Summary
Background
In countries with low baseline burdens of hepatitis B and C viruses (HBV, HCV), high levels of migration can impact the burden of viral hepatitis. The screening and treatment of migrants requires different methods and sensitivities than broad-based programs. We aimed to estimate the prevalence of HBV and HCV among migrants in EU-27 countries in 2024. The Ukrainian Refugee Crisis was also quantified.
Methods
Using the United Nations 2024 migrant stock data, we estimated the migrant population for each EU-27 country by five-year age and sex cohorts by country of birth. These distributions were multiplied by five-year age and sex prevalence estimates in the country-of-birth models maintained by the Polaris Observatory. The difference between the 2024 and 2020 Ukrainian migrant stock was quantified to estimate the impact of the Ukrainian Refugee Crisis.
Findings
In 2024, there were an estimated 1.73 million (UI: 1.04–2.66 million) migrants living with HBV and 1.03 million (UI: 757,000–1,559,000) living with anti-HCV in the EU-27, corresponding to migrant prevalences of 2.73% (UI: 1.6–4.2%) and 1.53% (UI: 1.2–2.5%) respectively. The Ukrainian Refugee Crisis is estimated to have resulted in an additional 43,000 (UI: 28,700–60,900) migrants living with HBV, and 154,000 (UI: 12,500–202,000) with HCV in the EU-27.
Interpretation
The burden of HBV and HCV among migrants and which communities are most affected in the EU-27 at the national level are vastly heterogeneous. These data provide evidence for policy makers to better understand the burden their community faces so that they can be better poised to develop culturally appropriate materials and outreach. While there is a great deal of uncertainty regarding the number of migrants by country, as well as the prevalence among these groups, this work provides direction towards which groups are most likely impacted at the EU-27 and national level.
Funding
John C Martin Foundation.
Keywords: Hepatitis B, Hepatitis C, Migration, Prevalence, Epidemiology, European Union, Modeling
Research in context.
Evidence before this study
Previous epidemiological studies among migrants living in high-income European countries have found that the prevalence of hepatitis B virus and hepatitis C virus (HBV and HCV) varies greatly depending on the population studied. Overall, these prevalence estimates are often similar, or slightly lower than the prevalence reported in the literature for countries of birth. However, prevalence can be higher among irregular migrants or refugees than the country of birth. Literature prevalence estimates are often not adjusted for age, nor are they able to consider, in the case of HBV, the impact of vaccination since the study survey date. As EU-27 countries bear a relatively low burden of HBV and HCV, but many countries host large number of migrants, these migrant populations have been shown to represent a significant portion of total infections in these destination countries. A recent meta-analysis reported a prevalence of 1.5% (CI: 1.1–2.0%) of anti-HCV among migrants living in high income countries, however similar studies for HBV focus on the region of origin or birth of the migrants. Databases on the number of migrants living in EU-27 countries were reviewed, including United Nations, EUROSTAT, and independent studies examining undocumented migrants. Ultimately the United Nations data were chosen due to the granularity of data available and the fact that it was comparable across all countries. For this modeling study, inputs were based on combination of an updated literature review and a Delphi process that used expert input to fill gaps and confirm data when available. A comprehensive literature review was done by searching PubMed using the terms “[country name] AND [(hepatitis B) OR HBV] AND [prevalence]” and “[country name] AND (‘prevalence’/exp OR prevalence) AND (‘hepatitis B’/exp OR ‘hepatitis B’ OR ‘HBV’/exp OR ‘HBV’)”. The same search was conducted for HCV, replacing HBV with HCV and hepatitis B with hepatitis C. The search was conducted from March 1, 2016 to November 1, 2023, as this built on our previous work (January 1, 1960 to March 1, 2016 for HBV, and January 1, 2000 to March 31, 2016 for HCV). Studies were selected for those that were most representative at the national population level, excluding studies done solely in non-representative groups. Non-indexed government reports and personal communications with country and territorial through the Polaris Observatory collaborators network were also utilized.
Added value of this study
We leverage the Polaris Observatory's work that combines systematic review and national expert interviews to produce and annually update 170 country specific models for HBV and 117 country specific models for HCV. These disease burden models annually estimate the changing prevalence by age and sex over time as a result of prevention and treatment. We utilize a standardized approach by using the most recent United Nations migrant stock data to quantify the migrant population for each EU-27 country, in five-year age and sex distributions by country of birth. These data were cross-multiplied by five-year age and sex prevalence estimates in the aforementioned country-of-birth models maintained by the Polaris Observatory. This method allows us to estimate the number of infections at the national and EU-27 level, while also providing insights into which migrant communities are most impacted by viral hepatitis. The difference between the 2024 and 2020 Ukrainian migrant stock was independently quantified to estimate the impact of the Ukrainian Refugee Crisis.
Implications of all the available evidence
The burden of viral hepatitis in the EU-27 is highly dependent on the composition of and the prevalence of viral hepatitis among the various migrant communities. While EU-27 totals help quantify the total burden, there is great heterogeneity at the national level of which migrant communities are most impacted, and how much these communities impact the national prevalence estimates. High-quality serosurveys at the national level are expensive and often have difficulty accurately quantifying the prevalence among migrants. The data presented here can directionally aid national experts in ensuring that their national estimates are considering the impact of migration. These data can be utilized by policy makers and patient advocates to know which migrant communities are most likely to be impacted by viral hepatitis and thus create culturally appropriate outreach to increase linkage to care and thus reduce future morbidity and mortality. There is the need for additional data among these communities, to bolster the current literature, and to add where evidence is gravely lacking such as the hepatitis delta virus.
Introduction
Migration within and to Europe has risen significantly in recent decades, reshaping the demographic profile of European countries and influencing the health and well-being of migrant and host communities. The World Health Organization (WHO) European Region hosts approximately 36% of the global international migrant population, representing the largest share of individuals living outside their country of birth.1 Epidemiological studies have found that the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) in migrants from around the world to high-income European countries mirrors or is lower than those reported for their country or region of birth.2,3 However, migrant populations bear a disproportionately higher burden of HBV and HCV, representing a significant proportion of chronic viral hepatitis infections in destination countries where the background prevalence is low.4, 5, 6, 7, 8, 9, 10 For instance, it has been estimated that 80%–90% of all HBV infections are attributed to foreign-born individuals.4,8,11 The elevated HBV infection rates primarily reflect a lack or incomplete immunization at birth or early childhood in high-prevalence countries. Regarding HCV infection, migrants account for approximately 14% of HCV cases in the EU/EEA, with some countries reporting over 50% of cases being foreign born.11 Non-drug use-related HCV infections among migrants are often a result from exposure to inadequately controlled healthcare settings at any stage in life.12 As countries have worked to better control HCV spread in healthcare settings, these infections are most likely to be found in the older population.12 Heterogeneous groups of migrants, including refugees, asylum seekers, and irregular migrants from around the world, have settled in European Union countries, predominantly in Western and Southern Europe (migrant groups defined in Appendix Section 4). There is a gap in accurately assessing HCV infection rates in the most vulnerable subgroups, such as asylum seekers, refugees, and irregular migrants.
