Abstract
The role of medical affairs (MA) in the pharmaceutical industry is undergoing a profound transformation, driven by the increasing complexity of healthcare ecosystems, the rise of digital technologies, and the need for enhanced stakeholder engagement. In alignment with the MA 2030 vision, AstraZeneca Spain has implemented a forward-thinking transformation strategy, positioning MA as a strategic healthcare partner through a structured, data-driven, and patient-centered approach. Our transformation journey has been anchored in five strategic priorities: boosting MA leadership, integrating end-to-end data and analytics, refocusing resources using data-backed strategies, aligning evidence generation with stakeholder needs, and orchestrating omnichannel scientific engagement. To achieve this, we developed and implemented innovative methodologies such as regional archetyping, healthcare account characterization, and stakeholder mapping to systematically analyze and tailor engagement strategies across different healthcare settings. A core component of this strategy is the CARABELA initiative, a structured approach aimed at fostering the optimization clinical pathways, promoting public–private collaborations, and driving practice-changing interventions to improve healthcare efficiency. In addition, we have transitioned from descriptive to predictive analytics through advanced real-world evidence models, ensuring that MA-led initiatives remain proactive and impact-driven. This transformation serves as a scalable framework for MA evolution globally, reinforcing its role as a catalyst for healthcare innovation. By integrating real-world data, digital engagement, and strategic collaboration, MA departments should position themselves to navigate the evolving healthcare landscape while delivering tangible benefits for patients, healthcare professionals, and systems worldwide.
Key Points
| Our medical affairs (MA) department has undergone a comprehensive transformation, becoming a strategic partner within the healthcare ecosystem by driving innovations that enhance patient care and system efficiency. |
| This transformation is fully aligned with and advances beyond the MA 2030 vision, emphasizing leadership elevation and data-driven decision-making to optimize stakeholder engagement. |
| We pioneered spearheading initiatives, including CARABELA and the healthcare model analysis, which integrate real-world data, clinical insights, and cross-functional collaboration to perform practice-changing interventions. |
The Medical Affairs 2030 Vision
The healthcare industry is in a new era characterized by rapid innovations and changing paradigms, and medical affairs (MA) departments worldwide are at a crucial juncture [1]. Before the COVID-19 worldwide health crisis of 2020, MA departments were already encouraged to assume new roles in response to the impact of digital technologies and “big data” in the biological sciences and become a third pillar of pharmaceutical companies at the same level of research and development (R&D) and commercial and market access [2]. Then, the challenges of the COVID-19 pandemic accelerated and reshaped this process [3] since operational pressure and scrutiny, new stakeholder expectations, and the reconfiguration of healthcare systems further pushed the demands for MA transformation as a strategic partner with proactive roles within each national healthcare ecosystem [4–7].
In 2022, the MA Professional Society (MAPS) Visionary Working Group envisioned such transformation as a goal towards 2030 [8]. Recently, Algazy et al. published a roadmap to achieve this MA 2030 vision by establishing five priorities that MA leaders should follow [9]. These priorities, together with the MAPS Visionary Working Group goal towards 2030 [8], are depicted in Table 1. Inspired by this vision and driven by the goal of providing value to the healthcare ecosystem, our organization embraced the challenge of anticipating the MA 2030 vision to pioneer such transformation at record-breaking speed and going beyond the goals established by Algazy et al. [9].
Table 1.
The MA 2030 vision: inspirational framework to evolve MA departments
| Key objectives according to the MAPS Visionary Working Group [8] | |
| 1 | Affirm MA role as leader of the organization’s scientific narrative |
| 2 | Engagement of broader stakeholder groups |
| 3 | Model of long-term relationships and dialogue with external stakeholders |
| 4 | Collection and analysis of strategic insights |
| 5 | Evolve team capabilities |
| 6 | Define metrics and key performance indicators |
| The top 5 priorities according to Algazy et al. 2023 [9] | |
| 1 | Boost medical affairs leadership to achieve next-level patient impact |
| 2 | Integrate end-to-end data and analytics |
| 3 | Refocus resources according to data-backed strategies |
| 4 | Align evidence-generation with stakeholder needs |
| 5 | Orchestrate medical engagement across channels |
MA, medical affairs; MAPS, Medical Affairs Professional Society
This report summarizes the practical transformation of MA in AstraZeneca (AZ) Spain into a strategic partner to all healthcare stakeholders, aligned with global trends, but also deeply rooted in our local realities. We describe our journey to leverage the power of data and technology, to communicate effectively and efficiently with different audiences, to adapt quickly and continuously to changing situations, and to influence key decision-makers and opinion leaders in the healthcare ecosystem. In the following pages, we share the foundations of how we reached (and even expanded) the MA 2030 vision, something that may serve as guidance or inspiration to other organizations.
