Abstract
The role of Medical Affairs (MA) has shifted from traditional evidence dissemination to an integrated process leveraging real-world data (RWD) and diverse data sources. To adapt and lead this shift, AstraZeneca (AZ) Spain recognized the essential need to identify gaps in evidence-generation capabilities and establish targeted strategies aligned with both global and local priorities. The objective of this work was to assess AZ Spain’s current evidence-generation capabilities and identify areas for improvement by using the so-called “Evidence Blueprint” framework. To do this, we conducted a systematic self-assessment following the Blueprint to evaluate performance across ten core areas. An approach to identify gaps and enhance evidence-generation processes was undertaken in four phases: defining capabilities, assessing maturity, developing a roadmap for improvement, and implementing changes. The self-assessment identified five priority areas, including two focused priorities—Innovative Value Strategies/Payer Evidence and RWD Vision and Strategy—as well as three improvement areas—Evidence Planning and Value Team Implementation, Research/Evidence Partnerships, and Patient-Centric Evidence. Tangible actions included the development of processes to assess outcome-based agreements, comprehensive mapping of existing national and regional databases to strengthen RWD strategies, creating a cross-functional strategy for evidence planning, establishing research partnerships leveraging European funding, and adopting patient-centric methodologies such as ethnographic studies and patient-authored publications. The initiatives undertaken by AZ Spain demonstrate the transformative potential of an Evidence Blueprint framework in addressing gaps and enhancing evidence-generation capabilities. By aligning local strategies with AZ’s MA 2030 vision, these efforts ensure continuous innovation, improvement of decision-making, and a more substantial contribution to the healthcare ecosystem.
Key Points
| AstraZeneca (AZ) Spain used a comprehensive self-assessment (the “Evidence Blueprint”) to identify gaps in evidence generation and implement strategies for improvement, advancing its alignment with AZ's global Medical Affairs 2030 vision. |
| Tangible actions included payer-focused strategies, leveraging real-world data sources, fostering research partnerships, and incorporating patient-centric methodologies. |
| The initiatives enhanced AZ Spain's evidence generation, fostering robust decision-making, earlier alignment with brand strategies, and sustainable innovation in healthcare. |
Evidence Generation and the Blueprint Initiative
Historically, the activities of Medical Affairs (MA) departments within pharmaceutical companies revolved around generating and communicating externally scientific evidence from clinical trials. In contrast, internal activities were limited to dissemination and evidence-based strategic assessment for other company departments. However, this role has evolved significantly over the years, transitioning to a more dynamic and integrated evidence-generation process [1]. The modern landscape of evidence generation demands a holistic approach that incorporates insights from diverse sources, aiming to drive strategic decision-making and deliver measurable value to healthcare stakeholders. Today, evidence generation extends beyond traditional clinical trials' boundaries to include real-world data (RWD), observational studies, patient-reported outcome measures (PROMs), patient-reported experience measures (PREMs), healthcare system analytics, and even novel data streams from emerging technologies.
At its core, evidence generation seeks a robust and comprehensive understanding of various medical aspects in real-life settings, such as disease epidemiology, therapeutic management, healthcare resource utilization, and patient quality of life. In today’s complex healthcare ecosystem, the evidence generated is essential to gain in-depth knowledge of pathologies, the use of medicines by healthcare professionals (HCPs), and the value they provide [2]. Adequate communication of these results is crucial, so that the evidence generated can inform the decisions of patients, prescribers, payers, and regulators. Agile and systematic evidence-generation processes are critical to meeting market demands. As defined by Cadogan et al. in 2023 [3], the concept of insights—the "why" behind observed trends—is central to evidence generation. Insights, especially those arising from real-world evidence (RWE), provide actionable intelligence supporting tactical and strategic decision-making.
The Evidence Blueprint initiative, developed by Larsen et al. [4], is a comprehensive framework for enhancing evidence-generation capabilities. This modular framework emphasizes the "glocal" approach—linking global strategies with local expertise—and focuses on ten core areas of evidence generation, including evidence planning and value team implementation; RWD vision and strategy; stakeholder-specific evidence generation; and patient-centric evidence strategies. This initiative aligns with the broader transformation in evidence generation advocated by European regulatory agencies, including the European Medicines Agency (EMA), as outlined in their Clinical Evidence 2030 framework [5], which defines key principles to ensure that evidence generation evolves in a way that supports regulatory, clinical, and payer decision-making by 2030. The EMA framework emphasizes patient-centric approaches, leveraging existing knowledge, defining clear research questions, embracing diverse data sources, fostering early collaboration, and ensuring transparency.
