Abstract
Background:
Overdose is a leading cause of pregnancy-associated mortality in the US. Our personally-tailored opioid-overdose (OOD) and medication for opioid use disorder (MOUD) education intervention has been shown to significantly improve MOUD/OOD knowledge in out-of-treatment persons using illicit opioids. We evaluated the ability of the intervention modified for peripartum (pregnant or within one year postpartum) individuals, the personally-tailored OOD and MOUD education (TOME) intervention, to increase MOUD (primary) and OOD (key secondary) knowledge.
Methods:
A six-site, two-arm, open-label, trial with 131 peripartum individuals receiving MOUD (methadone or buprenorphine) randomized to TOME, a 15-minute, computer-facilitated, individually-tailored intervention, or Control. TOME participants received education on MOUD and OOD questions they missed in a pre-test. Control participants received SAMHSA handouts on OOD and MOUD. All participants were scheduled for a 3-week post-test.
Results:
Participants were enrolled in MOUD for an average of 15.6 months (SD=20.4) at baseline, with 30.5% enrolled in methadone and 69.5% enrolled in buprenorphine treatment. On the pre-test, participants answered 66.7% of the MOUD and 82.1% of the OOD questions correctly on average. Linear regressions indicated that participants’ MOUD (X2=33.96, p<0.001) and OOD (X2=45.78, p<0.001) knowledge increased significantly more in the TOME, relative to Control, group.
Conclusions:
In a sample of peripartum patients enrolled in MOUD for a substantial length of time, TOME significantly increased MOUD and OOD knowledge. Taken together with past research, these findings suggest that there are gaps in MOUD and OOD knowledge in individuals with opioid use disorder that can be addressed with brief personally-tailored education.
Trial Registration:
Clinical Trials.gov http://www.clinicaltrials.gov; Identifier: NCT06262347
Keywords: opioid, overdose, pregnant, postpartum, education, medication for opioid use disorder
1.0. Introduction
From 2010 to 2017, opioid-related maternal deaths increased by 220% (Sanjanwala et al., 2023) and drug overdose is now a leading cause of pregnancy-associated mortality in the United States (Bruzelius & Martins, 2022). Interventions to decrease overdose risks in peripartum individuals are lacking and are needed in order to address the epidemic of opioid-related overdoses (Bruzelius & Martins, 2022). Our team has developed and tested a brief personally-tailored opioid overdose prevention education and naloxone distribution (PTOEND) intervention designed to reduce mortality by not only encouraging the use of naloxone but also educating individuals about factors that increase risk for opioid-overdose (OOD), including modifiable behaviors that increase risk (Winhusen et al., 2016; Winhusen, Wilder, et al., 2020). In addition, the PTOEND was designed to promote medication for opioid use disorder (MOUD) engagement, which is effective for preventing OOD and death from other causes (Larochelle et al., 2019; Sordo et al., 2017) but is underutilized (Volkow & Wargo, 2018) in part due to inaccurate perceptions and stigma (Madden et al., 2021; Peterson et al., 2010; Uebelacker et al., 2016; Zaller et al., 2009).