Recognizing the complex health challenges migrants face, including disparities in access to healthcare and the need for tailored health interventions, the WHO Regional Committee for Europe has adopted an Action Plan for Refugee and Migrant Health.13 This initiative underscores the critical importance of updated, reliable, accessible, granular, and timely refugee and migrant health data for public health planning to guide the development and implementation of migrant-sensitive health policies across diverse health domains.9
This study aimed to estimate the prevalence of HBV (HBsAg-positive) and HCV (anti-HCV-positive) infections among migrants in the European Union, EU-27 countries (Appendix Section 4) in 2024, using population data and country-level modeling outputs. Additionally, we calculated the difference between the stock of migrants born in Ukraine between 2020 and 2024 to examine the impact of the Ukraine Refugee Situation and provide timely insights necessary for shaping viral hepatitis action plans targeting migrant health at the EU level. This is particularly important not only due to the large number of migrants from Ukraine entering the EU but also given that their population carries a higher burden of viral hepatitis in comparison to many EU countries. In 2015, it was estimated that the prevalence of HCV in Ukraine was 5% and the prevalence of HBV was 1.3% at the population level.14,15
Methods
Data selections and modeling methods
The Polaris Observatory maintains 170 country-specific HBV disease burden and transmission models—the PRoGReSs model—that are updated annually with data from in-country experts. PRoGReSs is a compartmental, deterministic, dynamic Markov model that estimates vertical and horizontal transmission, the impact of HBV prophylaxis programs, disease progression, all-cause mortality, liver-related deaths, and the impact of HBV treatment on disease progression and transmission. It was developed in Microsoft Excel and Microsoft Visual Basic (Microsoft Corporation, Redmond, WA, United States) to quantify the annual HBV-infected population by disease stage, sex, and age in a country. The disease stages considered in the PRoGReSs model are chronic hepatitis B, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation. Excel was selected due to its transparency, flexibility, and widespread availability. Details on the search strategy, study selection criteria, data collection and processing, and modeling were previously published.14 The Appendix outlines details of data and model updates since the most recent publication.
The Polaris Observatory also maintains 117 country-specific HCV disease burden models—the Bright model—that are updated following the same methodology described for the PRoGReSs models. The Bright model was also developed in Microsoft Excel and Microsoft Visual Basic (Microsoft Corporation, Redmond, WA, United States). Details on the data inputs and modeling have been previously published.15 The Bright model forecasts the viremic HCV and anti-HCV prevalence and disease burden from 1950 to 2050 accounting for annual incidence, mortality, and cure. The modeled outputs include new chronic infections, by fibrosis stage (F0, F1, F2, F3), compensated cirrhosis (F4), decompensated cirrhosis, hepatocellular carcinoma, liver-related deaths, and liver transplantation. Anti-HCV was chosen as the prevalence indicator as there is a great deal of uncertainty regarding the number of migrants who have been treated within the destination countries, which would have a large impact on the viremic prevalence.
When the authors refer to country models in the manuscript, it refers to the aforementioned country-specific models for HBV and HCV. For HCV, all of these models include country specific prevalence estimates and demographic data, with 79/117 having received feedback from in-country experts. For HBV, 150/170 country model are based on country-specific prevalence estimates and demographic data, with 100 of them having received feedback from in-country experts. In 20 of the HBV country models, the prevalence estimates are based on regional averages, however, the demographic and vaccination data are all country specific. The countries in this last group account for 1% of the global population.
Migrants and data analyses
For the purpose of this analysis, we use up-to-date migration data to estimate the overall number of international migrants (people living outside their country of birth) in the EU-27 in 2024.
The UN reports the international migrant stock in 2024, by sex, country of destination, and country of birth (Appendix: Migrant Stock Data).16 The 2020 release provides five-year-age groups distribution for both sexes by country of destination (Appendix: Migrant Stock Data).17 Five-year age and sex cohorts were utilized as the data were available in this format. Furthermore, this allows the HBV country models to estimate the impact of vaccination and other methods to prevent mother to child transmission on the prevalence of HBV in the younger age cohorts by country of birth. The international migrant stock, as defined in the UN methodology, was equated with the total foreign-born population, in all countries of destination with the exception of Czechia, and were utilized to estimate the age and sex distributions of the migrant population by the country of birth for each destination country in the EU-27 (Equation 1). This population thus would include asylum seekers, refugees, regular migrants, and at least a portion of the irregular migrant population. The sex specific age distribution by the country of destination was applied to the total male and female migrants by country of birth (Equation 1). The 2024 data release included significantly higher shares of migrants with the country of birth listed as “Others” than the 2020 release for eight countries, these were adjusted proportionally based on the 2020 release (Appendix, Appendix: Migrant Stock Data).
These now standardized age and sex data were then cross multiplied by the five-year age and sex prevalence distribution, by country of birth, from the 2024 country specific modeled estimate (Equation 1).
Estimating the prevalence of HBV and HCV by country of origin and destination by age and sex:
| (1) |
where is the number of migrants of sex and age group from country of origin living with hepatitis in destination country . is the share of migrants of sex and age group among all migrants of sex living in destination country . is the number of migrants of sex from country of origin living in country of destination . is the prevalence of hepatitis among sex and age group in the country of origin .
For countries of birth, for which there were migration data but no country model, population weighted regional average age and sex prevalence distributions were created and then applied to the migration data for missing countries by the region they are a part of (Appendix). The sum of these age and sex data resulted in the total number of positive cases by country of birth in each country of destination for the 2024 estimates (Equation 1).
To quantify the impact of the Ukraine Refugee Situation on the burden of HBV and HCV in the EU-27, we extracted the number of Ukrainians (migrant stock) from the UN database, in each EU-27 country in 2020 and 2024. We then took the difference between the number of Ukrainians living in each EU-27 country between 2024 and 2020. The aforementioned methodology (Equation 1) was then applied to the age and sex adjusted net number of Ukrainian migrants, to estimate the prevalence among this specific new migrant population.