The Transformation of Our MA Department
The first steps towards the transformation required a careful analysis of our working scenario, which led to a detailed outside assessment and a deep study of our internal structures, methodologies, and resources. For this purpose, a cross-functional team was appointed to a transformation office that developed a research strategy to fully understand our reality and provide the foundations that would lead to orchestrating a short-, medium-, and long-term pathway for this transformational journey. The internal evaluation and external assessment of the Spanish healthcare ecosystem was systematic and has been reported in a previous publication [10]. In short, this program identified all current trends of the healthcare stakeholders in Spain—within patients, healthcare professionals (HCPs), and institutions—providing an information map and a summary of key insights about their needs and motivations. These data, together with our internal assessment and the analysis of macrotrends that impact the pharmaceutical industry (Fig. 1), led to the identification of opportunities for improvement and formed the basis for a strategic revision of the MA model in our organization (Fig. 2).
Fig. 1.
Macrotrends that impact the pharmaceutical industry
Fig. 2.
Opportunities for improvement identified in medical affairs
On the basis of our analysis, we defined a new mindset and structure for the MA function that was aligned with our vision and company values. We aim to put the patient at the center of everything we do, to provide innovative and value-added solutions to healthcare stakeholders through science and innovation, to foster a lifelong learning culture, to collaborate internally and externally with trust and transparency, and to be accountable for our results and impact. We structured our mindset and pillars in work streams that cover the five priorities delineated by Algazy et al. [9] for MA transformation (Table 1): (1) boost medical affairs leadership to achieve next-level patient impact, (2) integrate end-to-end data and analytics, (3) refocus resources according to data-backed strategies, (4) align evidence-generation with stakeholder needs, and (5) orchestrate medical engagement across different channels.
Boost Medical Affairs Leadership to Achieve Next-Level Patient Impact
This priority of the MA 2030 vision entails elevating the role of MA to a strategic leadership position within the company, ensuring it drives decision-making and innovation that directly impacts patient outcomes [8, 9]. The boost of our MA department leadership to meet real-world patient trends is required by involving MA central teams and in-field medical staff in earlier preparatory activities for clinical development and product launches. In-field medical engagement is a core activity of the MA department that demands an upgrade from the traditional roles of the medical science liaison (MSL) [7, 11–13]. Therefore, we developed a new profile in our company: the strategic scientific advisor (SSA).
While the role of MA central teams will be detailed in an upcoming publication, the responsibilities of the SSAs were described in a recent article [14]. The SSA transcends the traditional MSL definition to become a trusted partner and advisor for all external stakeholders, who can provide strategic insights, tailored solutions, and value-added services that address their needs and expectations. The SSA is also a key source of medical intelligence and feedback for the internal teams, who can inform and influence medical strategy and tactics.
To achieve next-level patient impact, we developed the CARABELA initiative, which is a structured approach aimed at driving optimization of the clinical pathways through the patient’s journey. By focusing on real-world healthcare challenges in each therapeutic area (TA), CARABELA enables the development of sustainable, impactful solutions that improve healthcare efficiency and patient outcomes. SSAs and MA central teams are key in leading these initiatives within their TAs.
Key Points of the Strategy to Boost MA Leadership
A profound understanding of the healthcare ecosystem is crucial for MA to evolve and drive patient-centered outcomes. By engaging directly with HCPs and other stakeholders, MA departments can identify their needs and align their activities to improve clinical outcomes and transform healthcare. This strategic leadership extends beyond traditional medical functions, involving collaboration with both internal and external innovation teams to deliver solutions that address the dynamic needs of the healthcare ecosystem. The strategy unfolds in four key stages: early access, pre-launch, launch, and post-launch [14]. Throughout these stages, MA teams collaborate with external experts, policymakers, and patient associations to: (1) gain a comprehensive understanding of the healthcare landscape, map stakeholders, and identify their specific needs; (2) foster strong, collaborative partnerships across the ecosystem, from payers to healthcare providers; and (3) develop and implement innovative solutions that add value and go beyond traditional therapies. To implement this strategy, SSAs are equipped with essential skills, such as healthcare knowledge, communication techniques, medical leadership, scientific expertise, and digital technology mastery, enabling them to drive transformation in their TA [14].
In AZ Spain, this new concept of leadership was born from the implementation of a new operational model across the entire company, emphasizing a shift in mindset and leadership within each functional area. The approach combines horizontal or multidisciplinary coordination, where cross-functional teams work collaboratively and continuously, organized by work levels (national, regional, autonomous community, and hospital accounts). These teams are tasked with developing, implementing, and monitoring medical plans for each specific area. Simultaneously, the model includes vertical or functional coordination, where functional teams (e.g., access, MA, and commercial) contribute their specialized expertise to ensure strategic alignment and coherence across all levels of the operational model.
MA plays a pivotal role in these teams by identifying needs and co-creating solutions that deliver direct benefits to patients. Achieving this requires a comprehensive, 360-degree understanding of the healthcare ecosystem at all levels. To this end, the 360-degree vision was defined using six descriptors, collaboratively developed by the cross-functional teams of each health area: (1) regional archetyping and access, (2) general characterization of the health area/account, (3) research, development and innovation (I + D + I, for its Spanish acronym) evaluation, (4) clinical care model analysis, and (5) mapping and characterization of stakeholders.