By addressing gaps in local capabilities and providing targeted roadmaps for improvement, the Evidence Blueprint empowers AstraZeneca (AZ) local affiliates to establish strategic evidence plans, enhance RWD utilization, and embed patient-centered science into their workflows. Ultimately, it is a benchmark for assessing and enhancing evidence-generation capabilities, enabling local affiliates to align with broader organizational goals while addressing specific regional challenges. This approach underpins AZ’s commitment to delivering innovative, patient-centered solutions and strengthening collaborations within the healthcare ecosystem.
AZ Spain’s evidence-generation approach embraces this holistic perspective, integrating scientific data, field intelligence, and stakeholder input. Evidence Generation is a division within AZ’s MA department, consisting in a specialized group of experts in areas such as epidemiology and methodology, who are dedicated exclusively to evidence-generation projects. While our teams operate independently, they are highly aligned with the broader MA strategy. Our sources of RWE extend beyond data generated in clinical trials and observational studies focused on disease epidemiology, patient management, and clinical outcomes, to incorporate diverse and complementary information streams. These include (1) analyses of care models, such as patient journeys, healthcare resource use, and associated costs; (2) process engineering methodologies; (3) insights into patient and HCP experiences, including PROMs and PREMs and ethnographic studies; (4) payer-specific data, such as pharmacoeconomic analyses and information generated in the context of outcome-based agreements (OBAs); (5) advanced technologies generating data through devices and patient apps; and (6) structured insights from stakeholder interactions.
By leveraging these diverse data sources, AZ Spain seeks to establish itself as a leader in evidence generation, driving innovation and fostering meaningful collaborations that ultimately benefit patients and healthcare systems. This paper describes AZ Spain's internal self-assessment and subsequent strategy to enhance its evidence-generation capabilities, aligning with the CARABELA initiative [6] and the broader strategic vision of transforming AZ Spain’s MA department under the MA 2030 vision [7]. Through these efforts, we aim to transform how diseases are managed and healthcare decisions are made.
Internal Self-Assessment and Action Plan
In 2022, Global AZ started an initiative to assess the performance of all their affiliates with respect to the Evidence Blueprint, aiming to identify areas for improvement. A cross-functional team in AZ Spain, including members of Evidence Generation, MA central teams, in-field MA, Market Access, Innovation and Digital Strategy, Marketing, Innovative Pricing, and Health Economics and Outcomes Research (HEOR), was set to conduct a comprehensive internal self-assessment to evaluate its performance across the ten Evidence Blueprint's core capabilities (Table 1). This process involved a systematic evaluation to identify strengths, gaps, and areas for development within each core area.
Table 1.
The Blueprint 10 core areas of evidence generation
| # | Core area | Description |
|---|---|---|
| 1 | Evidence planning and Value Team implementation | Implementation of value-focused evidence-planning teams to align local and global strategies |
| 2 | RWD vision and strategy | Development of a vision and actionable strategy for RWD use and integration |
| 3 | Research/evidence partnerships | Building partnerships with research organizations to enhance evidence generation efforts |
| 4 | Data and evidence communication/publications and RWE roundtables | Facilitating communication of evidence through publications and roundtables to key stakeholders |
| 5 | IVS/payer evidence | Creating IVS and evidence to support payer and reimbursement decisions |
| 6 | Epidemiology/data science/stats/digital health | Using epidemiology, data science, and digital tools to advance evidence generation |
| 7 | Study delivery/local tools | Enhancing local study delivery capabilities with tailored tools and resources |
| 8 | PROM/patient-centric evidence | Focusing on PROMs and patient-centric measures to enrich evidence |
| 9 | Evidence IT systems | Developing robust IT systems to support evidence management and analysis |
| 10 | Internal training platform | Providing comprehensive internal training platforms to build evidence-generation skills |
PROM patient-reported outcome measure, IT information technology, IVS Innovative Value Strategies, RWD real-world data, RWE real-world evidence
AZ Spain adopted the multi-step approach outlined in the Evidence Blueprint initiative [4] to address the gaps and priorities identified in the internal self-assessment. This structured strategy was designed to guide affiliates through capability enhancement and ensure alignment with local and global objectives. The Evidence Blueprint framework allowed each participating country to determine how best to implement the necessary changes to improve evidence-generation capabilities. In the case of AZ Spain, we opted for a model based on annual qualitative monitoring of the agreed initiatives and activities. This model required minimal formal governance and process formalization to keep operations simple. The work was undertaken by the existing team, with no additional budget allocated beyond the regular funding for ongoing tasks. The core changes were implemented in four consecutive key phases: defining capabilities, assessing maturity, developing a roadmap for improvement, and implementing changes.
