role in Maintaining Intracellular Redox Balance
To determine the role of xCT in the import of cystine/cysteine for GSH synthesis, we first measured cysteine and GSH levels in whole retinas and then mapped cysteine and GSH content in the different layers of the retina in six weeks and nine month WT and xCT KO mice. Although there were no differences in cysteine and GSH levels between WT and xCT KO retinas, we found localized changes to cysteine and GSH in the different retinal layers as a result of loss of xCT. There were decreased levels of cysteine and GSH in the outer nuclear layer of six-week xCT KO retinas compared to age-matched WT retinas, and decreased levels of GSH in the same layer in the nine-month xCT KO retinas, which might suggest that photoreceptors soma, where mitochondria reside, may be more susceptible to oxidative damage. Unexpectedly, xCT loss resulted in an increase in cysteine and GSH levels in the photoreceptor OS at six weeks of age and an increase in GSH levels in the OPL at six weeks and nine months of age. This suggests a potential compensatory mechanism by the 6-week xCT KO retinas involving cysteine or GSH accumulation directly from the choroidal blood supply.44,45 This could be achieved via import through alternative cyst(e)ine transporters such as the alanine-serine-cysteine-threonine transporter 1 and 2, system L amino acid transporter 2, and the excitatory amino acid transporter 1–346–49 or generation of cysteine through the transsulfuration pathway.50 Further work could examine the different mechanisms used by the retina to maintain GSH homeostasis, and how these might alter with age or disease
Given the localized changes in cysteine and GSH distribution, we examined what effect this might have on ROS production in the retina and in particular on mitochondrial ROS production. We showed that basal ROS levels were elevated in six-week-old xCT KO retinas compared to age-matched WT retinas and were in fact similar to those seen in the 9-month-old WT and xCT KO retinas, demonstrating that young xCT KO retinas are exposed to oxidative stress at levels typically experienced by older retinas. However, although we hypothesized that the source of ROS was from the mitochondria, there was on the contrary a significant reduction in mitochondrial ROS production through RET. Why and how a decrease in mitochondrial ROS production via RET occurs and affects the retina is unclear at this stage. It is possible that, given the heightened levels of overall ROS in the retina, the mitochondria may respond by reducing ROS production. Although this might mitigate further oxidative stress, physiological levels of ROS have been shown to play an important role in the intracellular signaling cascade in a number of processes,51 such as initiation of transcriptional stress responses against hypoxia, heat, unfolded proteins, and proteolysis,52 post-translational regulation of a number of cysteine-rich proteins,53,54 signal transduction cascades,55 regulation of arrangement of the ETS to maximize efficiency of the respiration of various fuels,51,56 and in the modulation of innate and acquired immune responses.55,57 Further work is required to characterize the contribution of physiological ROS on retinal function and determine how a decrease in mitochondrial ROS may impact the normal physiological functions of the retina.
If the mitochondria are not the source of increased basal ROS in xCT KO retinas, this suggests that other sources of ROS are responsible for the overall heightened levels of ROS in the xCT KO retina at six weeks of age. These could include intracellular lysosomes, xanthine oxidases, and nicotinamide adenine dinucleotide phosphate oxidase (NOX),58–60 which have been implicated in the development of a number of retinal diseases, including glaucoma, ischemic retinopathy, and AMD.61,62 Mitochondrial dysfunction is known to activate NOX in other tissues,63 and NOX has been identified as a major source of oxidative stress in the outer retina in particular.60 This suggests that in the xCT KO retina, altered mitochondrial ETS complex activity may similarly activate NOX, contributing to increased ROS production.6,21
Changes in mitochondrial ROS reflect alterations in mitochondrial substrate utilization and function, providing evidence of a physiological impact of xCT on cellular metabolism. In contrast, elevated total ROS indicates a broader oxidative challenge in the knockout retinas, leading to cell damage and disruptions. This increased oxidative stress likely drives the observed changes in mitochondrial activity and ROS production, highlighting how these processes are interlinked and can amplify oxidative stress in the retina. Additionally, both total and mitochondrial ROS are influenced by glutathione availability and are thus directly affected by loss of xCT function. Although our study has emphasized mitochondrial ROS, assessing both total and mitochondrial ROS are essential for a comprehensive understanding of xCT's role in maintaining retinal homeostasis.