Race and ethnicity data were not collected or analyzed as they are sociocultural constructs with inconsistent definitions across the 27 countries of destination, let alone the 234 countries of birth. Indigenous peoples and nations were implicitly included in the analysis but were not explicitly described due to lack of data.
Uncertainty analysis
For each country-of-birth model, the 95% uncertainty intervals (UIs) of HBV transmission and disease burden in the country of birth were considered (Appendix). The low prevalence estimate was defined by utilizing the low prevalence estimate in the country of birth, low transmission probabilities, and high progression rates in the country-of-birth model. This process resulted in the lowest prevalence based on the limits of all points of uncertainty that lead to a low prevalence. The high prevalence estimate used the high prevalence estimate in the country-of-birth, high transmission probabilities, and low progression rates. These rates were then applied to the foreign-born population by country of birth in each destination country.
For HCV, prevalence UIs were calculated with Crystal Ball (release 11.1.3708.0), an Excel add-in by Oracle. Beta-PERT distributions were used for all uncertain inputs. Monte Carlo simulation was used to estimate 95% UI. The uncertainty around prevalence for each country was calculated based on range inputs for prevalence, transition rates, and mortality rate (Appendix). Once these rates were calculated, they were then applied to the foreign-born population by country of birth in each destination country.
Role of funding source
The funder had no role in the data collection, analysis, interpretation, writing of the manuscript, decision to submit for publication, or any aspect pertinent to the study.
IRB approval
This study was a retrospective analysis of previously collected published or unpublished aggregate data. No human subjects were involved nor were any identifiable information accessed over the course of the study. IRB approval was thus deemed not necessary and informed consent was not applicable.
Results
2024 analysis
Of the 234 countries of birth reported by the UN, there were 169 country level HBV PRoGReSs models, and 116 country level Bright Models were available for the HCV analysis. Country specific PRoGReSS models represented 98.4% of all migrants and 99.3% of estimated HBV infections among migrants to the EU-27 in 2024, and Bright models represented 87.5% of migrants and 86.6% of HCV infections among migrants.
In 2024, we estimate that there were 1.73 million (UI: 1.04–2.66 million) migrants living with HBV and 1,033,000 (UI: 757,000–1,559,000) living with anti-HCV in the EU-27, corresponding to migrant prevalences of 2.73% (UI: 1.6–4.2%) and 1.63% (UI: 1.2–2.5%) respectively (Table 1).
Table 1.
Prevalence of HBV and HCV among migrants in the EU-27, 2024.
| Country | HBV prevalence (%) | Total HBV infections | HCV prevalence (%) | Total HCV infections |
|---|---|---|---|---|
| Austria | 2.40% (1.4%–3.5%) | 55,900 (32,500–82,300) | 1.33% (1.0%–2.1%) | 31,000 (22,700–50,000) |
| Belgium | 2.59% (1.6%–3.9%) | 60,800 (37,200–90,600) | 1.33% (0.9%–2.5%) | 31,200 (21,900–57,700) |
| Bulgaria | 1.68% (0.8%–2.4%) | 5000 (2400–7100) | 1.65% (1.2%–2.4%) | 4900 (3500–7200) |
| Croatia | 2.35% (1.1%–3.0%) | 12,400 (6000–15,900) | 1.25% (1.1%–1.6%) | 6600 (5700–8500) |
| Cyprus | 2.51% (1.4%–3.6%) | 5100 (2700–7300) | 1.85% (1.4%–2.6%) | 3700 (2900–5300) |
| Czechia | 1.75% (1.1%–2.7%) | 17,900 (11,000–27,800) | 2.94% (2.3%–4.3%) | 30,200 (23,900–44,600) |
| Denmark | 2.46% (1.4%–3.8%) | 20,800 (12,200–31,800) | 1.28% (0.9%–2.1%) | 10,900 (7800–18,200) |
| Estonia | 1.80% (0.5%–2.5%) | 3700 (1100–5000) | 2.88% (2.0%–3.5%) | 5900 (4100–7100) |
| Finland | 2.23% (1.4%–3.2%) | 11,400 (7000–16,700) | 1.57% (1.2%–2.4%) | 8100 (6000–12,200) |
| France | 3.28% (2.1%–5.0%) | 301,000 (189,000–461,000) | 1.50% (1.0%–2.3%) | 138,000 (92,500–212,000) |
| Germany | 2.68% (1.5%–4.0%) | 449,000 (251,000–667,000) | 1.84% (1.3%–2.8%) | 308,000 (222,000–467,000) |
| Greece | 4.74% (2.7%–7.5%) | 67,600 (38,000–107,000) | 2.13% (1.7%–2.9%) | 30,300 (24,400–41,900) |
| Hungary | 2.26% (1.8%–2.9%) | 15,600 (12,100–19,900) | 1.56% (1.3%–2.1%) | 10,700 (8900–14,600) |
| Ireland | 1.93% (1.2%–3.0%) | 23,500 (14,300–36,900) | 1.26% (0.9%–1.9%) | 15,300 (11,400–22,600) |
| Italy | 3.77% (2.4%–6.0%) | 247,000 (157,000–393,000) | 1.75% (1.4%–2.4%) | 115,000 (91,200–159,000) |
| Latvia | 2.16% (0.8%–3.0%) | 4800 (1600–6700) | 2.97% (2.2%–3.6%) | 6500 (4700–7900) |
| Lithuania | 2.02% (0.9%–2.8%) | 3500 (1600–5000) | 3.23% (2.4%–4.0%) | 5700 (4300–6900) |
| Luxembourg | 1.82% (1.2%–2.5%) | 6300 (4200–8700) | 0.98% (0.7%–1.5%) | 3400 (2500–5200) |
| Malta | 2.02% (1.2%–3.2%) | 4000 (2400–6300) | 1.18% (0.9%–1.7%) | 2400 (1700–3400) |
| Netherlands | 2.92% (1.8%–4.2%) | 86,400 (53,200–125,000) | 1.31% (0.9%–2.3%) | 38,600 (26,100–68,900) |
| Poland | 0.91% (0.6%–1.3%) | 15,900 (9800–23,100) | 2.88% (2.3%–3.8%) | 50,200 (40,200–66,100) |
| Portugal | 5.47% (4.0%–7.0%) | 61,600 (44,500–78,900) | 1.56% (1.1%–2.6%) | 17,500 (12,100–29,600) |
| Romania | 1.11% (0.7%–1.6%) | 7300 (4500–10,500) | 1.29% (1.0%–1.8%) | 8500 (6400–11,600) |
| Slovakia | 1.04% (0.6%–1.4%) | 3400 (2000–4700) | 2.13% (1.6%–2.8%) | 6900 (5200–9200) |
| Slovenia | 2.17% (1.2%–3.0%) | 6800 (3800–9400) | 1.14% (1.0%–1.5%) | 3600 (3100–4800) |
| Spain | 1.75% (1.1%–3.4%) | 155,000 (94,200–298,000) | 1.21% (0.9%–1.7%) | 108,000 (78,900–155,000) |
| Sweden | 3.30% (1.8%–4.8%) | 75,000 (40,700–109,000) | 1.42% (1.0%–2.8%) | 32,300 (22,400–62,700) |
| EU total | 2.73% (1.6%–4.2%) | 1,727,000 (1,036,000–2,655,000) | 1.63% (1.2%–2.5%) | 1,033,000 (757,000–1,559,000) |
HBV—HBsAg+, HCV—anti-HCV+.