Phase 1: Defining Capabilities
In the first phase, the focus on defining capabilities involved identifying and describing best-in-class evidence-generation capabilities, defining critical success factors, and recognizing potential roadblocks. This stage was instrumental in fostering alignment, engagement, and a shared commitment among team members, ensuring a clear understanding of the importance of implementing the necessary changes.
Phase 2: Assessing Maturity
The second phase centered on assessing maturity, where a comprehensive evaluation of internal capabilities was carried out. This included defining the current level of ambition and capability locally, assessing gaps, and prioritizing areas that required immediate attention. For each of the ten evidence-generation capabilities evaluated, the assessment utilized a modular framework to score maturity levels using a ranking of four competency descriptors: emerging, evolved, advanced, and leading. Each country’s affiliate evaluated its maturity position focusing on five aspects: (1) processes, (2) tools and documents, (3) people/role descriptions, (4) key performance indicators (KPIs), and (5) stakeholders and communications. This benchmarking process allowed us a comprehensive understanding of current capabilities and their alignment with strategic aspirations.
Phase 3: Developing a Roadmap
The third phase involved developing a roadmap tailored to local needs. A Change Lead spearheaded this process, ensuring the roadmap incorporated local strategies and workload considerations. Transformational activities were defined to address capability gaps, timelines were established for implementing these changes, and a business case for implementation was developed to secure organizational support.
Phase 4: Implementing Changes
The final phase, implementing changes, required assigning specific tasks to local teams, securing the necessary resources, and establishing mechanisms for monitoring progress. Regular progress reviews and consultations with the Change Lead were implemented to identify and address roadblocks. Input from global subject matter experts was sought as needed, ensuring that local efforts aligned with global best practices.
AZ Spain’s adaptation of this approach emphasized the importance of flexibility and customization to address the unique needs of the local healthcare ecosystem. Assigning dedicated Change Leads ensured accountability and focus, while continuous progress monitoring provided opportunities to refine strategies and improve outcomes. By adhering to the Evidence Blueprint’s methodology, AZ Spain demonstrated its commitment to enhancing evidence-generation capabilities and driving impactful changes in the healthcare environment.
Gaps and Priorities Identified in the Self-Assessment
Our internal self-assessment identified five areas for growth, categorized into two focused priority areas and three additional modules requiring improvement. The two primary priorities consisted in the development of Innovative Value Strategies (IVS)/Payer Evidence (area 5) and the refinement of an actionable plan regarding RWD Vision and Strategy (area 2). These areas were selected for their critical importance in supporting payer decision-making, fostering value-based healthcare collaborations, and establishing the frameworks necessary for evidence-based decision-making within the local context.
In addition to these focused priorities, the assessment highlighted three key areas for further development. The first was Evidence Planning and Value Team Implementation (area 1), to align cross-functional evidence-planning processes more effectively with strategic objectives. The second was Research/Evidence Partnerships (area 3), recognized for their potential to foster external collaborations and drive innovative evidence-generation projects. Finally, PROM/Patient-Centric Evidence (area 8) emerged as a priority due to its critical role in enriching evidence through direct patient insights and fostering patient-centered initiatives.
These five areas were prioritized based on their alignment with AZ Spain’s strategic goals and their relevance to the evolving needs of the local healthcare ecosystem. Collectively, they represent significant opportunities to enhance AZ Spain’s evidence-generation capacity, strengthen external partnerships, and effectively address unmet healthcare challenges.
Focused Priority Areas
IVS/Payer Evidence (Area/Module 5)
Reimbursement negotiations with payers are evolving towards an increased emphasis on agreements based on product value. This shift underscores the need for internal cross-functional structures and processes capable of assessing whether OBAs will be required early in the product lifecycle. Furthermore, these structures are tasked with identifying evidence gaps that must be addressed to effectively define success criteria for OBA constructs.
The action planning for this module was designed to ensure that payer evidence generation is a systematic, strategic, and scalable process. The capability gap development in this area followed a structured timeline of short-term (6–12 months), mid-term (12–24 months), and long-term (24+ months) objectives, with a clear focus on defining systematic processes, enhancing collaboration, and leveraging data-driven insights (Table 2). The plan aimed to proactively assess OBA needs, strengthen engagement with global reimbursement teams and regulatory bodies, and integrate technological frameworks in the hospital systems to support decision-making. By implementing these strategies, AZ Spain sought to align its evidence-generation efforts with the evolving demands of payers, ensuring robust preparation for OBAs and strengthening its position in value-based healthcare negotiations.
Table 2.
