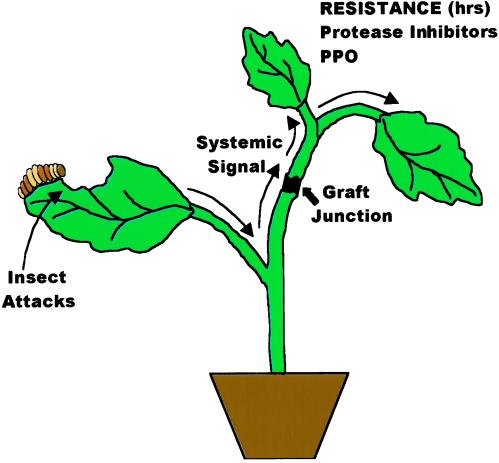
Over 100 species of plants exhibit systemic wound signaling that induces the production of defensive chemicals in leaves and stems (1). The signaling is caused by herbivore attacks and also occurs in response to some pathogens (2–5). The most intensively studied systemic signaling response is that found in species of the Solanaceae family, where a systemic wound signal that is graft transmissible regulates the expression of defensive proteinase inhibitors (PIs) and polyphenol oxidase genes (6) (Fig. 1). On wounding, an 18-aa polypeptide, called systemin (7), interacts with a cell-surface receptor (8, 9) to initiate a signaling cascade that includes the release of linolenic acid (18 carbon atoms) from plant cell membranes and its subsequent conversion to 12 oxo-phytodienoic acid (OPDA; 18 carbon atoms) and jasmonic acid (JA; 12 carbon atoms) (10) through the well known octadecanoid pathway (11). JA activates the expression of several signaling pathway genes that up-regulate JA synthesis and the production of H2O2 (12) leading to the synthesis of PIs. OPDA and JA synthesis is analogous to elements of the inflammatory pathway in animals in which arachidonic acid (20 carbon atoms) is converted to prostaglandins (13). Systemin is considered a mobile long-distance signal, and OPDA isomers (14) and JA have been considered to be localized signals produced in target cells. Li et al., in a recent issue of PNAS (15), use two mutants defective in the wound-signaling pathway to provide evidence that JA or a derivative may also act as a long-distance transmissible signal for wound signaling.
Jasmonic acid or a derivative may act as a long-distance transmissible signal for wound signaling.
Figure 1.
Illustration of the systemic wound response to insect attacks through a graft junction. PPO, polyphenol oxidase.
Systemin is produced from the C-terminal region of a 200-aa precursor called prosystemin (16) and is active at femtomols per plant in inducing proteinase inhibitor synthesis in young excised tomato plants (6). When radioactive systemin is applied directly to wounds, it is found in the phloem, where it is thought to be transported through the plant (6, 17). How far systemin can travel in the phloem and how it would be transported from the phloem to the outside of distal leaf cells to activate defense genes have not been established. However, systemin does plays a key role in long-distance signaling, because tomato plants transformed with an antisense prosystemin gene do not exhibit a systemic wound response (16). Conversely, tomato plants transformed with the prosystemin gene in its correct orientation under the 35S CaMV promoter (called prosystemin sense plants) produce prosystemin in leaves throughout the plants. Systemin is apparently released constitutively, and the plants behave as if they were in a permanently wounded state, producing, with time, extraordinarily high levels of PIs in leaves in the absence of wounding (18). Wild-type plants grafted as scions onto sense plant rootstocks also express high levels of defense genes in the absence of wounding, indicating that a systemic signal (systemin) is produced by the sense rootstocks and is transported to the scions (18).
In similar grafting experiments, Li et al. (15) further investigated signal transmission by using two mutant tomato lines (19, 20), spr-2 and jai-12. The spr-2 plants are deficient in signaling the wound-induced systemic expression of PI genes, but the genes are expressed when the plants are exposed to methyl jasmonate (MeJA) or JA, indicating that the plants have a lesion in the octadecanoid pathway upstream from JA. The jai-12 plants are deficient in jasmonate perception, or a component involved in JA signaling, and they are only weakly active in expressing PI genes in response to wounding or MeJA/JA treatments. The authors (15) initially used several combinations of grafted wild-type spr-2, and jai-12 plants as either scions or rootstocks. The rootstock leaves were wounded, and the leaves of both the rootstocks and scions were assayed for inducible PI-II mRNA and protein to determine whether a transmissible signal would pass through the graft. They found that wounding the jai-12 rootstocks sent a transmissible signal to wild-type scions, but jai-12 scions grafted to wounded wild-type rootstocks did not induce PIs. These results were in accord with the defect of the jai-12 plants being deficient in JA perception, with respect to PI synthesis, but being able to produce large amounts of JA to signal PI synthesis in wild-type scions. When wild-type plants were used as scions on spr-2 rootstocks, no transmissible signal was generated when the spr-2 leaves were wounded. On the other hand, spr-2 scions responded to wounds on wild-type rootstocks by producing PI-II mRNA and protein.
The authors (15) then grafted spr-2 and jai-12 as scions onto prosystemin sense plant rootstocks, which can send a transmissible signal to wild-type plants. The spr-2 scions received the signal and accumulated PI-II protein, but the jai-12 mutant plants did not, again indicating that the transmissible signal through the graft junction could be JA or a related oxylipin.