The highest prevalence of HBV among migrants, 5.47% (UI: 4.0–7.0%), was estimated to be in Portugal, but the largest absolute number of migrants living with HBV was in Germany, 449,000 (UI: 251,000–667,000) (Fig. 1, Table 1). For HCV, the highest prevalence among migrants, 3.23% (UI: 2.4–4.0%), was estimated to be in Lithuania, while the largest absolute number of migrants living with anti-HCV was in Germany, 308,000 (UI: 222,000–467,000) (Fig. 1, Table 1).
Fig. 1.
Prevalence of HBV and HCV among immigrants in the EU-27 in 2024. HBV—HBsAg+, HCV—anti-HCV+. Microsoft Excel for Mac Version 16.99.2 screen shots reprinted with permission from Microsoft Corporation, Powered by Bing ©GeoNames, Microsoft, OpenStreetMap, TomTom.
There was great heterogeneity in the leading country of birth for HBV in the EU-27. Seven countries, (Austria, Bulgaria, Cyprus, Denmark, Germany, the Netherlands, and Sweden), had Syria as the country of birth with the greatest absolute number of migrants living with HBV while three had Romania (Belgium, Hungary, and Spain) and three had Ukraine (Czechia, Poland, and Slovak Republic) (Appendix, S6). There was less heterogeneity in the leading country of birth for HCV in the EU-27 with sixteen countries, (Austria, Belgium, Bulgaria, Czechia, Denmark, Finland, Germany, Hungary, Ireland, Italy, Lithuania, the Netherlands, Poland, Portugal, Romania, and Slovak Republic), having Ukraine as the country of birth with the greatest absolute number of migrants living with HCV (Appendix, S7).
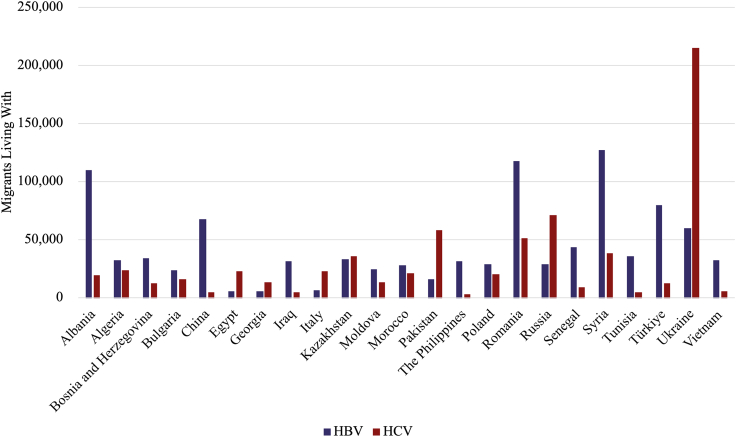
Twenty-three countries of birth represented 58% of migrants living with HBV, and 68% of migrants living with HCV in the EU-27 (Fig. 2). The leading countries of birth for migrants living with HBV in the EU-27 were Syria, Romania, and Albania (Fig. 2 and Fig. 3a). The prevalence of HBV among migrants from Sub-Saharan Africa was estimated to be 8.8% (UI: 5.6–13.2%), while it was 3.2% (UI: 1.6–4.5%) for those born in West Asia & North Africa (Appendix, S5). East and South East Asia & Oceania had the second highest pooled prevalence (5.7% (UI: 4.1–8.2%)) (Appendix, S5). However, as there were fewer migrants from this region, they were fourth in terms of migrants living with HBV (Fig. 3a).
Fig. 2.
Top 23 countries of birth for migrants living with HBV or HCV in the EU-27, 2024. HBV—HBsAg+, HCV—anti-HCV+.
Fig. 3.