The results of a pre-post study of PTOEND suggested that it significantly increased OOD and MOUD knowledge and increased treatment readiness in out-of-treatment persons using illicit opioids who had experienced an OOD (Winhusen, Wilder, et al., 2020). In addition, the intervention was found to significantly decrease expected difficulty in avoiding drug use (Winhusen, Wilder, et al., 2020), which is important since improved self-efficacy has been associated with improved substance use outcomes (Witkiewitz et al., 2022). The initial study of PTOEND was with out-of-treatment persons using illicit opioids but it is also applicable for people enrolled in MOUD. First, while MOUD significantly decreases the risk of OOD, people enrolled in MOUD still overdose and the risk of overdose is heightened when MOUD is discontinued (Brandt et al., 2023). OOD education, including information about risk factors for overdose, has been found to decrease overdose-risk behaviors (Winhusen, Wilder, et al., 2020) and, thus, would be expected to reduce OOD risk in individuals who continue to use illicit opioids while enrolled in MOUD or who relapse when they stop MOUD treatment. Second, the elements of PTOEND that increased readiness for MOUD should also serve to improve engagement and retention in MOUD. There is consensus that retention needs to be improved but no evidence-based interventions for increasing agonist / partial agonist MOUD retention have been established (Chan et al., 2020). Stigma is one of the most commonly cited barriers to MOUD retention by individuals with OUD (Anvari et al., 2022; Mackey et al., 2020; Randall-Kosich et al., 2020). The PTOEND, which corrects stigma-eliciting misperceptions (e.g., MOUD is “just replacing one drug with another”, etc.), is hypothesized to reduce internalized stigma. Another common barrier to MOUD engagement/retention is inaccurate perceptions of MOUD, including myths about its side effects and efficacy (Randall-Kosich et al., 2020). PTOEND has been found to increase an individual’s belief in their ability to avoid drug use, which may be the indirect result of correcting MOUD myths, including providing information about its effectiveness (Winhusen, Wilder, et al., 2020). The present pilot trial tested the impact of a modified version of the PTOEND intervention, referred to as the personally-Tailored OOD and MOUD Education (TOME) intervention, on MOUD knowledge, OOD knowledge, internalized stigma, and drug self-efficacy in peripartum individuals enrolled in MOUD. Modifications included updating the knowledge assessment/ education to reflect changes in the drug supply (i.e., the current high prevalence of fentanyl and increasing prevalence of xylazine) and the addition of items specific to pregnancy.
2.0. Methods
2.1. Design
This pilot study was a randomized (target N=120) controlled intent-to-treat (ITT) clinical trial. Eligible participants were randomized in a 1:1 ratio to TOME or Control, stratifying on site. The randomization sequence used a randomized block design (blocks of size 2 or 4). All participants were scheduled to complete a week 3 follow-up visit for this pilot study.
2.2. Study Sites
This trial used the infrastructure of The National Drug Abuse Treatment Clinical Trials Network (CTN) Medication treatment for opioid use disorder in expectant mothers (MOMs) trial (Winhusen, Lofwall, et al., 2020). Six MOMs sites, located in Florida, Pennsylvania, South Carolina, Tennessee, Utah, and West Virginia, participated.
2.3. Participants
Participants were recruited during a 4-month period (June-October 2024) through various methods including advertisements, flyers, clinic referrals, a review of upcoming clinic appointments, and word-of-mouth at the sites. All participants were given a thorough explanation of the study and signed an informed consent form approved by the institutional review board of record (University of Cincinnati). Eligible participants were pregnant or within 12 months postpartum, were enrolled in MOUD at one of the six study sites or at an affiliated clinic and were able to provide consent in English. Participants with suicidal or homicidal ideation requiring immediate attention or who were prisoners (i.e., currently in jail, prison or in an inpatient overnight facility required by a court of law) or had a pending legal action that could result in incarceration based on self report were excluded.
2.4. Procedures
After signing consent, participants completed screening and baseline assessments and, if eligible, were randomized. Participants received the assigned intervention following randomization and completed a follow-up visit 3 weeks later. Except for the demographics assessment, all assessments were completed at the baseline and week 3 visits. Participants were reimbursed up to $75.
2.5. Measures
Demographic information was assessed with the PhenX Toolkit (Hamilton et al., 2011).
