Correction: Pharmacological Reports
10.1007/s43440-025-00739-0
In Table 1 of this article, images in the second column "Structure" were incorrectly arranged.
Table 1.
A summary of the main properties of key proteasome inhibitors based on their chemical structure and mechanism of action.
| Proteasome inhibitor | Structure | Chemical origin | Inhibition | Targeted protea-some subunit | Administration | Class | Half-life/ applied dosage | References |
|---|---|---|---|---|---|---|---|---|
| Bortezomib (PS-341) |

|
Boronate | Reversible | CT-L | Intravenous, Subcutaneous | I | 110 min (1 µmol/L for 30 min) up to 6.8 h (1 mg/m2, first day)-32.5 h (1 mg/m2, eleventh day)* | [18, 27, 52] |
| Ixazomib (MLN9780/ MLN2238) |
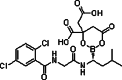
|
Boronate | Reversible | CT-L | Intravenous, Oral | I |
18 min (1 µmol/L for 30 min) up to 3.6–11.3 days (once weekly 0.8–3.95 mg/m2)* |
[27, 53, 54] |
| Delanzomib (CEP-18770) |

|
Boronate | Reversible | CT-L | Intravenous, Subcutaneous, Oral | I | 62 h (0.1–1.8 mg/m2) | [28, 30, 55] |
| Carfilzomib (PR-171) |

|
Epoxy-ketone | Irreversible | CT-L | Intravenous | II | < 60 min (2–8 mg/kg) | [29, 39, 56] |
| Oprozomib (ONX0912/ PR-047) |

|
Epoxyk-etone | Irreversible | CT-L | Intravenous, Oral | II | < 90 min (30 mg/kg) | [29, 39, 57] |
| Marizomib (salinosporamide A/ NPI-0052) |
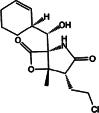
|
β-lactone | Irreversible |
CT-L, T-L, C-L |
Intravenous, Oral | II | < 30 min (0.3–0.8 mg/m2) | [50] |
*The reported values of the half-life vary dependently on the administrated dose, therapy regimen, duration of treatment and the day of sample collection
The original article has been corrected.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.