Influence of xCT on Mitochondrial Health, Content, and Activity
Given that xCT KO retinas displayed a disruption in glutamate homeostasis, with glutamate accumulating in areas where mitochondria are abundant, this suggested that loss of xCT may contribute to altered mitochondrial function.18,43 To investigate this, we first examined the mitochondrial coupling control ratio or the degree to which translocated electrons in the mitochondrial intermembrane space are used by F0-F1 ATP-synthase.42 We found that mitochondrial coupling was similar between WT and xCT KO retinas at both six weeks and nine months of age, and that coupling improved with age in the WT retinas, suggesting that mitochondrial health and phosphorylating ability is largely unaffected by loss of xCT even at nine months of age. Although mitochondrial coupling is known to decline with advanced age,64 and in other studies has been shown to be apparent in 2-year-old mice,37 this suggests that much older mice would be required in our study to see an age related decline in mitochondrial coupling.
Interestingly, total mitochondrial content measured by citrate synthase activity increased with age and in response to loss of xCT function. Mitochondrial content can increase in cells through biogenesis, or creation of new mitochondria (fusion), or through fission of individual mitochondria.65 With age, typically, biogenesis declines, as levels of key biogenesis regulator proteins decrease.66 This decline in mitochondrial biogenesis is also often reflected in the loss of mitochondrial fission-related proteins in the whole retina.67 However, in the photoreceptors, electron microscopy reveals that fusion proteins, such as dynamin-like GTPase (Opa1), and fission proteins, such as fission 1 protein, are upregulated specifically within the photoreceptor inner segment, alongside increased mitochondrial fragmentation, at 12-months compared to 1-month in C57BL/6 mice.68 Kam et al.68 postulate that this increase in mitochondrial enzymes specifically in the outer retina reflects an attempt by photoreceptors to maintain mitochondrial function despite declining mitochondrial quality. Therefore, although more work is required, our findings might suggest that the increased citrate synthase activity in WT and xCT KO retinas at nine months compared to six weeks, and in nine-month-old xCT KO retinas compared to nine-month-old WT retinas, may compensate for the changes in mitochondrial activity seen in xCT KO retinas. It should be pointed out that although citrate synthase is commonly used as a proxy for mitochondrial content, there are limitations, as citrate synthase activity can vary with age,69,70 as we have observed in our own data, making it a potentially confounding marker in some experimental contexts. However, identifying a more suitable alternative remains challenging. As highlighted in other studies, citrate synthase activity remains a widely accepted and validated marker for estimating mitochondrial content,37,40,71 despite its known limitations.
We measured the activity of the individual electron transport chain complexes and found age and genotype differences. Complex I activity increased with age for both WT and xCT KO. This suggests that with age, the mitochondria may increase its utilization of complex I substrates (such as malate, pyruvate, or glutamate via α-ketoglutarate) to drive and maintain overall mitochondrial activity. Between genotypes, complex I activity was significantly increased in xCT KO retinas relative to WT retinas at 6-weeks of age. This coincides with the age that glutamate accumulation was evident in xCT KO retinas.18 This early increase in CI activity at 6-weeks in xCT KO retinas is potentially indicative of increased glutamate utilization by the mitochondria, facilitated by the increased available local supply, stimulating cellular metabolic changes that typically occur in older WT retinas. Such findings raise the possibility that six-week-old xCT KO retinas exhibit signs of early aging in the form of changes in cellular metabolism and increases in complex I activity.
We speculate that this increased mitochondrial utilization of glutamate represents a compensatory metabolic adaptation in xCT KO and aged retinas aimed at supporting energy production and modulate redox balance. As shown in our previous work,18 and further supported by Figure 6 in the current study, glutamate accumulates in photoreceptor inner segments where mitochondria are densely localized. Glutamate can be metabolized via conversion to α-ketoglutarate, enabling its entry into the TCA cycle as an anaplerotic substrate to sustain oxidative phosphorylation.72,73 This pathway not only supports ATP generation via forward electron movement but also improves mitochondrial coupling efficiency. Consistent with this, our high-resolution respirometry data showed a significant increase in Complex I activity at 6 weeks in xCT KO retinas (Fig. 6A), which coincides with elevated glutamate levels suggesting enhanced glutamate oxidation. This increased metabolic flux through CI may improve mitochondrial efficiency in the short term and could partly explain why ROS production via reverse electron transport is reduced in young xCT KO mice despite overall elevated ROS levels in the retina (Fig. 8).