Knowledge about MOUD and OOD
The primary outcome was MOUD knowledge as measured by the Opioid Overdose and Treatment Awareness Survey (OOTAS). The OOTAS is comprised of 4 sections: 1) opioid-overdose risk factors; 2) signs of an opioid-overdose; 3) how to respond to an opioid-overdose; and 4) misconceptions about MOUD (Winhusen et al., 2016). The key secondary outcome was OOD knowledge as measured by the first three sections of the OOTAS. In non-peripartum populations, the OOTAS has been used to measure MOUD and OOD knowledge and knowledge change (Winhusen et al., 2016; Winhusen, Wilder, et al., 2020). For the present trial, the OOTAS was modified to include two additional questions specific to pregnancy (i.e., are methadone and buprenorphine recommended during pregnancy and can infants be born addicted as a result of taking methadone or buprenorphine during pregnancy). The OOTAS was also modified to include questions about fentanyl (i.e., does fentanyl increase overdose risk) and xylazine (i.e., does xylazine increase overdose risk) to reflect changes in the United States drug supply in recent years. As with the original OOTAS, these questions were based on face validity with education targets derived from informal discussions about common patient knowledge deficits with clinicians and staff, who, in this case, had years of experience treating pregnant persons with OUD. The modified OOTAS includes 10 true-false questions to assess MOUD knowledge and 31 true-false questions to assess opioid-overdose knowledge. Responses are converted to a percentage of correct answers from 0–100% where higher percentages mean more knowledge.
Drug self-efficacy
The Thoughts About Abstinence measure, which has been shown to have predictive validity (Hall et al., 1991; Ondersma et al., 2009), was used to assess participants’ drug self-efficacy at baseline and week 3. The instrument uses a single item to assess estimated difficulty in avoiding drug use (0 = “Very Easy” to 9 = “Very Difficult”) with a higher score indicating greater self-efficacy. This item was used to assess difficulty in avoiding drug use in our pre-post study of PTOEND (Winhusen, Wilder, et al., 2020).
Internalized stigma
The Methadone Maintenance Treatment Stigma Mechanisms Scale (MMT-SMS) is a self-report questionnaire assessing three dimensions of stigma: anticipated, enacted, and internalized stigma (Smith et al., 2020). The MMT-SMS has demonstrated good internal consistency and validity (Smith et al., 2020). The MOUD education component of TOME is expected to decrease internalized stigma by countering stigma-inducing myths (e.g., methadone/BUP is just replacing one drug with another, etc.) and, thus, the internalized stigma score is the outcome of interest. The subscale measuring internalized stigma consists of seven items with response options of 1 (Strongly disagree) to 5 (Strongly agree). The scale is scored by taking an average of the responses, with a higher number indicating greater internalized stigma. The MMT-SMS was designed for use with any MOUD medication, and the wording was modified to assess stigma related to the medication that the participant was receiving.
2.6. Study Treatments
All randomized participants were offered a Narcan® Nasal Spray kit.
2.6.1. TOME
Intervention
Like PTOEND, TOME is a computer-facilitated education intervention in which a REDCap survey (Harris et al., 2019) assesses an individual’s knowledge of MOUD and OOD risk factors, signs of OOD, and how to respond to OOD. The program automatically generates a personally-tailored report that provides a list of the questions missed and why missed items were incorrect; a sample MOUD report is provided in Figure 1. A research staff member who had at least a bachelor’s degree and completed approximately 2 hours of TOME training met with each TOME participant individually for ≤15 minutes to review the reports and to provide copies to the participant. Research staff were provided with a sample script for introducing the feedback report to participants with recommended answers to potential participant questions.
Figure 1.

Sample MOUD Feedback report
2.6.2. Control
Control participants received three SAMHSA handouts: 1) “Opioid Overdose Prevention Toolkit: Safety Advice for Patients and Family Members”; 2) “Opioid Overdose Prevention Toolkit: Recovering from Opioid Overdose”; and 3) “Medication-Assisted Treatment for Opioid Addiction: Facts for Families and Friends”.