Region Archetyping
This task characterizes each region on the basis of five key qualitative and quantitative variables (Table 2): access complexity, decision-making centralization, innovation profile, protocolization level, and HCP’s prescribing freedom. Access complexity refers to the challenges in navigating the approval process, including the steps, interlocutors, and timelines required. Decision-making centralization assesses whether decisions are made at the account/hospital or centralized regional health council level. The innovation profile considers the degree to which new treatments and innovations are adopted to improve patient care, while protocolization assesses how regulated HCP prescribing practices are. Understanding these factors allows for strategic differentiation of approaches based on each region’s unique needs and characteristics.
Table 2.
Variables of region archetyping
| Area | Definition | Criteria used in the characterization of each region |
|---|---|---|
| Access complexity | Difficulty of the access process based on the required steps, interlocutors, and timing |
Data protection and access procedures, clinical effectiveness and efficiency Concentration of stakeholder access (regional health ministry/healthcare area/hospital) |
| Degree of decision-making centralization | Organizational structure of each autonomous community for the drug approval and usage process | Decision map in each autonomous community: decentralized (account/hospital), centralized (regional health ministry), or mixed |
| Innovative profile | Degree of belief in innovation and bringing new value treatments to improve patient health |
Approach focused on payer’s priorities: budget optimization, cost-effectiveness, and outcomes orientation Expertise in integrated value solutions Patient access to innovative diagnostics Access to integrated patient data databases Specialized areas by pathology Centers of Excellence in the autonomous community Innovative solutions implemented by the regional administration (e.g., telemedicine) HCPs, primary care/hospital pharmacists incorporation of digital solutions in their daily work (e.g., videoconference apps) Improvement in healthcare processes (current and past) Electronic health records |
| Degree of protocolization | Degree of regulation/agreements that guide the HCP’s prescription in their autonomous communities/healthcare areas/hospitals |
Pathologies with clinical protocols (e.g., harmonization guidelines, etc.), in hospital/healthcare area application Degree of protocol updates (autonomous community/healthcare area/hospital level) based on available evidence and market developments |
| HCP’s prescription preferences | Degree of clinical attitudes and practices alignment with the recommendations |
KPIs/incentive mechanisms for HCPs to regulate drug spending and/or incentivize improvements in patient health Degree of compliance with protocols by HCPs |
HCPs, healthcare professionals; KPIs, key performance indicators
By systematically evaluating these factors, region archetyping provides a data-driven foundation for differentiating engagement strategies across diverse healthcare landscapes. This structured approach can be customized to reflect the nuances of different national and regional healthcare systems. While some countries may exhibit highly centralized decision-making structures with strict protocolized care pathways, others may have more decentralized frameworks where prescribing autonomy and regional variability play a larger role. Similarly, market access complexity and the level of digital transformation may differ significantly between countries, requiring a flexible approach to archetyping. By applying a standardized yet adaptable framework, MA teams can generate insights that inform tailored strategies, ensuring that evidence generation, stakeholder engagement, and innovation adoption align with the realities of each specific healthcare ecosystem.
General Characterization of the Health Area/Account
Once the regional archetype has been established, it is essential to proceed with a structured characterization of the healthcare area or hospital (account). This characterization involves a 360-degree evaluation, covering the hospital environment, resources, patient demographics, and competitive intelligence. It examines the hospital’s infrastructure, including the availability of specialized units and their coordination. It also assesses the HCPs involved in each hospital specialty and primary care, as well as the presence of residents and nursing staff. An analysis of the available infrastructure includes the number of consultations carried out in specific units, the presence of specialized nursing consultations, day hospitals dedicated to particular areas, and the number of affiliated primary care centers and specialized centers linked to the hospital.
Data on the patient population attended are examined in detail, including annual admissions, recurrence rates, mortality, and how patients are distributed across different care settings, including primary care, emergency services, and specialties. In addition to these medical resources, the characterization provides insights into HCP’s ability to digitalize patient data, integrate health records, and adopt innovative clinical solutions.
The characterization also includes an analysis of the competitive landscape, detailing collaborations and initiatives led by other organizations. For instance, partnerships between research groups and pharmaceutical companies are evaluated to understand their impact on local healthcare dynamics. This thorough account characterization helps ensure that strategic action plans are tailored to each healthcare environment’s specific resources, needs, and challenges, enabling better decision-making and collaboration with key stakeholders.
Research, Development, and Innovation Evaluation
The research, development, and innovation (I + D + I, for its Spanish acronym) area evaluates the healthcare account’s capabilities in fostering I + D + I, which is crucial for advancing medical science and improving clinical outcomes. This assessment focuses on quantitative and qualitative aspects related to evidence generation and innovation activities within the institution. Together, these criteria provide a comprehensive overview of the hospital’s contribution to medical innovation, its research capabilities, and its engagement in advancing healthcare through scientific evidence and process innovation.
Key criteria include whether the hospital has an associated research center and dedicated research units. Its capacity for conducting independent clinical studies, coupled with the involvement in external partnerships with research organizations, are other critical factors. Furthermore, the number of clinicians with relevant publications and involvement in clinical trials are crucial markers of the hospital’s research activity.
Qualitatively, the I + D + I profile considers the hospital’s involvement in innovative care models and technologies. These include experience in integrated value solutions programs, big data, and artificial intelligence (AI). The adoption of telemedicine and other process innovations is also examined. Lastly, participation in specific innovative projects, particularly those designed to enhance healthcare processes, reflects the institution’s commitment to continuous improvement and its readiness to adopt new methodologies and technologies in patient care.