IVS/payer evidence action planning
| Phase | Outcome | Activity | Critical success factors |
|---|---|---|---|
| Short term (6–12 months) |
Systematic process to assess OBA needs for each product established Gaps anticipated earlier to ensure timely data availability |
Proactively identify payer evidence data requirements Strengthen the network of evidence-collecting institutions/registries Leverage insights from other countries |
Build on existing registry work Leverage ATLAS as a platform for payer discussions Establish relationships with registry owners Foster an internal mindset supporting IVS |
| Midterm (12–24 months) |
Market fit assessed with global reimbursement teams, through early engagement. Value teams standardized across brands. Simplified understanding of OBAs across teams |
Maintain/increase communication with global teams regarding reimbursement dossiers and OBA needs Develop a simple OBA assessment method and provide training |
Leverage and adapt outcome-based models from market access global teams |
| Long term (24 + months) | Technology frameworks integrated in hospital systems that could be used for OBAs assessed |
Discuss the need for outcome-based IVS for all new products Explore collaborations with industry associations (FarmaIndustria) and other pharmaceutical companies |
Leverage partnerships with industry associations and pharma companies to drive national-level changes |
OBA outcome-based agreement, IVS Innovative Value Strategies
To address this challenge, AZ Spain undertook several tangible actions. A local procedure was developed that included working processes and established a cross-functional team to evaluate OBA needs early in the planning cycle and, where needed, to generate the necessary evidence and define its use in OBAs. The procedure was intended to ensure the timely generation of required evidence and to define the strategy to develop OBAs, taking into account interactions with key stakeholders, including global teams, regulators, and hospitals. Another tangible action was the creation of payer-focused evidence strategies using local case studies to contextualize and tailor the approach to local market needs. Furthermore, the ATLAS platform, a recently in production proprietary tool containing public and private local RWE data on disease state, treatment patterns, and health system burden, is expected to play a critical role in supporting payer evidence discussions in the future.
RWD Vision and Strategy (Area/Module 2)
AZ Spain identified critical needs and opportunities for improving its RWD vision and strategy. The current landscape of data sources in Spain is characterized by significant regional variability in data availability, quality, and industry access. Addressing this fragmentation was essential to refining and expanding existing evidence-based strategies and leveraging RWD to support decision-making across the product lifecycle. In line with the Clinical Evidence 2030 framework proposed by the EMA [5], AZ Spain's strategy emphasizes the need to enhance RWD integration, ensuring its evidentiary value across different research questions.
To this end, AZ Spain supported the establishment of a “FarmaIndustria” Working Group in 2023, dedicated to advancing RWE strategies. Efforts were also directed towards enhancing knowledge of existing data sources, such as national public databases (e.g., CMBD/Ministerio de Sanidad [8], utilized for the ATLAS platform) and regional sources (e.g., Andalusia, Valencia), to maximize their potential use. Furthermore, RWE initiatives were aligned with our ongoing transforming care efforts to identify opportunities for practice change, including perception research and other potential RWE-linked projects [7, 9]. AZ also began integrating sustainability outcomes into its RWE initiatives, reflecting their growing importance to decision-makers. Additionally, partnerships with innovative organizations such as technological companies and startups resulted in projects exploring advancements in artificial intelligence (AI), machine learning (ML), and natural language processing (NLP), such as Diabetic@ [10] and FAITHFUL [11].
The action planning for this module was designed to enhance RWD accessibility, integration, and strategic application over short-term (6–12 months), mid-term (12–24 months), and long-term (24+ months) phases. The plan prioritized early access to local RWD, the development of federated data models, and strengthening governance around data quality and privacy (Table 3). Over time, efforts were focused on advancing AI-driven analytics, improving collaboration with regulatory bodies, and fostering partnerships for government-to-government data discussions. Through this structured approach, AZ Spain aimed to overcome regional disparities, optimize the use of RWE, and support impactful healthcare decisions that drive meaningful practice change across the product lifecycle.
Table 3.