a. Prevalence of HBV among immigrants in the EU-27 by region and country of birth in 2024. HBV—HBsAg+. Sub-Saharan Africa: SEN-Senegal, OTH-Other Countries, CIV-Côte d'Ivoire, AGO-Angola, NGA-Nigeria, GIN-Guinea, MLI-Mali, GHA-Ghana, CMR-Cameroon, CPV-Cabo Verde, SOM-Somalia, ERI-Eritrea, COM-Comoros, ETH-Ethiopia, GNB-Guinea-Bissau, MDG-Madagascar, MOZ-Mozambique, ZAF-South Africa, TGO-Togo, COG-Congo, Republic of the, COD-Democratic Republic of Congo, M …-Mauritania, GMB-Gambia, MUS-Mauritius. West Asia & North Africa: SYR-Syria, TUR-Türkiye, TUN-Tunisia, DZA-Algeria, IRQ-Iraq, MAR-Morocco, OTH-Other Countries, GEO-Georgia, EGY-Egypt, LBN-Lebanon, SDN-Sudan, A …-Armenia. Eastern Europe: ROU-Romania, UKR-Ukraine, POL-Poland, RUS-Russia, MDA-Moldova, BGR-Bulgaria, OTH-Other Countries, BLR-Belarus. Southern Europe: ALB-Albania, BIH-Bosnia and Herzegovina, SRB-Serbia, PRT-Portugal, GRC-Greece, ITA-Italy, MKD-North Macedonia, OTH-Other Countries, ESP-Spain. East and South East Asia & Oceania: CHN-China Mainland, VNM-Vietnam, PHL-The Philippines, IDN-Indonesia, OTH-Other Countries, THA-Thailand, KOR-Republic of Korea, KH …-Cambodia, M …-Mongolia. Central and Southern Asia: KAZ-Kazakhstan, IND-India, AFG-Afghanistan, BGD-Bangladesh, PAK-Pakistan, IRN-Iran, KGZ-Kyrgyzstan, LKA-Sri Lanka, TJK-Tajikistan, UZB-Uzbekistan, OTH-Other Countries. Americas: VEN-Venezuela, OTH-Other Countries, COL-Colombia, DOM-Dominican Republic, SUR-Suriname, BRA-Brazil, ECU-Ecuador. Northern Europe: GBR-United Kingdom, OTH-Other Countries. Western Europe: OTH-Other Countries, CHE-Switzerland, DEU-Germany, FRA-France. b. Prevalence of HCV among immigrants in the EU-27 by region and country of birth in 2024. HCV—anti-HCV+. Eastern Europe: UKR-Ukraine, RUS-Russia, ROU-Romania, POL-Poland, BGR-Bulgaria, MDA-Moldova, BLR-Belarus, CZE-Czechia, HUN-Hungary, S …-Slovakia. West Asia & North Africa: SYR-Syria, DZA-Algeria, EGY-Egypt, MAR-Morocco, GEO-Georgia, TUR-Türkiye, IRQ-Iraq, ARM-Armenia, TUN-Tunisia, OTH-Other Countries, AZE-Azerbaijan, SDN-Sudan, LBY-Libya. Central and Southern Asia: PAK-Pakistan, KAZ-Kazakhstan, IND-India, AFG-Afghanistan, UZB-Uzbekistan, KGZ-Kyrgyzstan, BGD-Bangladesh, LKA-Sri Lanka, TJK-Tajikistan, IRN-Iran, Blank-Other Countries. Southern Europe: ALB-Albania, BIH-Bosnia and Herzegovina, PRT-Portugal, SRB-Serbia, OTH-Other Countries, GRC-Greece, HRV-Croatia. Americas: USA-United States, COL-Colombia, VEN-Venezuela, OTH-Other Countries, ECU-Ecuador, ARG-Argentina, BRA-Brazil, DOM-Dominican Republic, HND-Honduras, CUB-Cuba, SUR-Suriname, MEX-Mexico, C …-Canada, PER-Peru. Sub-Saharan Africa: OTH-Other Countries, SEN-Senegal, CMR-Cameroon, COM-Comoros, GHA-Ghana, CIV-Côte d'Ivoire, SOM-Somalia, ZAF-South Africa, GAB-Gabon, ERI-Eritrea, NGA-Nigeria, MLI-Mali, AGO-Angola, GIN-Guinea, COD-Democratic Republic of Congo, CPV-Cabo Verde, MDG-Madagascar, BDI-Burundi, ETH-Ethiopia, GMB-Gambia. East and South East Asia & Oceania: VNM-Vietnam, CHN-China Mainland, OTH-Other Countries, PHL-The Philippines, MNG-Mongolia, THA-Thailand, KHM-Cambodia, AUS-Australia, IDN-Indonesia. Western Europe: DEU-Germany, FRA-France, OTH-Other Countries, CHE-Switzerland. Northern Europe: GBR-United Kingdom, OTH-Other Countries, LTU-Lithuania, EST-Estonia.
The leading countries of birth for migrants living with HCV in the EU-27 were Ukraine, Russia, and Pakistan (Fig. 3b). The highest prevalence among migrants living with HCV, were among those born in Central and Southern Asia, 2.9% (UI: 2.3–4.0%), with the second highest being among those born in Eastern Europe, 2.5% (UI: 1.9–3.2%) (Appendix, S5). The prevalence among those born in West Asia & North Africa was estimated to be 1.3% (0.8–2.6%), but due to the large number of migrants from this region, they are second in absolute number of cases (Fig. 3b).
Country level migrant prevalence estimates, including the number of people living with HBV and HCV (Table S6 and S7) by country of destination and birth, can be found in the Appendix as well as at www.cdafound.org/polaris/migration/. Countries for birth with less than 50 infections estimated were excluded in these tables as to not contribute to further stigma.
Ukraine Refugee Situation
Utilizing the UN international migrant stock dataset, it was found that in 2024 there were 3.67 million more Ukrainian migrants living in the EU-27 when compared to 2020. While Germany (1,147,000), Poland (923,000), Czechia (359,000), Romania (167,000), and Italy (153,000) have seen the largest absolute increase in Ukrainian migrants since 2020 (Table 2); Poland (53%), Czechia (35%), Slovak Republic (34%), Romania (25%), and Bulgaria (17%) have the largest share of total 2024 migrants among Ukrainian migrants since 2020. Latvia was found to have fewer Ukrainian migrants in 2024 compared to 2020, 2600 (Table 2).
Table 2.
The impact of Ukrainian Refugee Situation.
| Country | Change in HBV infections among Ukrainian migrants | Change in HCV infections among Ukrainian migrants | Change in Ukrainian migrants |
|---|---|---|---|
| Austria | 1100 (710–1500) | 3400 (2800–4500) | 85,000 |
| Belgium | 1000 (690–1500) | 3600 (2900–4700) | 79,500 |
| Bulgaria | 490 (330–690) | 1700 (1400–2200) | 49,800 |
| Croatia | 310 (210–440) | 1200 (1000–1600) | 24,800 |
| Cyprus | 3 (2–4) | 16 (13–20) | 200 |
| Czechia | 5100 (3400–7200) | 17,400 (14,200–22,900) | 359,000 |
| Denmark | 410 (280–590) | 1400 (1100–1800) | 31,200 |
| Estonia | 6 (4–8) | 60 (50–80) | 460 |
| Finland | 810 (540–1100) | 2600 (2100–3400) | 60,500 |
| France | 830 (550–1200) | 3700 (3000–4900) | 67,300 |
| Germany | 14,400 (9600–20,400) | 51,900 (42,100–68,100) | 1,147,000 |
| Greece | 16 (11–20) | 80 (60–100) | 1200 |
| Hungary | 160 (110–230) | 720 (580–940) | 12,700 |
| Ireland | 1400 (930–2000) | 4600 (3700–6000) | 114,000 |
| Italy | 2100 (1400–2900) | 6600 (5400–8700) | 153,000 |
| Latvia | 30 (40–20) | 100 (130–80) | 2600 |
| Lithuania | 290 (200–410) | 1300 (1000–1700) | 23,300 |
| Luxembourg | 3 (2–4) | 10 (10–20) | 230 |
| Malta | 9 (6–12) | 30 (20–40) | 640 |
| Netherlands | 1400 (970–2100) | 5100 (4100–6700) | 112,000 |
| Poland | 8600 (5700–12,100) | 33,800 (27,500–44,400) | 923,000 |
| Portugal | 90 (60–130) | 430 (350–570) | 6700 |
| Romania | 1600 (1000–2200) | 3800 (3100–5000) | 167,000 |
| Slovakia | 1200 (790–1700) | 4600 (3700–6000) | 111,000 |
| Slovenia | 5 (3–7) | 20 (18–30) | 380 |
| Spain | 1400 (950–2000) | 4900 (4000–6500) | 107,000 |
| Sweden | 410 (270–570) | 1300 (1100–1700) | 33,800 |
| EU total | 43,000 (28,700–60,900) | 154,000 (125,000–202,000) | 3,667,000 |
HBV—HBsAg+, HCV—anti-HCV+.