In conclusion, the results of this work suggest that xCT contributes to glutamate export and the maintenance of cysteine levels and GSH levels to localized layers within the retina. Young xCT KO retinas exhibit signs of redox imbalance, metabolic imbalance, and mitochondrial dysfunction, highlighting the xCT KO mouse to be a useful model to study accelerated aging. However, these changes are less obvious with age suggesting that compensatory mechanisms exist which enable the retina to adapt and normalize to the wild type, thus ensuring a balance between meeting the high metabolic needs of the retina and redox balance.
Supplementary Material
Acknowledgments
Disclosure: L.J. Knight, None; R.M. Martis, None; P.J. Donaldson, None; M.L. Acosta, None; J.C. Lim, None
References
- 1. Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980; 255: 2372–2376. [PubMed] [Google Scholar]
- 2. Bridges RJ, Natale NR, Patel SA. System xc⁻ cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012; 165: 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999; 274: 11455–11458. [DOI] [PubMed] [Google Scholar]
- 4. Lall MM, Ferrell J, Nagar S, Fleisher LN, McGahan MC. Iron regulates L-cystine uptake and glutathione levels in lens epithelial and retinal pigment epithelial cells by its effect on cytosolic aconitase. Invest Ophthalmol Vis Sci. 2008; 49: 310–319. [DOI] [PubMed] [Google Scholar]
- 5. Sagara JI, Miura K, Bannai S. Maintenance of neuronal glutathione by glial cells. J Neurochem. 1993; 61: 1672–1676. [DOI] [PubMed] [Google Scholar]
- 6. Sato H, Shiiya A, Kimata M, et al.. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005; 280: 37423–37429. [DOI] [PubMed] [Google Scholar]
- 7. Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006; 26: 10514–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kranich O, Hamprecht B, Dringen R. Different preferences in the utilization of amino acids for glutathione synthesis in cultured neurons and astroglial cells derived from rat brain. Neurosci Lett. 1996; 219: 211–214. [DOI] [PubMed] [Google Scholar]
- 9. Bridges RJ, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012; 64: 780–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bannai S, Takada A, Kasuga H, Tateishi N. Induction of cystine transport activity in isolated rat hepatocytes by sulfobromophthalein and other electrophilic agents. Hepatology (Baltimore). 1986; 6: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 11. Martis RM, Knight LJ, Donaldson PJ, Lim JC. Identification, expression, and roles of the cystine/glutamate antiporter in ocular tissues. Oxid Med Cell Longev. 2020; 2020: 4594606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martis RM, Knight LJ, Acosta ML, et al.. Early onset of age-related changes in the retina of cystine/glutamate antiporter knockout mice. Exp Eye Res. 2023; 227: 109364. [DOI] [PubMed] [Google Scholar]
- 13. Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol (Baltimore). 1994; 152: 3578–3585. [PubMed] [Google Scholar]
- 14. Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci. 2007; 27: 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martis RM, Donaldson PJ, Lim JC. Corneal opacities in mice exposed to repeated contact procedures during ocular examinations. Clin Exp Optom. 2020; 103: 307–311. [DOI] [PubMed] [Google Scholar]
- 16. Martis RM, Li B, Donaldson PJ, Lim JC. Early onset of age-related cataracts in cystine/glutamate antiporter knockout mice. Invest Ophthalmol Vis Sci. 2021; 62: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu RG, Lim JC, Donaldson PJ, Kalloniatis M. Characterization of the cystine/glutamate transporter in the outer plexiform layer of the vertebrate retina. Eur J Neurosci. 2008; 28: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 18. Knight LJ, Martis RM, Donaldson PJ, Acosta ML, Lim JC. Changes in glutamate and glutamine distributions in the retinas of cystine/glutamate antiporter knockout mice. Mol Vision. 2023; 29: 274–288. [PMC free article] [PubMed] [Google Scholar]
- 19. Rego AC, Sancha Santos M, Oliveira CR. Glutamate-mediated inhibition of oxidative phosphorylation in cultured retinal cells. Neurochem Int. 2000; 36: 159–166. [DOI] [PubMed] [Google Scholar]
- 20. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martis RM, Donaldson PJ, Li B, Middleditch M, Kallingappa PK, Lim JC. Mapping of the cystine–glutamate exchanger in the mouse eye: a role for xCT in controlling extracellular redox balance. Histochem Cell Biol. 2019; 152: 293–310. [DOI] [PubMed] [Google Scholar]