Healthcare Model Analysis
Understanding the clinical processes that patients undergo (from diagnosis to treatment and follow-up) is essential for identifying inefficiencies and areas for improvement in healthcare delivery. For each stage of the patient’s journey, the relevant HCPs and departments involved are mapped, characterized and analyzed. This process plays a pivotal role in aligning medical interventions with patient needs, ensuring that all stakeholders involved in patient management contribute efficiently.
Healthcare model analysis is rooted in the CARABELA methodology, which has been extensively described in our previous publications [10, 14, 15]. In short, it follows a structured four-phase approach in each disease area. The first phase analyzes and characterizes existing management models for a targeted disease, with a scientific committee selecting pilot centers to study healthcare processes, clinical practices, and patient experiences. The second phase validates improvement areas and proposed solutions through national workshops, where key indicators identified in the first phase are refined. The third phase involves regional meetings across Spain, engaging multidisciplinary teams to co-create and optimize the proposed solutions. In some cases, an additional quantitative consensus step ensures further validation of key indicators. The final phase focuses on implementation, integrating validated models into real-world practice, forming a comprehensive framework for sustainable healthcare transformation.
To implement the final phase of the CARABELA methodology, the CARABELA Playbook was developed as a digital platform to enable healthcare centers across Spain to collect, evaluate, and monitor key clinical management indicators. Participating centers define and receive summaries of findings and expected improvements, allowing them to prioritize and implement tailored solutions, whether technological or process-based, according to their local context.
The data generated using Playbook is fed into the Account Clinical Pathway (ACP), which is the main analysis tool of the healthcare model analysis. As such, Playbook and the ACP are key methodologies for identifying inefficiencies and optimizing patient care at the hospital or healthcare area level. By analyzing several ACP variables, MA teams work with the stakeholders to co-create strategies addressing the needs of each healthcare account, ensuring that scientific collaborations and medical initiatives align with local priorities. In addition, the ACP incorporates real-world data that helps identify key opportunities for evidence generation, as has been recommended [16, 17].
Collaborative engagement through the Playbook and ACP fosters the co-creation of strategic solutions and action plans for each TA, ensuring medical interventions are scientifically robust, aligned with healthcare priorities, and optimized for clinical and economic efficiency. In addition, the platform enables impact measurement, reinforcing AZ Spain’s commitment to catalyze healthcare ecosystem improvement.
Stakeholder Mapping and Characterization
A fundamental task of MA is the mapping of stakeholders, ensuring that the most relevant and influential healthcare professionals, decision-makers, and key external experts (KEEs) within each account are strategically engaged. Each healthcare institution presents a unique network of stakeholders whose roles, influence, and decision-making processes vary significantly. To optimize engagement and evidence generation, it is essential to identify and prioritize these stakeholders on the basis of a structured set of segmentation criteria (Table 3).
Table 3.
Segmentation variables for stakeholder mapping
| Category | Definition | Profile evaluation |
|---|---|---|
| Institutional relevance | Defines the level of relevance of the KEE in the scientific ecosystem | Assigned profile based on scientific impact: international/national, regional, local, future prospect |
| Clinical practice influence | Assesses the level of influence of the KEE within their TA | Influence score calculated on the basis of clinical impact |
| Scientific research profile | Evaluates the extent of KEE participation in scientific research | Assigned profile based on research contribution: international/national, regional, local, future prospect |
| Digital relevance | Measures the level of social media influence of the KEE | Digital score based on influence variables: followers and activity |
KEE, key external expert; TA, therapeutic area
Institutional relevance defines a stakeholder’s significance within the scientific and healthcare community, categorizing them as international, national, regional, or local. Clinical practice influence assesses their role in shaping treatment decisions and guidelines within their TA. The research profile considers the stakeholder’s involvement in scientific research, publications, and clinical trials, further categorized by their geographical impact. Digital influence evaluates their presence and engagement on social media, quantified through metrics such as the number of followers and activity levels.
The stakeholder mapping process provides a systematic framework of KEE categorization. However, beyond this classification, it is critical to assess how their knowledge, perception, and engagement with a product evolve over time. The Awareness, Positioning, and Differentiation index (APDi) serves as a quantitative tool for tracking this evolution, enabling a dynamic understanding of how HCPs engage with scientific advancements and the role of a product in clinical practice. The APDi is calculated using a structured questionnaire that assesses three key dimensions: awareness, positioning, and differentiation. Each dimension is evaluated through a set of questions related to disease knowledge, therapeutic practices, and the perception of treatment attributes.
Awareness quantifies the extent of a KEE’s understanding of the disease and the scientific rationale behind a given therapy. Positioning assesses how the product is integrated into the stakeholder’s treatment paradigm, while differentiation measures how the product is perceived relative to available alternatives. As a product progresses through its life cycle, the APDi facilitates longitudinal monitoring of these perceptions, ensuring that engagement strategies remain evidence-based and tailored to evolving stakeholder insights. By integrating stakeholder mapping with APDi-based assessments, MA teams can optimize scientific communication and engagement strategies, thereby reinforcing the translation of evidence into clinical practice.