Data Analysis
All statistical tests were two-tailed with a 5% Type I error rate and completed using SAS (SAS Institute, Inc.). Treatment comparisons for demographics and baseline characteristics were completed with Pearson’s chi-squared tests, Fisher’s exact tests, and Cochran-Armitage tests for categorical data, depending on the nature of the data. Wilcoxon rank-sum tests and Student’s t-tests were used for numeric data, depending on which test’s assumptions were more appropriate. Each primary and secondary outcome was tested for a treatment effect using available data from all randomized participants. Each test was a linear regression where the response variable was the week-3 outcome value and the predictor of interest was a binary indicator of treatment (1 for TOME, 0 for control). When Likert-style outcome results did not reasonably support linear regression, an ordinal logistic generalized linear regression was used. Supporting predictors were initially the baseline outcome value, site and site*treatment; site and site*treatment were dropped when not significant. There were no significant site or site*treatment interaction effects.
Results
3.1. Participants
From June – October 2024, 131 participants were randomized (Figure 2), surpassing the target sample size of 120. The number of participants randomized varied across the six study sites, with a range of 6 – 31 randomized participants per site. The study completion rate was 93.9% with no significant treatment group difference. There were no statistically significant differences between the treatment groups on any demographic or baseline characteristic. Participants were on average 31 (SD=4.9) years of age, with most being non-Hispanic (92.4%) and White (89.3%), which is consistent with the racial and ethnic composition of the patient populations treated at the study sites. The largest proportion of participants were postpartum (60.8%) and enrolled in buprenorphine treatment (70%). Treatment length was relatively substantial at baseline, with an average of 15.6 months (SD=20.4) overall and an average of 15.7 (SD=10.7) months for methadone-enrolled and 15.5 (SD=23.5) months for buprenorphine-enrolled participants. The majority of participants had a high school education (62.6%). For employment, 31.3% reported being employed and 27.5% reported being unemployed. Finally, 46.6% reported being married or living with a partner while 36.6% reported never being married.
Figure 2.

CONSORT chart of participant flow from screening to 3-week follow-up
3.2. MOUD/OOD Knowledge (Primary and Key Secondary Outcomes)
Figure 3 depicts the average % correct answers at baseline and week 3 for the MOUD and OOD knowledge assessments as a function of treatment arm. At baseline, the sample as a whole answered 66.7% of the MOUD questions correctly and 82.1% of the OOD questions correctly, with weaker knowledge for how to appropriately respond to an overdose (74.0%) and OOD risk factors (79.6%) than for knowledge of signs of overdose (89.2%). TOME, relative to Control, significantly increased MOUD (X2(1)=33.96, p<.0001) and OOD (X2(1)=45.78, p<.0001) knowledge. TOME, relative to Control, significantly increased each of the three OOD knowledge subscales: opioid-overdose risk factors (X2(1)=14.01, p<.001), signs of an opioid overdose (X2(1)=9.16, p<.01), and how to respond to an opioid overdose (X2(1)=43.48, p<.0001). Supplement, Table S1 provides the performance on each MOUD and OOD question as a function of treatment and time. As can be seen in Table S1, at baseline, participants were aware that methadone and buprenorphine are recommended for pregnant individuals with OUD (97% correct). In contrast, at baseline, only around 11.5% of participants knew that babies cannot be born addicted, with striking improvement on this item in the TOME (61.9%) vs Control (23.3%) groups at week 3.
Figure 3.