- 22. Mattapallil MJ, Wawrousek EF, Chan C-C, et al.. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalloniatis M, Fletcher EL. Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol. 1993; 336: 174–193. [DOI] [PubMed] [Google Scholar]
- 24. Marc RE, Liu WL, Kalloniatis M, Raiguel SF, van Haesendonck E. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990; 10: 4006–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalloniatis M, Tomisich G. Amino acid neurochemistry of the vertebrate retina. Progr Retin Eye Res. 1999; 18: 811–866. [DOI] [PubMed] [Google Scholar]
- 26. Kalloniatis M, Tomisich G, Marc RE. Neurochemical signatures revealed by glutamine labeling in the chicken retina. Vis Neurosci. 1994; 11: 793–804. [DOI] [PubMed] [Google Scholar]
- 27. Shivashankar G, Lim JC, Acosta ML. Proinflammatory cytokines trigger biochemical and neurochemical changes in mouse retinal explants exposed to hyperglycemic conditions. Mol Vision. 2020; 26: 277–290. [PMC free article] [PubMed] [Google Scholar]
- 28. Shivashankar G, Lim JC, Acosta ML. Glyceraldehyde-3-phosphate dehydrogenase and glutamine synthetase inhibition in the presence of pro-inflammatory cytokines contribute to the metabolic imbalance of diabetic retinopathy. Exp Eye Res. 2021; 213: 108845. [DOI] [PubMed] [Google Scholar]
- 29. Marc RE, Murry RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. J Neurosci. 1995; 15: 5106–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moeremans M, Daneels G, Van Dijck A, Langanger G, De Mey J. Sensitive visualization of antigen-antibody reactions in dot and blot immune overlay assays with immunogold and immunogold/silver staining. J Immunol Methods. 1984; 74: 353–360. [DOI] [PubMed] [Google Scholar]
- 31. Acosta ML, Kalloniatis M. Short- and long-term enzymatic regulation secondary to metabolic insult in the rat retina. J Neurochem. 2005; 92: 1350–1362. [DOI] [PubMed] [Google Scholar]
- 32. Sun D, Vingrys AJ, Kalloniatis M. Metabolic and functional profiling of the ischemic/reperfused rat retina. J Comp Neurol. 2007; 505: 114–130. [DOI] [PubMed] [Google Scholar]
- 33. Chang LY, Ardiles AO, Tapia-Rojas C, et al.. Evidence of synaptic and neurochemical remodeling in the retina of aging degus. Front Neurosci. 2020; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gnaiger E. Oxygen calibration by DatLab 6. Mitochondrial Physiol Netw. 2016; 19: 39–48. [Google Scholar]
- 35. Power ASC, Pham T, Loiselle DS, Crossman DH, Ward M-L, Hickey AJ. Impaired ADP channeling to mitochondria and elevated reactive oxygen species in hypertensive hearts. Am J Physiol Heart Circ Physiol. 2016; 310: H1649–H1657. [DOI] [PubMed] [Google Scholar]
- 36. Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules. 2015; 5: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han WH, Gotzmann J, Kuny S, et al.. Modifications in retinal mitochondrial respiration precede type 2 diabetes and protracted microvascular retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 3826–3839. [DOI] [PubMed] [Google Scholar]
- 38. Gnaiger E, Arnould T, Detraux D, Storder J. Mitochondrial respiratory states and rates. MitoFit Preprint Arch. Posted online March 15, 2019. 10.26124/mitofit:190001.v5. [DOI] [Google Scholar]
- 39. Gnaiger E. Mitochondrial pathways and respiratory control: an introduction to OXPHOS analysis. Bioenergetics Commun. 2020; 2020: 2. [Google Scholar]
- 40. Eigentler A, Draxl A, Wiethüchter A, Kuznetsov A, Lassing B, Gnaiger E. Laboratory protocol: citrate synthase. A mitochondrial marker enzyme. MiPNet. 2012; 1704: 1–11. [Google Scholar]
- 41. Benz R, McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J. 1983; 41: 381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005; 1706: 1–11. [DOI] [PubMed] [Google Scholar]
- 43. Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008; 1189: 58–69. [DOI] [PubMed] [Google Scholar]
- 44. Yam M, Engel AL, Wang Y, et al.. Proline mediates metabolic communication between retinal pigment epithelial cells and the retina. J Biol Chem. 2019; 294: 10278–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013; 19: 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewerenz J, Hewett SJ, Huang Y, et al.. The Cystine/Glutamate Antiporter System xc− in Health and Disease: From Molecular Mechanisms to Novel Therapeutic Opportunities. Antioxid Redox Signal. 2013; 18: 522–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001; 902: 156–163. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003; 84: 1332–1339. [DOI] [PubMed] [Google Scholar]