Integration of End-to-End Data and Analytics in Cross-Functional Plans
Establishing end-to-end data and analytics capabilities is a top priority for MA leaders, ensuring that insights derived across the product lifecycle are effectively leveraged to guide decision-making and stakeholder engagement. The comprehensive data gathered from the CARABELA Playbook, ACP, and APDi serve as the foundation for designing account-specific action plans, ensuring full alignment with each healthcare account’s evolving needs. This process is dynamic and cross-functional, continuously adapting to environmental changes and stakeholder feedback. Using the CARABELA Playbook and ACP, we structure patient journeys and assess healthcare process inefficiencies, while APDi provides insights into stakeholder perceptions, awareness, and scientific alignment over time. The integration of these tools ensures that action plans are not only tailored but also strategically co-created with key stakeholders, maximizing scientific impact and healthcare outcomes.
This structured approach feeds into our transforming care methodology, an initiative fully embedded in our strategic scientific plan at the account, regional, and national levels. Within this model, data-driven strategic objectives are translated into scientific engagement strategies and action plans that optimize clinical decision-making and enhance healthcare efficiency. The SSA plays a key role in this process, orchestrating these activities in collaboration with cross-functional teams, as detailed in our previous publication [14].
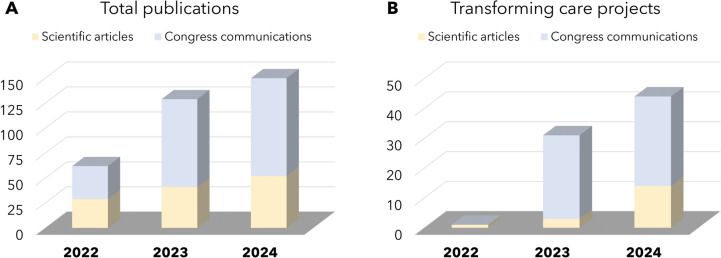
The impact that these strategies are having on patient outcomes and the overall healthcare ecosystem can be inferred by the significant increase in scientific output associated with the transformation of the AZ Spain MA department (Fig. 3). From 2022 to 2024, the total number of scientific articles and congress communications has steadily risen (Fig. 3A), with a notable increase in publications related to transforming care projects led by SSAs (Fig. 3B). The average impact factor of our publications has also improved, from 5.0 in 2022 and 2023 to 5.6 in 2024, reflecting the growing influence of our work on the healthcare community. Particularly, the CARABELA initiative has already led to seven ongoing national projects, addressing conditions such as severe asthma, chronic obstructive pulmonary disease, heart failure, chronic kidney disease, amyloidosis, and immunosuppression [15, 18–22]. CARABELA has improved chronic disease management by establishing quality indicators, enhancing clinical outcomes, and enabling continuous analysis and implementation of solutions that improve healthcare system efficiency across diverse settings via locally led workshops.
Fig. 3.
Evolution in the amount of scientific articles and congress communications from 2022 to 2024: A total number of publications; B publications related to the transforming care methodology
Differentiation of Medical Engagement According to Data-Backed Strategies
According to the MA 2030 vision, strategic planning should be more ambitious, focusing on measurable progress and optimizing resource allocation to areas with the highest potential for improving patient care [8, 9]. Planning should be backed on advanced analytics and digital tools to ensure that every engagement is tailored to individual needs and leads to meaningful improvements in patient care [9]. MA should lead efforts to produce real-world evidence (RWE) aligned with the needs of various stakeholders, such as HCPs, payers, and regulators. Beyond informing regulatory and reimbursement decisions, RWE plays a crucial role in identifying gaps in clinical practice and optimizing patient care pathways, serving as the foundation for strategic interventions that drive system-wide transformation. Innovative approaches should ensure the timely delivery of relevant and impactful evidence for each disease area.
The CARABELA framework serves as a catalyst for the self-assessment and transformation of healthcare teams at both local and regional levels, ultimately driving impact at a national scale. Building upon insights derived from RWE, CARABELA translates real-world findings into actionable strategies, ensuring that clinical management improvements are evidence-based and aligned with evolving healthcare priorities. Upon request, structured workshops are conducted with the CARABELA Playbooks, facilitated by the corresponding SSA and involving key stakeholders engaged in the management of the targeted pathology. These workshops provide a systematic approach for healthcare teams to analyze their organizational models, clinical pathways, and unmet needs. By leveraging insights from the CARABELA Playbooks, teams collaboratively identify challenges and develop tailored, practical solutions aligned with their specific operational contexts. This process fosters a structured framework for public–private collaboration, enabling the co-creation of both traditional and innovative interventions aimed at enhancing healthcare efficiency and improving patient outcomes.