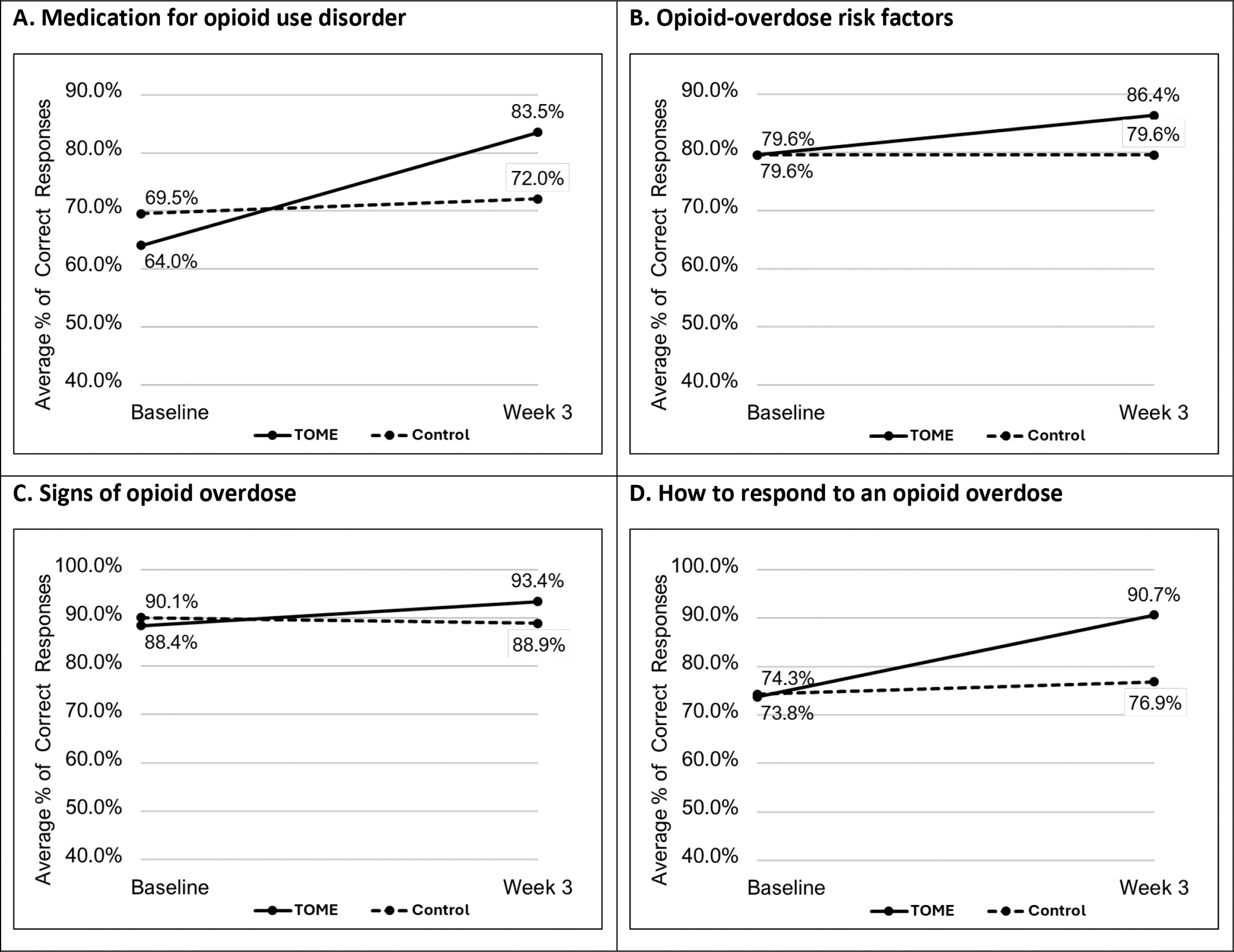
Average proportion of correct answers for knowledge assessments as a function of time and treatment arm
3.3. Drug self-efficacy and internalized stigma
Average MOUD-related internalized stigma was 1.7 (0.8) on a scale of 1 to 5 at baseline with no significant treatment effect found for change from baseline to week 3 (X2(1)=0.84, p=.36). Average expected difficulty in avoiding drug use was 2.6 on a scale of 0 to 9 at baseline, with no significant treatment effect found for change from baseline to week 3 (X2(1)=0.29, p=.59).
Discussion
This pilot randomized controlled trial comparing TOME, an education intervention designed to improve MOUD and OOD knowledge, to Control in peripartum individuals enrolled in MOUD found that TOME significantly increased both MOUD (primary outcome) and OOD (key secondary) knowledge. A significant effect was not observed for internalized stigma related to receiving MOUD or for expected difficulty in avoiding drug use.