RWD Vision and Strategy action planning
| Phase | Outcome | Activity | Critical success factors |
|---|---|---|---|
| Short term (6–12 months) |
ATLAS usage Access to local RWD improved Earlier anticipation of data needs to ensure timely availability Methods to analyze impact of transforming care initiatives defined |
Update and expand data inventory via ATLAS Explore engagement opportunities with IT decision-makers and registries Measure the impact of solutions, considering both clinical and business KPIs |
Leverage learnings from other affiliates and ensure cross-functional partnership to support ATLAS implementation and development Identify the appropriate governance route for patient-centric data collection/publication Address data quality/privacy hurdles |
| Midterm (12–24 months) |
Linkage among different data sources improved Federated models for patient matching deployed in hospitals |
Work with hospitals and data source owners to improve data usability for research purposes Leverage surveys to generate patient management data |
Collaborate with Global to share best practices |
| Long term (24 + months) |
Global and internal capabilities in data analytics/ML/AI enhanced More vigorous data advocacy with external experts |
Continue exploring partnerships with private data firms (AI/NLP) to create detailed datasets Explore opportunities for government-level discussions on RWD |
Leverage learnings from other affiliates to strengthen advocacy efforts |
AI artificial intelligence, IT information technology, KPI key performance indicator, MC medical center, ML machine learning, NLP natural language processing, RWD real-world data
Modules for Improvement
Evidence Planning and Value Team Implementation (Area/Module 1)
AZ Spain identified the need for earlier alignment of evidence plans with brand strategies to enhance integration and improve decision-making throughout the product lifecycle. This need was addressed in the context of a broader initiative simultaneously taking place in the company, aimed at accelerating AZ Spain’s transformation. This initiative used a combination of external and internal assessments, including aspects related to evidence generation [9].
The external assessment, conducted to identify market trends and stakeholder needs, emphasized the increasing complexity of market access due to tightening budgets and a shift towards consultative selling over traditional commercial approaches. Stakeholders now expect value-added services beyond the pharmaceutical product and are often willing to only pay for value, which may require generating evidence to show that the drug provides the expected benefits (outcome-based funding models). This underscores the need for more comprehensive evidence strategies.
The inside assessment identified opportunities for improvement in key organizational areas, highlighting the potential for greater systematization and end-to-end traceability in core processes related to launching and marketing new drugs. It also revealed areas where stronger collaboration between national and regional levels could enhance operational efficiency, ensuring more seamless execution of core processes and optimized resource allocation. Additionally, the engagement model presented an opportunity to become more integrated and responsive to stakeholder needs, fostering a holistic customer view and shared organizational objectives. Feedback from internal surveys of Brand Teams further underscored the value of refining team structures and work processes to strengthen alignment between central teams and in-field roles. Enhancing planning, coordination, and execution was recognized as a key step toward achieving greater synergy in evidence-planning efforts, ultimately driving more effective and cohesive strategies.
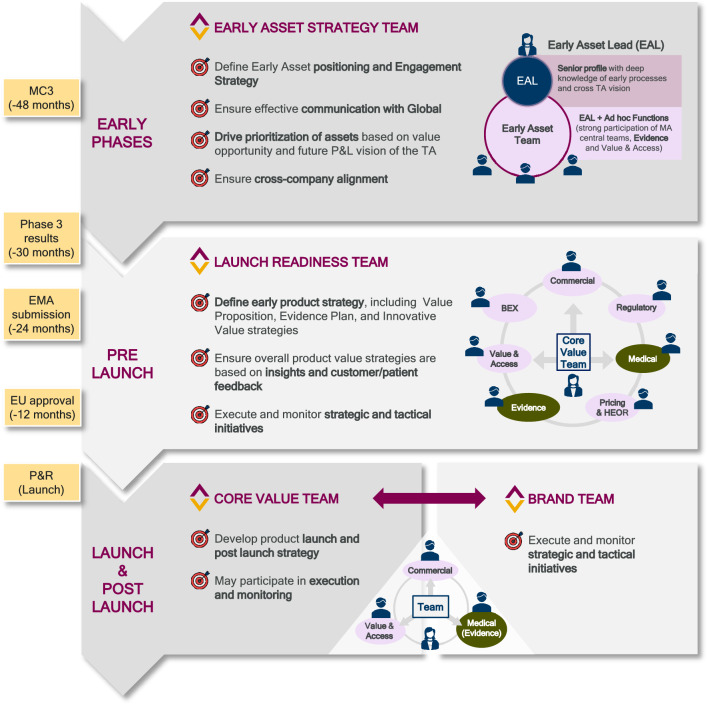
Tangible actions were undertaken to address these needs, including the systematization and standardization of cross-functional teams along the product lifecycle (Fig. 1). These changes in the way AZ Spain MA operates within the Spanish context ensured evidence generation became a key contributor across all model phases, starting as early as 12–18 months before phase 3 readouts. These teams included the Early Asset Strategy Team, the Launch Readiness Team, the Core Value Team, and the aforementioned Brand Team, each designed to address specific needs across the product lifecycle. The Early Asset Strategy Team focuses on early positioning and engagement strategies to ensure an optimal time to market. The Launch Readiness Team ensures early positioning of the assets by aligning product value strategies with customer feedback during the pre-launch phase. Finally, the Brand Team and Core Value Team prepare the ground for a successful launch and executes strategic and tactical initiatives post-launch.
Fig. 1.