The practical solutions implemented through CARABELA are designed to address key challenges in healthcare delivery by focusing on four strategic areas. First, proactive screening and early diagnosis initiatives facilitate early disease identification through systematic patient screening and simple diagnostic tools in community settings. Second, guideline adoption at the practice level ensures that evidence-based recommendations are seamlessly integrated into clinical workflows, supported by top-down quality improvement initiatives and clinical decision-support tools. Third, advanced specialist pathways and personalized care optimize transitions between care settings, promote precision diagnosis and treatment, and incorporate remote monitoring strategies for high-risk patients. Finally, world-class policy leadership initiatives work to expand public health and social science research (PHSSR), drive disease policy advancements, and support health system modeling to guide resource allocation and long-term planning. By addressing these areas, CARABELA ensures that medical engagement is aligned with real-world healthcare needs, fostering sustainable improvements in patient care and health system efficiency.
Alignment of Evidence Generation with Stakeholders’ Needs
In today’s evolving therapeutic landscape, expanding RWE is fundamental to generating meaningful insights that drive healthcare decision-making. A comprehensive and scalable approach is required to integrate diverse data sources, including claims databases, electronic medical records, clinical trials, and disease registries, into a unified evidence-generation framework. To ensure impactful outcomes, close collaboration across key areas of RWE, health economics, and outcomes research is essential. This integration ensures that the resulting evidence is not only robust but also directly applicable to real-world clinical and payer environments.
By refining evidence generation strategies, our MA department transitioned from traditional descriptive analyses to predictive modeling, enabling a more proactive approach to healthcare management to achieve optimal pricing and reimbursement and therapeutic positioning. Our new evidence generation plans focus on capturing in-depth data on disease burden, healthcare resource utilization, and treatment patterns to build a compelling value story. This approach addresses knowledge gaps by collecting comprehensive data on patient population characteristics, disease progression, healthcare resource utilization, and costs associated with the target disease.
This model enhances decision-making by providing early insights that support clinical and payer strategies. In addition, our efforts extend beyond data collection, emphasizing the importance of making RWE both accessible and actionable. By tailoring data visualization tools and interactive evidence platforms, we ensure that key stakeholders, including patients, regulators, HCPs, and payers, can efficiently interpret and apply scientific findings to their respective domains.
By embedding evidence generation into a dynamic, stakeholder-centered approach, we reinforce our commitment to delivering data-driven solutions that align with healthcare priorities. The impact of these initiatives, along with further advancements in our evidence strategies, will be explored in upcoming publications.
Orchestration of Medical Engagement Across Channels
Enhancing data visualization across various channels will enable quicker understanding of the evidence and its implications for clinical practice, facilitating data-driven decision-making [9]. Our new medical omnichannel strategy focuses on creating seamless and integrated customer experiences using personalized real-time insights. At its core, this strategy is designed to meet the needs of HCPs throughout the product lifecycle (from early access to post-launch). A key aspect of the strategy is leveraging data and insight analytics, allowing the integration of medical activities into the entire company. This enables a unified and enriched view of performance metrics, ensuring that medical and commercial teams are aligned.
Personalization is another essential pillar, as it allows us to adapt medical and scientific content to the specific needs of each stakeholder. Our omnichannel strategy is built on orchestration, ensuring that all interactions are coordinated across both physical and digital channels. The methodology includes detailed situational analysis, profiling of stakeholders, and a customized omnichannel engagement plan for each person involved. We continuously review and adapt our plans on the basis of KPIs, feedback, and outcomes, ensuring continuous improvement in engagement and the delivery of value. Our medical omnichannel strategy will be described in detail in an upcoming publication.
Discussion
This article describes the MA transformation of AZ Spain. Aligning with the MA 2030 vision [8] and the priorities outlined by Algazy et al. [9], we have redefined the role of MA as a strategic partner within the healthcare ecosystem. On the basis of a comprehensive analysis of the Spanish healthcare ecosystem and our internal capabilities, we implemented data-driven strategies, enhanced cross-functional collaboration, and fostered stakeholder engagement. This transformation has been driven by five key strategic workstreams, which have allowed us to implement innovative methodologies to improve patient outcomes and optimize healthcare delivery.
A key aspect of this transformation has been the structured integration of strategic methodologies, enabling a systematic approach to characterizing and engaging healthcare accounts. Region archetyping and account characterization allow for in-depth understanding of healthcare areas, including their I + D + I capacities, healthcare model analysis, and institutional resources. The healthcare model analysis, powered by the CARABELA Playbook and the ACP, provided an actionable framework for continuous assessment and improvement of clinical care models. In addition, the APDi facilitates a dynamic evaluation of stakeholder perceptions, ensuring that scientific engagement was evidence-based and responsive to evolving healthcare needs. This structured approach has strengthened our ability to tailor interventions and optimize medical engagement to drive a meaningful healthcare transformation.
In this sense, initiatives such as CARABELA have allowed us to bridge the gap between pharmaceutical innovation and real-world healthcare challenges demanding a greater focus on accessibility and patient welfare [3, 15, 23]. By partnering and fostering collaboration with scientific societies and policymakers we have become a pillar of the Spanish healthcare ecosystem, co-creating solutions that address systemic inefficiencies while staying focused on patient care [8]. The introduction of the SSA role has been instrumental in catalyzing these initiatives [14], facilitating engagement with healthcare teams and supporting the implementation of tailored interventions. Through structured workshops and stakeholder-driven analysis, CARABELA has enabled the deployment of practical solutions to foster proactive screening and early diagnosis, optimize transitions of care, integrate digital monitoring tools, and drive policy changes that enhance patient outcomes.