The randomized sample had substantial time in treatment with participants having been enrolled in MOUD for an average of 15.6 months (SD=20.4) at baseline. Despite this, the participants only answered 66.7% of the MOUD questions correctly at baseline on average. A pre-post study of PTOEND, the intervention on which TOME was based, found that out-of-treatment individuals having experienced an opioid overdose answered 66.9% of MOUD questions correctly at baseline and that PTOEND significantly increased MOUD knowledge (Winhusen, Wilder, et al., 2020). It is interesting that baseline MOUD knowledge for out-of-treatment participants was almost identical to that of the present sample despite their relative longevity on treatment. This suggests that patients receiving MOUD may have MOUD knowledge gaps. At baseline, participants answered 82.1% of the OOD questions correctly, with weaker knowledge for how to appropriately respond to an overdose (74.0%) and OOD risk factors (79.6%) than for knowledge of signs of overdose (89.2%). TOME significantly improved OOD knowledge including knowledge of the appropriate response to overdose. This is critical as incorrect overdose response knowledge can cause further harm. TOME’s results are consistent with findings from the pre-post study of PTOEND which found that participants answered 79.8% of the questions correctly on average at baseline and that PTOEND significantly increased opioid overdose knowledge (Winhusen, Wilder, et al., 2020). Taken together, these two studies suggest that individuals with OUD may have OOD knowledge gaps.
In the present trial, TOME was not found to significantly decrease internalized stigma related to the receipt of MOUD or expected difficulty in avoiding drug use. This lack of effect may be due to floor effects. Baseline average internalized stigma was 1.7 (0.8), for which 1 is the minimum possible score. Baseline average expected difficulty in avoiding drug use was 2.6 (2.7), on a scale of 0 to 9. The low internalized stigma and expected difficulty in avoiding drug use might be due to the relatively long period of MOUD treatment in this sample or to the dedication of the study sites to caring for this population. In addition, the lack of treatment effect observed for expected difficulty in avoiding drug use may be due to the use of a single item to assess self-efficacy. While overall self-efficacy was strong in this population, a more detailed measure, for example, assessing self-efficacy in specific circumstances (e.g., during stressful life events, etc.) might have revealed areas in which participants experienced less self-efficacy. These areas might have been impacted by the intervention, but this is uncertain given that the hypothesized impact of the intervention on self-efficacy is through an indirect mechanism (i.e., better understanding the effectiveness of MOUD will increase participants’ perceived ability to avoid drug use).
This study had several strengths and limitations. Study strengths include the use of a randomized controlled design and recruitment from multiple sites, which should serve to increase the generalizability of the study findings. The relatively large sample size (N=131) is another strength along with the small number of screen failures, rapid enrollment, and minimal participant drop-out, suggesting that this type of intervention is palatable to clinical sites and their patients. A significant limitation is that the participant sample was primarily White and non-Latinx and, thus, it is unknown whether the results would generalize to a more racially and ethnically diverse population. Another limitation is that this pilot trial did not include a treatment outcome (e.g., retention, etc.) and, thus, it is unknown whether the increase in knowledge leads to improved outcomes. A final limitation is that the control participants were provided with educational materials but were not explicitly instructed to read them nor were they asked about whether they had read them at follow-up; more explicit instruction and follow-up might have improved patient engagement with the materials and, hence, improved MOUD/OOD knowledge more than observed in this pilot trial.