- 49. Wagner CA, Lang F, Bröer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001; 281: C1077–C1093. [DOI] [PubMed] [Google Scholar]
- 50. Markand S, Tawfik A, Ha Y, et al.. Cystathionine beta synthase expression in mouse retina. Curr Eye Res. 2013; 38: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scialò F, Fernández-Ayala DJ, Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol. 2017; 8: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scialò F, Sriram A, Fernández-Ayala D, et al.. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016; 23: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kruk JS, Vasefi MS, Heikkila JJ, Beazely MA. Reactive oxygen species are required for 5-HT-induced transactivation of neuronal platelet-derived growth factor and TrkB receptors, but not for ERK1/2 activation. PLoS One. 2013; 8: e77027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang J, Wang X, Vikash V, et al.. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016; 2016: 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82: 47–95. [DOI] [PubMed] [Google Scholar]
- 56. Guarás A, Perales-Clemente E, Calvo E, et al.. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 2016; 15: 197–209. [DOI] [PubMed] [Google Scholar]
- 57. Roy J, Galano J-M, Durand T, Le Guennec J-Y, Chung-Yung Lee J. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017; 31: 3729–3745. [DOI] [PubMed] [Google Scholar]
- 58. Lacy F, Gough DA, Schmid-Schönbein GW. Role of xanthine oxidase in hydrogen peroxide production. Free Radic Biol Med. 1998; 25: 720–727. [DOI] [PubMed] [Google Scholar]
- 59. Boya P, Kaarniranta K, Handa JT, Sinha D. Lysosomes in retinal health and disease. Trends Neurosci. 2023; 46: 1067–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Usui S, Oveson BC, Lee SY, et al.. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009; 110: 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fan Gaskin JC, Shah MH, Chan EC. Oxidative stress and the role of NADPH oxidase in glaucoma. Antioxidants (Basel). 2021; 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chan EC, Liu GS, Dusting GJ. Redox mechanisms in pathological angiogenesis in the retina: roles for NADPH oxidase. Curr Pharm Des. 2015; 21: 5988–5998. [DOI] [PubMed] [Google Scholar]
- 63. Dikalov SI, Li W, Doughan AK, Blanco RR, Zafari AM. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am J Physiol Regul Integr Comp Physiol. 2012; 302: R1134–R1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Porter C, Hurren NM, Cotter MV, et al.. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015; 309: E224–E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010; 11: 872–884. [DOI] [PubMed] [Google Scholar]
- 66. Zhao L, Feng Z, Zou X, Cao K, Xu J, Liu J. Aging leads to elevation of O-GlcNAcylation and disruption of mitochondrial homeostasis in retina. Oxid Med Cell Longev. 2014; 2014: 425705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jendrach M, Pohl S, Vöth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev. 2005; 126: 813–821. [DOI] [PubMed] [Google Scholar]
- 68. Kam JH, Jeffery G. To unite or divide: mitochondrial dynamics in the murine outer retina that preceded age related photoreceptor loss. Oncotarget. 2015; 6: 26690–26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coggan AR, Abduljalil AM, Swanson SC, et al.. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol. 1993; 75: 2125–2133. [DOI] [PubMed] [Google Scholar]
- 70. Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. 1996; 93: 15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vigelsø A, Andersen NB, Dela F. The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training. Int J Physiol Pathophysiol Pharmacol. 2014; 6: 84–101. [PMC free article] [PubMed] [Google Scholar]
- 72. Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002; 277: 30409–30412. [DOI] [PubMed] [Google Scholar]
- 73. Aubert S, Bligny R, Douce R, Gout E, Ratcliffe RG, Roberts JKM. Contribution of glutamate dehydrogenase to mitochondrial glutamate metabolism studied by 13C and 31P nuclear magnetic resonance. J Exp Bot. 2001; 52: 37–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.