To illustrate these efforts, an internal survey performed in 2023 with 140 KEEs benchmarked AZ Spain’s performance against competitors across scientific leadership, partnership, patient centricity, and MA in-field expertise. The results confirmed AZ’s leadership position across all our TAs, including cardiovascular, metabolism, renal, and respiratory/immunology. AZ consistently ranked first or among the top two companies in most domains, reflecting a strong perception of scientific leadership and excellence in medical engagement compared with competitors. The ranking trend from surveys conducted in 2021 and 2022 showed marked improvements in most KPIs across all TAs. Notably, the results also indicated sustained increase in patient centricity and SSA expertise, underscoring the positive impact of the MA transformation described in this article.
Another fundamental aspect of MA transformation is the integration of data analytics and RWE into decision-making processes, enabling a shift from reactive to predictive engagement strategies [2]. By aligning insights from ACP, the CARABELA Playbook, and APDi with broader scientific and business strategies, AZ Spain ensures that every stakeholder interaction is backed by robust data, fostering an evidence-driven transformation in MA. We have transitioned to a proactive and data-driven model that allows for personalized and adaptable engagement strategies, ensuring that scientific discourse remains aligned with national healthcare priorities [4].
The integration of our omnichannel strategy has further expanded our ability to engage stakeholders efficiently, providing customized scientific content through digital and in-person interactions. Furthermore, our evidence generation efforts have evolved beyond descriptive analyses to predictive modeling, reinforcing the role of MA in informing clinical decision-making. These developments will be explored in greater depth in upcoming publications, detailing our advancements in evidence generation and medical engagement across different channels.
Conclusions
Looking ahead, MA will need to continually adapt to new scientific, technological, and healthcare trends, while staying proactive and data-driven to meet the changing needs of all stakeholders [4, 17]. As we move forward, our focus will remain on deepening collaborations with external partners, further refining predictive analytics capabilities, and driving evidence-based transformation in healthcare delivery through scalable, impact-driven solutions. The AZ Spain MA model could serve as a replicable and adaptable framework for MA transformation. The model is already being implemented in several Latin-American affiliates and could be applicable to other healthcare systems world-wide, offering practical guidance for global MA teams seeking to align with the MA 2030 vision [8]. By leveraging real-world data, optimizing omnichannel engagement strategies, and reinforcing cross-functional collaborations, we are committed to shaping the future of healthcare through medical innovation and patient-centric solutions.
Acknowledgements
The authors thank Ánchel González Barriga and Blanca Piedrafita, from Medical Science Consulting (Spain), for providing editorial support, in the form of medical writing and assembling tables/figures based on the authors’ detailed directions, collating author comments, copyediting, fact-checking, and referencing.
Declarations
Conflicts of interest
All authors declare being employed by AstraZeneca.
Funding
This work has been funded by AstraZeneca Spain.
Availability of data and material
All data generated during this study are included in the published article or by reasonable request to the corresponding author.
Author contributions
All authors contributed to the conception and design of the study, as well as the investigation and analysis performed. The first draft of the manuscript was written by Ana Pérez Domínguez. All authors critically reviewed, edited, and commented on the manuscript until the final version was obtained. The final version of the manuscript was read and approved by all authors.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.Evers M, Ghatak A, Holt E, Ostojic I, Pradel C, Suresh B, et al. A vision for Medical Affairs in 2025. McKinsey & Company [Internet]. 2019. https://www.mckinsey.com/~/media/mckinsey/industries/life%20sciences/our%20insights/a%20vision%20for%20medical%20affairs%20in%202025/a-vision-for-medical-affairs-in-2025.pdf. Accessed 16 Oct 2024.
- 2.Plantevin L, Schlegel C, Gordian M. Reinventing the role of medical affairs. A strategic overhaul of medical affairs can help pharma companies win in an era of big data medicine. Bain & Company [Internet]. 2017. https://www.bain.com/contentassets/f4702d29aeba409cbc425ab418d130f8/bain_brief_reinventing_the_role_of_medical_affairs.pdf. Accessed 13 Feb 2025.
- 3.Furtner D, Shinde SP, Singh M, Wong CH, Setia S. Digital Transformation in medical affairs sparked by the pandemic: insights and learnings from COVID-19 era and beyond. Pharm Med. 2022;36(1):1–10. 10.1007/s40290-021-00412-w. (Epub 20211231). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gores M, Berkels R. Whitepaper. Their finest hour: medical affairs in a disrupted world. IQVIA Inc. [Internet]. 2022. https://www.iqvia.com/library/white-papers/their-finest-hour-medical-affairs-in-a-disrupted-world. Accessed 13 Feb 2025.