We estimate that the Ukraine Refugee Situation resulted in an additional 43,000 (UI: 28,700–60,900) people living with HBV and an additional 154,000 (UI: 125,000–202,000) people living with HCV in the EU-27 (Table 2). The impact of the Ukraine Refugee Situation in the EU-27 has been much more pronounced with HCV when compared to HBV. The change in the Ukrainian migrant stock between 2020 and 2024 alone make up 15% of the total estimated number of migrant cases of HCV while only 2.5% of HBV migrant cases.
Discussion
The analysis presented in this paper leveraged robust modeling (e.g., PRoGReSs and Bright models) combined with extensive data from the UN database. These methods enabled age- and sex-specific prevalence estimates and accounted for variations in migration patterns, vaccination histories, and healthcare access in countries of birth.
The 2016 European Centre for Disease Prevention and Control (ECDC) epidemiological assessment provided critical insights into the prevalence and burden of hepatitis in these communities.2 However, migration trends, reasons for migration, and pathways have undergone significant shifts driven by geopolitical crises, economic changes, and dynamic migration policies. These shifts directly impact the demographic composition and health needs of migrant populations, highlighting the importance of having transparent, up-to-date, granular data.
A recent systematic review and meta-analysis estimated an anti-HCV prevalence of 1.5% (95% CI: 1.1–2.0%) among migrants. While this review included empirical studies from Australia, Canada, Switzerland, the UK, and the US along with EU-27 member states it is quite similar to the results of our modeling exercise, 1.63% (UI: 1.2–2.5%), in the EU-27.18 This study also provides evidence of the great heterogeneity in migrant population as the anti-HCV prevalence estimates for migrants in Italy ranged from 0.2 to 0.4.5%.
A review of HBV among migrants found that the prevalence in Western Europe among migrants from Sub-Saharan Africa ranged from 3.7 to 15%, comparable to the 8.8% (UI: 5.6–13.2%) that the current study found at the EU-27 level.19 However, this same study found HBV prevalence of 6.94–36.7% among migrants from Eastern Europe, while our study reported a prevalence of 1.9% (UI: 1.2–2.5%). Similarly, a study from the Netherlands found prevalence among Chinese migrants of 8.7% while our study reported an estimated a prevalence of 6.6% (UI: 5.7–7.4%) among migrants from China living in the Netherlands.
A recent ECDC systematic review found HBV prevalence among migrants of 3.51% in Belgium compared to 2.59% (UI: 1.6–3.9%) in the current study, 6.52% in Denmark vs 2.46% (1.4–3.8%), 1.40% in Finland vs 2.23% (UI: 1.4–3.2%), 5.52% in France vs 3.28% (2.1–5.0%), 3.75% in Germany vs 2.68% (UI: 1.5–4.0%), 5.00% in Greece vs 4.74% (UI: 2.7–7.5%), 10.94% in Italy vs 3.77% (UI: 2.4–6.0%), 6.20% in Malta vs 2.02% (UI: 1.2–3.2%), 4.06% in the Netherlands vs 2.92% (UI: 1.8–4.2%), 12.24% in Spain vs 1.75% (UI: 1.1–3.4%), and 1.59% in Sweden vs 3.30% (UI: 1.8–4.8%).20 In only two of the eleven countries, Finland and Sweden were our base estimates higher than the estimates presented in this review. There is a high possibility that these higher estimates found in the literature are primarily due to selection bias in the migrant groups studied, which likely have high prevalences and may not be representative of the migrant population as a whole.
A study from Italy that examined the prevalence of HBV and HCV among refugees and undocumented migrants generally reported higher prevalences, than reported in the current work.21 Migrants from sub-Saharan Africa showed an HBV prevalence of 14% vs 8.9% in the current study, Eastern Europe showed 6.1% vs 1.9%. The Italian literature estimate for migrants from Northern Africa was lower in HBV 2.5% vs than the current study estimate of 3.2% while the “India–Pakistan” area was comparable for HBV 3.2% vs 3.5%. However, our regional estimates combine North Africa and West Asia. Migrants from sub-Saharan Africa showed an HCV prevalence of 3.8% vs the current estimate of 2.4%, Eastern Europe showed 6.1% vs 2.5%, “India–Pakistan” area 7.1% vs 2.9%, and Northern Africa 2.5% HCV vs 1.3%. Our regional estimates include all of Central and South Asia in the estimate compared to “India–Pakistan” area.