Implication of MA and evidence generation in cross-functional teams during the product’s development lifecycle in Spain. BEX Business Excellence, EAL Early Asset Lead, EMA European Medicines Agency, EU European Union, HEOR Health Economics and Outcomes Research, MA Medical Affairs, MC3 Marketing Company Consultation and Communication, P&L Profit and Loss, P&R Pricing and Reimbursement, TA therapeutic area
Product lifecycle teams are aligned with all stages of product development, ensuring that each phase has a dedicated expert team for oversight. Strategic tactical planning is developed to define comprehensive plans for each team throughout the product’s lifecycle, following a pre-established work methodology. Operational execution and monitoring forums are held periodically to ensure that teams’ activities align with strategic goals and continuously track the progress to adapt to changing healthcare ecosystem conditions and stakeholder needs.
All these efforts enhanced the anticipation and preparation of information and dossiers required to adapt and fully comply with the latest national and European regulatory changes in scientific consultations on medicinal products and technologies. The new European regulation European Union (EU) Regulation 2021/2282 on Health Technology Assessment [12] establishes a structured framework for joint clinical assessments at the EU level, emphasizing early and continuous dialogue between regulatory agencies and industry stakeholders. This regulation demands a higher degree of alignment in evidence-generation strategies across multiple markets, requiring pharmaceutical companies to adopt a proactive and standardized approach to data collection and submission. Additionally, it reinforces the need for robust RWE to support value-based assessments and market access decisions.
At the national level, Spain is advancing new legislation on Health Technology Assessment and evidence-based decision-making [13], which will increase the focus on regional and national scientific consultations. The draft regulation introduces a more structured and transparent evaluation process, requiring pharmaceutical companies to engage earlier with regulatory authorities and demonstrate the clinical and economic impact of their therapies more rigorously. This shift underscores the growing importance of integrating health economic evaluations and RWE into the regulatory and reimbursement landscape.
By refining its evidence planning model, AZ Spain has positioned itself ahead of these regulatory changes, ensuring that data generation efforts are aligned with both national and European requirements. The systematization of cross-functional teams, the development of structured evidence-planning tools such as "one-pagers," and the integration of brand strategies with early evidence generation, have strengthened the company’s ability to meet evolving compliance expectations. These efforts not only enhance regulatory preparedness but also improve strategic engagement with health authorities, reinforcing AZ Spain’s role in shaping the future of evidence-based healthcare decision-making.
Research/Evidence Partnerships (Area/Module 3)
We identified the opportunity to expand our collaborative efforts beyond traditional database owners and study-specific site engagements. By broadening the scope of partnerships, AZ Spain sought to establish long-term collaborations that could generate impactful RWE. Recognizing the potential of such partnerships, we implemented a series of tangible actions to address these needs.
An Innovation Hub was established to foster collaboration between internal departments and external institutions. This initiative aimed to integrate diverse expertise and promote innovative research endeavors. Strategic partnerships were also forged with key research institutions and consortia by leveraging European funding opportunities. Notable collaborations include the following: the FAITHFUL project [11], which focuses on AI solutions for early heart failure detection, supported by EIT Health (2023-SUD-3860); the PRECISEU initiative [14], which seeks to advance personalized medicine by connecting innovation ecosystems across Europe, funded under the HORIZON-EIE-2023-CONNECT-03-01 program; and the UMBRELLA project [15], which aims to create a comprehensive framework for holistic and patient-centric stroke management, supported by the Innovative Health Initiative Joint Undertaking (grant agreement No. 101172825).
These strategic initiatives have enabled AZ Spain to engage in cutting-edge research, enhance its evidence-generation capabilities, and contribute meaningfully to the broader scientific community.
PROM/Patient-Centric Evidence (Area/Module 8)
We recognized the need to increase the integration of patient-centric methodologies into our evidence-generation processes, moving beyond reliance on quality-of-life questionnaires. Several key initiatives were implemented to address this need and ensure that patient perspectives were central to evidence development.
Ethnographic studies using semi-structured interviews were introduced to capture deeper insights into patient experiences. Collaborations with patient associations were established to co-develop evidence frameworks and lead large-scale macro-surveys, ensuring that patient voices directly informed research initiatives. Furthermore, AZ Spain emphasized the creation of plain-language summaries and patient-authored publications to enhance accessibility and engagement. For example, publications by Rubio et al. [16] and Galindo Izquierdo et al. [17], which involved patients and patient associations in their preparation and authorship, have provided critical insights into patient experiences in heart failure and lupus, respectively, and have demonstrated the organization’s commitment to this approach.