- 5.Fulford-Smith A, Leah E, Azroyan A, De Abadal M, Loew D, Hildemann S. Medical affairs transformation in specialty pharma: next-level collaboration at the core. Pharm Med. 2022;36(2):63–9. 10.1007/s40290-022-00419-x. (Epub 20220306). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furtner D, Hutas G, Tan BJW, Meier R. Journey from an enabler to a strategic leader: integration of the medical affairs function in ESG initiatives and values. Pharm Med. 2023;37(6):405–16. 10.1007/s40290-023-00485-9. (Epub 20230718). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajadhyaksha VD. Medical affairs post-COVID 19: are we ready to take the baton? Perspect Clin Res. 2020;11(3):124–7. 10.4103/picr.PICR_164_20. (Epub 20200706). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MAPS Visionary Working Group. The Future of Medical Affairs 2030. Medical Affairs Professional Society [Internet]. 2022. https://medicalaffairs.org/futuremedical-affairs-2030/. Accessed 16 Oct 2024.
- 9.Algazy J, Garcia A, Ryan S, Westra A, Zemp A. A vision for medical affairs 2030: five priorities for patient impact. McKinsey & Company [Internet]. 2023. https://www.mckinsey.com/industries/life-sciences/our-insights/a-vision-for-medical-affairs-2030-five-priorities-for-patient-impact#/. Accessed 13 Feb 2025.
- 10.Iglesias I, Marinich JA, Anechina LR, Cortes M, Perez Dominguez A. Understanding the national healthcare ecosystem to position medical affairs as a strategic element: lessons learned from AstraZeneca Spain. Pharm Med. 2024. 10.1007/s40290-024-00542-x. (Epub 20241223). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia Garcia C, Riosalido Montero M, Sastre V, Gonzalez Del Castillo A, Matesanz MA. The medical science liaison role in spain: opinion of the commercial department personnel. Ther Innov Regul Sci. 2023;57(5):1030–9. 10.1007/s43441-023-00533-1. (Epub 20230607). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez Del Castillo A, Garcia C, Matesanz-Marin A, Gomez-Sanchez MJ, Sastre V. The medical science liaison role in Spain: a survey about the opinion of healthcare professionals. Ther Innov Regul Sci. 2022;56(1):96–103. 10.1007/s43441-021-00333-5. (Epub 20210820). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sastre V, Matesanz-Marin A, Garcia C, Gonzalez Del Castillo A. The medical science liaison role in Spain: a nationwide survey. Perspect Clin Res. 2022;13(1):48–53. 10.4103/picr.PICR_53_20. (Epub 20210712). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RegaderaAnechina L, Iglesias I, Marinich JA, Diago J, Pérez Domínguez A. The Evolution of in-field medical affairs: introducing the strategic scientific advisor. Pharmaceut Med. 2025. 10.1007/s40290-025-00551-4. (Epub 20250221). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escalada J, Carretero Gomez J, Anguita M, de Sequera P, Garcia-Rio F, Davila I, et al. Enhancing the management of chronic diseases in clinical practice: the CARABELA methodology. J Healthc Qual Res. 2024;39(5):336–9. 10.1016/j.jhqr.2024.06.001. (Epub 20240622). [DOI] [PubMed] [Google Scholar]
- 16.Cadogan AA, Lau J, Wnorowski S, Kelsch GR, Oreper J, Chavez L, et al. Defining insights. Ther Innov Regul Sci. 2023;57(6):1229–37. 10.1007/s43441-023-00554-w. (Epub 20230705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLoughlin M, Jonkman M, Zivkov M. Moving to an integrated, holistic approach to insights-related activities in medical affairs. A MAPS white paper. Medical Affairs Professional Society, [Internet]. 2021. https://medicalaffairs.org/wp-content/uploads/2022/05/Holistic-Integrated-Insights.pdf. Accesed 28 Mar 2025.
- 18.Crespo-Lessmann A, Marqués-Espi JA, Dominguez-Ortega J, Perez de Llano L, Blanco-Aparicio M, Santiñá M, et al. Quality indicators in the rational management of severe asthma: a Spanish multidisciplinary consensus. J Healthc Qual Res. 2023;38(5):277–83. 10.1016/j.jhqr.2023.03.003. (Epub 20230330). [DOI] [PubMed] [Google Scholar]
- 19.de Miguel-Diez J, Manglano JD, Mediavilla I, Escudero L, Alonso-Ortíz MB, Boixeda R, et al. CARABELA-COPD: a novel approach for the transformation and improvement of the healthcare process in COPD management in Spain. Open Respir Arch. 2025;7(1): 100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco ÁG, Soto JF, Mediavilla I, Leal M, Anguita M, Committee C-HS. CARABELA-HF: new frontiers in the optimization of heart failure clinical management in Spain. REC CardioClinics. 2024;60(2):145-148.
- 21.Salgueira M, Ruiz P, Diago JI, Maraver MP, Committee C-CS. Healthcare models, quality indicators and quality of care in the management of patients with chronic kidney disease in Spain: results from the CARABELA-CKD initiative. Nefrología. 2024;45(4):279-350. [DOI] [PubMed]
- 22.Crespo-Lessmann A, ScientificCommittee Carabela SA. Enhancing the management of severe asthma in Spain: the CARABELA Initiative disclosed. Open Respir Arch. 2025;7(1):100378. 10.1016/j.opresp.2024.100378. (Epub 20241114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howells M. Fostering a culture of lifelong learning. AstraZeneca [Internet]. 2023. https://www.astrazeneca.com/media-centre/articles/2023/fostering-culture-lifelong-learning.html. Accessed 18 Feb 2025.