In 2024, it was estimated that there were 2.7 million (UI: 1.9–3.5 million) HBV and 3.2 million (UI: 2.8–3.7 million) anti-HCV infections in the EU-27.22 While there is a great deal of heterogeneity in the base prevalence estimates for countries within the EU-27, if they capture some, none, or all of the migrants living with viral hepatitis in the country, we can estimate that 64% (UI: 53–76%) of the people living with HBV, and 33% (UI: 27–42%) of those living with HCV are among migrants in the EU-27.22 These shares will vary greatly by country and require further analysis of the baseline prevalence estimates to ensure that the migrant populations are being properly accounted for. This population often faces structural and legal barriers to accessing healthcare. They require cultural and linguistic competence from those carrying out the screening and linkage to care. Identified barriers for migrants accessing viral hepatitis care are low levels of knowledge and health literacy, which may be amplified if language barriers exist.23,24 Working with community-based organizations and in community spaces can aid in bridging gaps in healthcare access but also must acknowledge the vast diversity of migrant communities in the EU.23 Strategies must be adapted to the local contexts, epidemiology, and realities of the migrant communities they serve. One example from Spain reported a high linkage to care among sub-Saharan African migrants screened for HBV in community settings and included community health workers.10
While Portugal can focus HBV efforts on migrants from Angola, Cabo Verde and Mozambique, Sweden will need to focus on migrants from Syria, Iraq and Somalia (Appendix). For HCV, Portugal will need to add Ukrainians to their focus communities, and Sweden will need to add Ukrainians and Pakistanis (Appendix). There are also subnational considerations; the country-of-birth composition of the migrant population in one region of a country may be significantly different than that in another region.25
Ukraine had an HBsAg prevalence of 1.3% in 2024, compared to 0.6% in the EU-27. In contrast, the HCV estimated prevalence in Ukraine in 2024 was 4.6% compared to 0.7% in the EU-27.22 This large difference in prevalence combined with the large absolute number of migrants is why they contribute much more substantially to the overall migrant prevalence in specific countries and the EU-27. The addition of tens of thousands of HBV (43,000 cases) and HCV (154,000 HCV cases) cases due to the Ukrainian refugee crisis, underscores the importance of population dynamics, including migration and displacement, to public health.
While limited by the available databases, it is important to distinguish irregular migrants and short-term or temporary migration from long-term or permanent migration. A recent study found that the prevalence of HBV among unaccompanied minors and undocumented pregnant women were significantly higher than the other groups of migrants examined in the study.26 European countries vary significantly in the amount and type of healthcare to which migrants are legally entitled, and few countries have national guidance on the testing of migrants for viral hepatitis. Despite close to universal population coverage in the EU, in some European countries, specific categories of migrants (i.e., without documentation or insurance) are not legally entitled to use public healthcare services.27 In many countries, there are restrictions on providing antiviral therapy to irregular migrants and those without health insurance. Additionally, migrants who are aware of their liver disease may not be linked to care in the country of destination or may be lost along the care cascade.28 The cascade of care is impacted at every point as many migrants are not being referred, or even if they are referred do not make it to specialist appointments. Frequently if not currently eligible for treatment, they are told that they are healthy and thus often stop attending HBV monitoring visits. Migrants may avoid seeking medical care for fear of being deported or losing precarious jobs. This issue is further complicated by the stigma of living with viral hepatitis. While one study showed evidence that migrants that have been living in a country longer tend to have better healthcare access a different Canadian study found that when there are screening programs newly arrived migrants can have higher rates across the cascade of care of HBV when compared to long term residents.29,30
Migrants living in the European region for a long time that are living with chronic HCV infection not caused by drug use are more likely to have advanced liver disease and hepatocellular carcinoma compared with non-migrants at the time of HCV diagnosis.12 This also holds true also for HBV infection and is likely due to missed or delayed diagnoses and possibly to infection at an earlier age.12,31
While the decision makers in countries are often concerned about the costs of treating migrants, a review has found that combination of screening of high-risk children and adults for HBV, vaccinating susceptible children, and linking chronic hepatitis B infected migrants to care are cost-effective interventions.32 Similarly, a review found that screening and treatment of HCV among migrants in the EU/EEA is effective and cost-effective, especially as prices have decreased since 2015.33
While the ECDC has set 2% prevalence in the country of birth as the thresholds for migrants to be screened for HBV and HCV, a study from the Netherlands found screening and treatment were cost-effective if the HBV and HCV prevalences were ≥0.41% and ≥0.22% respectively.34,35 Utilizing these cost-effectiveness thresholds in the current study would result in 99.2% of migrants living with HBV in the EU-27 and 99.8% living with HCV being cost-effective to screen and treat. Needless to say, the current study provides evidence that we should screen and treat all migrants in the EU-27, and based on previous literature that this approach would be cost-effective. Studies in these populations will also reduce the uncertainty that is reflected in our analysis.
This analysis has several limitations, with the biggest one being the uncertainty surrounding the number of migrants. The UN data would include at minimum of the irregular migrant population as it is based on the foreign-born population estimates.16 The Pew Research Center and the Clandestino project used the residual method to estimate the total number of unauthorized migrants in a country.36,37 This method subtracts the total number of authorized immigrants in a country from the total number of foreign-born reported via census in the country. In the Pew Research Center analysis, this methodology accounted for 60% of all estimated unauthorized migrants.36 Based on these previous analyses on undocumented migrants, the 2024 data presented in this manuscript would be missing 1.68–2.06 million migrants or 2.8–3.5% of the total migrant population in the EU-27. These populations are likely at higher risk for viral hepatitis and stigma and more likely to face barriers when accessing healthcare services. Additionally, some of these migrants move quite often and, for a variety of reasons, may not want to be registered. Thus, the estimates presented here may underestimate the actual burden among the migrant community in the EU-27, as approximately 2 million individuals at the highest risk of having viral hepatitis were excluded in the estimates presented in this analysis.
Another limitation of the current study is that we do not include emigration. This is why the UN 2024 migrant stock was chosen for the analysis, as there is much movement within the EU-27 as well as into and out of the borders annually, which becomes much more difficult to model and quantify.
While the age and sex distribution of migrants was available by country of destination, there may be significant variances in the age and sex distribution by country of birth. This could cause the estimates presented here to be higher or lower than the actual situation. However, these uncertainties derived by some estimations based on the country of residence age and sex distribution could be balanced by the assumption that the prevalence in the migrants is the same as the country of birth, which could overestimate the viral hepatitis burden.
There are also limitations inherent in the modeling. The biggest source of uncertainty in either model is the baseline prevalence estimates. While the authors sourced wide ranges for the prevalence estimates and scored the studies as a method to show our confidence in the underlying data, there is still a great deal of uncertainty.14,15 There is also the issue of availability of data, for HCV in particular there is a paucity of high-quality data available in sub-Saharan Africa. Neither model includes the impact of co-infections, which have been shown to greatly accelerate disease progression, which in the long run would decrease the prevalence estimates. Although we have utilized the highest quality studies to inform our progression rates, they may not be representative of all populations. While we update the models annually, there is the possibility that we would miss emerging trends, such the emergence and immediate impact of COVID-19.