In conclusion, TOME, a brief (≤15 minutes), computer-facilitated intervention provided by a trained research staff member significantly increased MOUD and OOD knowledge in peripartum individuals, a population in which opioid-overdose deaths have risen dramatically in recent years. Together with past research, these findings suggest that there are MOUD and OOD knowledge gaps in individuals with OUD that can be addressed with brief personally-tailored education. Future research may be warranted to evaluate TOME in peripartum individuals who are early in MOUD treatment (e.g., <3 months) or who have OUD and are not yet engaged in MOUD treatment to determine whether it will also decrease internalized stigma related to the receipt of MOUD and expected difficulty in avoiding drug use, improve linkage to MOUD and MOUD treatment retention, and assist with improved health literacy on opioid overdoses.
Supplementary Material
Highlights.
We modified opioid-overdose and MOUD education for peripartum individuals (TOME)
Randomized trial of TOME vs. Control (SAMHSA handouts) for MOUD peripartum patients
TOME vs. Control significantly increased opioid-overdose and MOUD knowledge
Brief, personally-tailored education can address knowledge gaps in OUD populations
Funding Source:
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Health through the NIH HEAL Initiative under award numbers UG1DA013732 to the University of Cincinnati (T. John Winhusen), UG1DA049444 (Adam Gordon and Gerald Cochran), UG1DA013727 (Kathleen Brady and Matthew Carpenter), UG1DA013720 (Jose Szapocznik, Daniel Feaster, and Lisa Metsch), and UG1DA049436 (Jane Liebschutz and Judith Feinberg). The Publications Committee of the National Drug Abuse Treatment Clinical Trials Network reviewed and gave approval for submission of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
Footnotes
Declarations of interest: Dr. Krans is an investigator on grants to Magee-Womens Research Institute from the National Institutes of Health, Gilead, and Merck outside of the submitted work. Dr. Lofwall has been a research advisor to Braeburn Pharmaceuticals, Berkshire Biomedical and Journey Colab on topics unrelated to this paper. Dr. Lofwall received an honorarium from Camurus for developing a research talk unrelated to the topic of this paper. The other authors report no conflicts of interest.
References
- Anvari MS, Kleinman MB, Massey EC, Bradley VD, Felton JW, Belcher AM, & Magidson JF (2022). “In their mind, they always felt less than”: The role of peers in shifting stigma as a barrier to opioid use disorder treatment retention [Research Support, N.I.H., Extramural]. J Subst Abuse Treat, 138, 108721. 10.1016/j.jsat.2022.108721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt L, Hu M-C, Liu Y, Castillo F, Odom GJ, Balise RR, Feaster DJ, Nunes EV, & Luo SX (2023). Risk of experiencing an overdose event for patients undergoing treatment with medication for opioid use disorder. American Journal of Psychiatry, 180(5), 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzelius E, & Martins SS (2022). US trends in drug overdose mortality among pregnant and postpartum persons, 2017–2020. JAMA, 328(21), 2159–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Gean E, Arkhipova-Jenkins I, Gilbert J, Hilgart J, Fiordalisi C, Hubbard K, Brandt I, Stoeger E, Paynter R, Korthuis P, & Guise J-M (2020). Retention Strategies for Medications for Addiction Treatment in Adults With Opioid Use Disorder: A Rapid Evidence Review. Rockville, MD: Agency for Healthcare Research and Quality; Retrieved from https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/mat-retention-strategies-rapid-review-1.pdf [PubMed] [Google Scholar]
- Hall SM, Havassy BE, & Wasserman DA (1991). Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol, 59(4), 526. [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, & Pan H (2011). The PhenX Toolkit: get the most from your measures. American journal of epidemiology, 174(3), 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, & Consortium RE (2019). The REDCap consortium: Building an international community of software platform partners. J Biomed Inform, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Stopka TJ, Xuan Z, Liebschutz JM, & Walley AY (2019). Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Mortality. Ann Intern Med, 170(6), 430–431. 10.7326/L18-0685 [DOI] [PubMed] [Google Scholar]