These efforts represent a significant step forward in embedding patient-centric methodologies into AZ Spain's evidence-generation strategy, ensuring that the outcomes are directly aligned with patients' needs and priorities.
Future Reflections and Implications for Medical Affairs
The findings of this self-assessment highlight the transformative impact of the Evidence Blueprint initiative on AZ Spain’s approach to evidence generation [4]. By systematically identifying gaps and prioritizing key areas for development, we have successfully implemented targeted action plans that align with local and global objectives. The emphasis on patient-centered research, strategic use of RWD, and cross-stakeholder collaboration closely aligns with the Blueprint initiative’s core areas, reinforcing the importance of structured, forward-looking evidence-generation strategies. Furthermore, this approach resonates with the vision outlined in the Clinical Evidence 2030 framework [5]. The integration of innovative methodologies, strategic partnerships, and patient-centric evidence approaches demonstrates AZ Spain’s commitment to transform MA and drive impactful changes in the healthcare ecosystem.
The strategies implemented by AZ Spain address critical gaps in evidence generation, including earlier alignment of evidence plans with brand strategies, the expansion of research partnerships, and the integration of patient-centric methodologies. These initiatives strengthen our position within the healthcare ecosystem and contribute to AZ’s global leadership in MA. By aligning with the MA 2030 vision [7, 18, 19], AZ Spain is pioneering approaches that will shape the future of evidence generation. The focus on RWE, patient engagement, and innovative partnerships positions the organization to respond effectively to evolving market and stakeholder needs. This study also highlights the importance of fostering a culture of innovation and adaptability within the healthcare system [20]. Future challenges, such as increased demand for sustainability-focused evidence and stricter data privacy regulations, can be mitigated through continuous MA evolution, enhanced collaborations, and leveraging global expertise. The success of the Evidence Blueprint initiative serves as a call to action for further cooperation across affiliates and continuous refinement of evidence-generation practices.
Conclusion
The tangible action plans implemented by AZ Spain represent a significant step forward in aligning evidence-generation strategies with the organization’s overarching goals. By addressing gaps, prioritizing key areas, and leveraging both local and global resources, we have set a new benchmark for evidence generation within MA. This paper provides the foundation for future evaluations of the outcomes and impacts of these strategies, which will be detailed in our subsequent publication. These insights will further demonstrate the value of the Evidence Blueprint initiative and its role in transforming evidence generation to benefit patients, stakeholders, and the healthcare ecosystem as a whole.
Acknowledgements
The authors thank Ánchel González Barriga and Blanca Piedrafita from Medical Science Consulting (Spain) for providing editorial support in the form of medical writing and assembling tables/figures based on the authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing.
Declarations
Funding
This work was funded by AstraZeneca Spain.
Conflict of interest
All authors declare being employed by AstraZeneca.
Availability of data and material
All data generated during this work are included in this published article.
Author contributions
All authors contributed to the conception and design of the project, as well as the investigation and analysis performed. APD wrote the first draft of the manuscript. All authors critically reviewed, edited, and commented on the document until the final version was obtained. All authors read and approved the final version of the article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.McLoughlin M, Jonkman M, Zivkov M. Moving to an integrated, holistic approach to insights-related Activities in Medical Affairs. A MAPS white paper. Medical Affairs Professional Society [Internet]. 2021. https://medicalaffairs.org/wp-content/uploads/2022/05/Holistic-Integrated-Insights.pdf. Accesed 28 March 2025.
- 2.Dagenais S, Russo L, Madsen A, Webster J, Becnel L. Use of real-world evidence to drive drug development strategy and inform clinical trial design. Clin Pharmacol Ther. 2022;111(1):77–89. 10.1002/cpt.2480. (Epub 20211128). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadogan AA, Lau J, Wnorowski S, Kelsch GR, Oreper J, Chavez L, et al. Defining insights. Ther Innov Regul Sci. 2023;57(6):1229–37. 10.1007/s43441-023-00554-w. (Epub 20230705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen K, Walton RN, Elsayed M, Ipatov A, Townsend-Holyoake F, Axelsson SFA, et al. A blueprint for success in real-world evidence: “glocal” approach to building capabilities and generating impactful evidence. Front Pharmacol. 2023;14:1233617. 10.3389/fphar.2023.1233617. (Epub 20231011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arlett P, Umuhire D, Verpillat P, Foggi P, Wändel Liminga U, Sepodes B, et al. Clinical Evidence 2030. Clin Pharmacol Ther. 2025. 10.1002/cpt.3596. (Epub 20250214). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escalada J, Carretero Gomez J, Anguita M, de Sequera P, Garcia-Rio F, Davila I, et al. Enhancing the management of chronic diseases in clinical practice: the CARABELA methodology. J Healthc Qual Res. 2024;39(5):336–9. 10.1016/j.jhqr.2024.06.001. (Epub 20240622). [DOI] [PubMed] [Google Scholar]
- 7.Regadera Anechina L, Iglesias I, Marinich JA, Diago J, Pérez Domínguez A. Pioneering the future of Medical Affairs: a strategic transformation to meet the healthcare ecosystem evolving trends. Pharm Med. 2025 (under review). [DOI] [PMC free article] [PubMed]
- 8.Ministerio de Sanidad. Consulta Interactiva del Sistema Nacional de Salud. Área de Inteligencia de Gestión. Portal Estadístico [Internet]. https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S. Accessed 11 Mar 2025.