In wild-type tomato plants, after a single wound, the systemic activation of defense genes is maintained for several hours. It seems unlikely that any signal could survive that long without amplification. In prosystemin sense plants, this would not seem to be a problem, because a low continual source of systemin is apparently generated constitutively, which could account for the transmissible signal over several days. But how do singly wounded wild-type plants maintain a long-term distal response? One possibility is that enough JA is synthesized at a single wound site in response to systemin and from damaged membranes and is transported throughout the plant to signal and maintain gene expression for several hours. A more attractive hypothesis is that wild-type plants can amplify the signal (21). Both systemin and JA are released at wound sites, systemin being processed from prosystemin and JA initially produced from the degradation of membranes, releasing linolenic acid, the precursor of JA. Both systemin and JA would move away from the site in a systemic manner, but with the smaller soluble JA likely being more mobile. Systemin would generate more JA, resulting in the amplification of systemin and oxylipins as a cascade along the stems and petioles. The process would eventually be limited by the presence of extracellular systemin-inactivating enzymes (22). A signal cascade initiated in a wounded wild-type rootstock grafted to an spr-2 scion would amplify all of the signals through the vascular bundles to the graft junction but could not proceed along the spr-2 stem. JA synthesis would continue in the leaves and stems of the rootstock, producing oxylipins that would be transmitted to the spr-2 scion for a much longer period than could be achieved by a single wound.
Several observations support the amplification hypothesis, including the requirement for the expression of the prosystemin gene for systemic wound signaling, the timing for the development of maximal transmission of the signal(s) out of the wounded leaf (approximately 60–90 min) (23), and the wound-induced expression of prosystemin in vascular bundles (21), specifically in phloem parenchyma cells (J. N.-V. and C.A.R, unpublished work). Several of the early wound- and JA-inducible genes are proteinases (13) that are candidates for processing prosystemin to systemin for export to the apoplast for interaction with its receptor to amplify signaling. The identification of the defective genes spr-2 and in jai-12 should contribute importantly to the understanding of the relationships of systemin and oxylipins in signaling the systemic wound response.
The report by Li et al. (15) has provided a fundamentally novel insight into the signaling cascade. The use of the two mutants in simple grafting experiments demonstrates the simplicity of experimental design that can provide clues to evaluate the complex biochemistry of wound signaling. The signaling pathway is complex and involves multiple organs, cell types, compartments, and signals. Although much has been learned about the signaling pathway, both the genetic and biochemical approaches will continue to be required to integrate knowledge of the various parts of the signaling pathway and to learn how nature has evolved such an elegant process in plants to defend against attacking herbivores and pathogens.
Footnotes
See companion article on page 6416 in issue 9 of volume 99.
References
- 1.Karban R, Baldwin I T. In: Induced Responses to Herbivory. Karban R, Baldwin I T, editors. Chicago, IL: Univ. of Chicago Press; 1997. pp. 108–115. [Google Scholar]
- 2.Patot K V, Holzer F M, Reisch B, Walling L L. Proc Natl Acad Sci USA. 1993;90:9906–9910. doi: 10.1073/pnas.90.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijayan Pl, Schokey J, Levesque C A, Cook R J, Browse J. Proc Natl Acad Sci USA. 1996;95:5473–7477. [Google Scholar]
- 4.Staswick P E, Yuen G Y, Lehman C C. Plant J. 1999;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Pennicncks I A, Thomma B P, Buchala A, Metrauz J-P, Brockaert W F. Plant Cell. 1998;10:3103–3110. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 7.Ryan C A. Biochem Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 8.Meindl T, Boller T, Felix G. Plant Cell. 1998;10:1561–1570. doi: 10.1105/tpc.10.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheer J M, Ryan C A. Plant Cell. 1999;11:1525–1535. doi: 10.1105/tpc.11.8.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer E E, Ryan C A. Plant Cell. 1992;4:29–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vick B A. In: Lipid Metabolism in Plants. Moore T S, editor. Boca Raton, FL: CRC; 1993. pp. 167–191. [Google Scholar]
- 12.Orozco-Cardenas M, Narvaez-Vasquez J, Ryan C A. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- 13.Bergey D, Howe G, Ryan C A. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stintzi A, Weber H, Reymond P, Browse J, Farmer E E. Proc Natl Acad Sci USA. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Li C, Lee G I, Howe G A. Proc Natl Acad Sci USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narvaez-Vasquez J, Orozco-Cardenas M L, Ryan C A. Plant Physiol. 1994;105:725–730. doi: 10.1104/pp.105.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGurl B, Pearce G, Orozco-Cardenas M, Ryan C A. Science. 1992;255:1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 18.McGurl B, Orozco-Cardenas M, Pearce G, Ryan C A. Proc Natl Acad Sci USA. 1994;91:9799–9802. doi: 10.1073/pnas.91.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe G A, Ryan C A. Genetics. 1999;153:1411–1421. doi: 10.1093/genetics/153.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Howe G A. Plant Physiol. 2002;127:1414–1417. [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan, C. A., Pearce, G., Scheer, J. & Moura, D. S. (2002) Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- 22.Janzik I, Maceroux P, Amrhein N, Schaller A. J Biol Chem. 2000;275:5193–5199. doi: 10.1074/jbc.275.7.5193. [DOI] [PubMed] [Google Scholar]
- 23.Nelson C, Walker-Simmons M, Makus D, Zuroske G, Graham J, Ryan C A. In: Mechanisms of Plant Resistance to Insects. Hedin P, editor. Washington, DC: Am. Chem. Soc.; 1983. pp. 103–122. [Google Scholar]