- Mackey K, Veazie S, Anderson J, Bourne D, & Peterson K (2020). Barriers and facilitators to the use of medications for opioid use disorder: a rapid review. Journal of general internal medicine, 35, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden EF, Prevedel S, Light T, & Sulzer SH (2021). Intervention stigma toward medications for opioid use disorder: a systematic review. Substance Use & Misuse, 56(14), 2181–2201. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Winhusen T, Erickson SJ, Stine SM, & Wang Y (2009). Motivation Enhancement Therapy with pregnant substance-abusing women: does baseline motivation moderate efficacy? Drug and Alcohol Dependence, 101(1–2), 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Schwartz RP, Mitchell SG, Reisinger HS, Kelly SM, O’Grady KE, Brown BS, & Agar MH (2010). Why don’t out-of-treatment individuals enter methadone treatment programmes? International Journal of Drug Policy, 21(1), 36–42. 10.1016/j.drugpo.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Kosich O, Andraka-Christou B, Totaram R, Alamo J, & Nadig M (2020). Comparing reasons for starting and stopping methadone, buprenorphine, and naltrexone treatment among a sample of white individuals with opioid use disorder. Journal of Addiction Medicine, 14(4), e44–e52. [DOI] [PubMed] [Google Scholar]
- Sanjanwala AR, Lim G, & Krans EE (2023). Opioids and Opioid Use Disorder in Pregnancy. Obstetrics and Gynecology Clinics, 50(1), 229–240. [DOI] [PubMed] [Google Scholar]
- Smith LR, Mittal ML, Wagner K, Copenhaver MM, Cunningham CO, & Earnshaw VA (2020). Factor structure, internal reliability and construct validity of the Methadone Maintenance Treatment Stigma Mechanisms Scale (MMT-SMS) [Research Support, N.I.H., Extramural]. Addiction, 115(2), 354–367. 10.1111/add.14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, & Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ, 357, j1550. 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, & Stein M (2016). Patients’ Beliefs About Medications are Associated with Stated Preference for Methadone, Buprenorphine, Naltrexone, or no Medication-Assisted Therapy Following Inpatient Opioid Detoxification [Research Support, N.I.H., Extramural]. J Subst Abuse Treat, 66, 48–53. 10.1016/j.jsat.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Wargo EM (2018). Overdose Prevention Through Medical Treatment of Opioid Use Disorders. Ann Intern Med, 169(3), 190–192. 10.7326/M18-1397 [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lofwall M, Jones HE, Wilder C, Lindblad R, Schiff DM, Wexelblatt S, Merhar S, Murphy SM, Greenfield SF, Terplan M, Wachman EM, Kropp F, Theobald J, Lewis M, Matthews AG, Guille C, Silverstein M, & Rosa C (2020). Medication treatment for opioid use disorder in expectant mothers (MOMs): Design considerations for a pragmatic randomized trial comparing extended-release and daily buprenorphine formulations. Contemp Clin Trials, 93, 106014. 10.1016/j.cct.2020.106014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Theobald J, Lewis D, Wilder CM, & Lyons MS (2016). Development and initial testing of a tailored telephone intervention delivered by peers to prevent recurring opioid-overdoses (TTIP-PRO). Health Education Research, 31(2), 146–160. 10.1093/her/cyw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Wilder C, Lyons MS, Theobald J, Kropp F, & Lewis D (2020). Evaluation of a personally-tailored opioid overdose prevention education and naloxone distribution intervention to promote harm reduction and treatment readiness in individuals actively using illicit opioids. Drug Alcohol Depend, 216, 108265. 10.1016/j.drugalcdep.2020.108265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Pfund RA, & Tucker JA (2022). Mechanisms of behavior change in substance use disorder with and without formal treatment. Annual review of clinical psychology, 18, 497–525. [DOI] [PubMed] [Google Scholar]
- Zaller ND, Bazazi AR, Velazquez L, & Rich JD (2009). Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities [Research Support, N.I.H., Extramural]. Int J Environ Res Public Health, 6(2), 787–797. 10.3390/ijerph6020787 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