- 9.Iglesias I, Marinich JA, Anechina LR, Cortes M, Perez Dominguez A. Understanding the National Healthcare Ecosystem to position medical affairs as a strategic element: lessons learned from AstraZeneca Spain. Pharmaceut Med. 2024. 10.1007/s40290-024-00542-x. (Epub 20241223). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merino JF, Blanco-Carrasco AJ, Rubio Almanza M, Cánovas Molina G, Brito Sanfiel MA, Merino JF, et al. Characterizing the clinical profile and prevalence of people with diabetes attended in the hospital setting by using unstructured healthcare data and natural language processing: the Diabetic@ study. Diabetes Res Clin Pract. 2025. May 2:112214. 10.1016/j.diabres.2025.112214. (online aheadead of print) . [DOI] [PubMed]
- 11.FAITHFUL. Future of Artificial intelligence trained for Heart Failure detection and upgrading latest technology. EIT health 2023-SUD-3860 [Internet]. 2023. https://eithealth.eu/product-service/faithful/. Accessed 19 Feb 2025.
- 12.THE EUROPEAN COMMISSION. Commission Implementing Regulation (EU) 2024/3169 of 18 December 2024 laying down rules for the application of Regulation (EU) 2021/2282 of the European Parliament and of the Council with regard to the procedures for joint scientific consultations on medicinal products for human use at Union level. [Internet]. 2024. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202403169. Accessed 19 Feb 2025.
- 13.Ministerio de Sanidad. Proyecto del Real Decreto por el que se regula la evaluación de tecnologías sanitarias. [Internet]. https://www.sanidad.gob.es/normativa/audiencia/docs/DG_54_24_Solicitud_informacion_publica_RD_EVALUACION_TECNOLOGIAS_SANITARIAS.pdf. Accessed 19 Feb 2025.
- 14.PRECISEU. PeRsonalised medicine Empowerment Connecting Innovation ecoSystems across EUrope. [Internet]. https://cordis.europa.eu/project/id/101161301. Accessed 21 Jan 2025.
- 15.UMBRELLA. Unleashing a comprehensive, holistic and patient centric stroke management for a better, rapid, advanced and personalised stroke diagnosis, treatment and outcome prediction. [Internet]. https://www.ihi.europa.eu/projects-results/project-factsheets/umbrella. Accessed 21 Jan 2025.
- 16.Rubio R, Palacios B, Varela L, Fernández R, Camargo Correa S, Estupiñan MF, et al. Quality of life and disease experience in patients with heart failure with reduced ejection fraction in Spain: a mixed-methods study. BMJ Open. 2021;11(12):e053216. 10.1136/bmjopen-2021-053216. (Epub 20211203). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galindo Izquierdo M, Borrás Blasco J, Pérez Ortega S, Salman-Monte TC, Vela-Casasempere P, Rodríguez Almaraz E, et al. Lack of awareness of systemic lupus erythematosus and its consequences in a cohort of moderate and severe patients in Spain: the LupusVoice study. Lupus. 2024;33(7):663–74. 10.1177/09612033241242886. (Epub 20240405). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Algazy J, Garcia A, Ryan S, Westra A, Zemp A. A vision for medical affairs 2030: Five priorities for patient impact. McKinsey & Company [Internet]. 2023. https://www.mckinsey.com/industries/life-sciences/our-insights/a-vision-for-medical-affairs-2030-five-priorities-for-patient-impact#/. Accessed 13 Feb 2025.
- 19.MAPS Visionary Working Group. The Future of Medical Affairs 2030. Medical Affairs Professional Society [Internet]. 2022. https://medicalaffairs.org/futuremedical-affairs-2030/. Accessed 16 Oct 2024.
- 20.Yisa MI. Fostering a culture of innovation in healthcare through advanced technology and ongoing training for professionals. Int J Sci Res Arch. 2024;13(02):3698–714. 10.30574/ijsra.2024.13.2.2638. [Google Scholar]