The analysis of the Ukrainian Refugee Situation has its limitations. The difference between the Ukrainian migrant stock by EU-27 county in 2024 and 2020 is used as a proxy for the impact of the Ukrainian Refugee Situation. While the majority of the difference here would result from the Ukrainian Refugee Situation, there were, of course, Ukrainians migrating before 2022. We applied the age and sex distribution of migrants by country of destination; however it has been previously shown that there are more women, youth, and elders in the recent Ukrainian migrants.38 This would cause the estimates presented here to overestimate the burden, specifically of HCV, among Ukrainian migrants. These migrants also face rapidly shifting realities as the Ukrainian Refugee Situation database shows there is significant movement of Ukrainian refugees weekly making final or definitive numbers challenging to quantify.39
In conclusion, the estimated burden of viral hepatitis in permanent longstanding migrants and the newcomers in the EU-27 for each country of birth is significant and these population are necessary to prioritize as countries work towards the elimination of HCV and HBV thus preventing liver cancer development. Unfortunately, data globally as well as among migrants on the prevalence of hepatitis delta remains scant. This is a particularly important topic for future research as the disease causes a significantly faster progression than HBV alone.40 Furthermore, genomic sequencing of HBV, HDV, and HCV could aid in tracing the modes and rates of transmission as well as the spread of particular lineages of those viruses.
While many countries have implemented free screening and treatment programs for Ukrainian refugees, programs for other migrants or even citizens are piecemeal and anything but universal.41 Health is a fundamental right of every human being, regardless of whether they were born in the country they reside in or even if they came to the country via unauthorized pathways. They should be considered by public health agencies, universities, and community organizations to test methodologies that work best for each migrant community in the destination country to screen and link communities to care. Access to care should not be determined by citizenship status nor by the country of origin of migrants. For the EU to succeed in eliminating viral hepatitis as a public health threat, vaccination, screening, and treatment must be made readily available to everyone within their borders.
Contributors
DMR-S conceived the study. DMR-S designed the methodology and was responsible for project administration. DMR-S and HR supervised the study. DMR-S and SH did the formal analysis. HR acquired funding. DMR-S, SH, and KR-S were responsible for data visualization. DMR-S and LK wrote the original draft. DMR-S, SH, ASV, HN, IG, HR, and KR-S had access to the underlying data and models. DMR-S, SH, ASV, HN, IG, HR, and KR-S accessed and verified the data. All authors curated data. All authors validated data. All authors reviewed and edited the manuscript. The authors were not precluded from fully accessing the data in the study, and they accept responsibility to submit for publication.
Data sharing statement
Country level migrant prevalence estimates, including the number of people living with HBV and HCV by country of destination and birth, will be publicly available indefinitely on www.cdafound.org/polaris/migration/ with publication. Countries for birth with less than 50 infections estimated were excluded in these tables as to not contribute to further stigma.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps, text, tables, and institutional affiliations.
Declaration of AI or AI-assisted technologies in the writing process statement
During the preparation of this work, the authors did not use any AI and AI-assisted technologies and will take full responsibility for the content of the publication.
Declaration of interests
DMR-S, SH, IG, HN, KR-S, ASV, and HR are all employees of Center for Disease Analysis Foundation. LK has received consulting fees from AbbVie. MB has received consulting fees from Gilead, AbbVie, and GSK. FN has been a consultant for Gilead and AbbVie. CAP reports consultancy fees from Roche Diagnostics and GSK. MCMN and AO have nothing to disclose.
Acknowledgements
The development of this manuscript was made possible by a research grant from the John C. Martin Foundation. The authors thank the Polaris Observatory Collaborators who provided inputs for individual country models used in this analysis. Without their contributions and review of country model outputs, this work would not have been possible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2025.101452.
Appendix A. Supplementary data
References
- 1.WHO Regional Office for Europe The health of refugees and migrants in the WHO European Region. 2023. https://www.who.int/europe/news-room/fact-sheets/item/the-health-of-refugees-and-migrants-in-the-who-european-region
- 2.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2016. Epidemiological Assessment of Hepatitis B and C Among Migrants in the EU/EEA. [Google Scholar]
- 3.Falla A.M., Ahmad A.A., Duffell E., Noori T., Veldhuijzen I.K. Estimating the scale of chronic hepatitis C virus infection in the EU/EEA: a focus on migrants from anti-HCV endemic countries. BMC Infect Dis. 2018;18(1):42. doi: 10.1186/s12879-017-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negro F., Mullhaupt B., Semela D., et al. The current and future burden of hepatitis B in Switzerland: a modelling study. Swiss Med Wkly. 2023;153 doi: 10.57187/smw.2023.40086. [DOI] [PubMed] [Google Scholar]
- 5.Razavi-Shearer D., Gamkrelidze I., Pan C.Q., et al. The impact of immigration on hepatitis B burden in the United States: a modelling study. Lancet Reg Health Am. 2023;22 doi: 10.1016/j.lana.2023.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuenca-Gomez J.A., Salas-Coronas J., Soriano-Perez M.J., Vazquez-Villegas J., Lozano-Serrano A.B., Cabezas-Fernandez M.T. Viral hepatitis and immigration: a challenge for the healthcare system. Rev Clin Esp (Barc) 2016;216(5):248–252. doi: 10.1016/j.rce.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Koc O.M., Kremer C., Bielen R., et al. Prevalence and risk factors of hepatitis B virus infection in Middle-Limburg Belgium, year 2017: importance of migration. J Med Virol. 2019;91(8):1479–1488. doi: 10.1002/jmv.25457. [DOI] [PubMed] [Google Scholar]
- 8.Duberg A.S., Lybeck C., Falt A., Montgomery S., Aleman S. Chronic hepatitis B virus infection and the risk of hepatocellular carcinoma by age and country of origin in people living in Sweden: a national register study. Hepatol Commun. 2022;6(9):2418–2430. doi: 10.1002/hep4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsen T.H., Sheron N., Zelber-Sagi S., et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 10.Picchio C.A., Nomah D.K., Rando-Segura A., et al. Community-based screening enhances hepatitis B virus linkage to care among West African migrants in Spain. Commun Med (Lond) 2023;3(1):182. doi: 10.1038/s43856-023-00420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2024. Monitoring of the Responses to the Hepatitis B and C Epidemics in EU/EEA Countries, 2023. [Google Scholar]
- 12.Kondili L.A., Craxi A. Migrants and hepatitis: a tale of two worlds. J Viral Hepat. 2023;30(8):634–637. doi: 10.1111/jvh.13866. [DOI] [PubMed] [Google Scholar]
- 13.WHO Regional Office for Europe . Action Plan for Refugee and Migrant Health in the WHO European Region 2023–2030. WHO Regional Office for Europe; Copenhagen: 2023. [Google Scholar]
- 14.Polaris Observatory Collaborators Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;8(10):879–907. doi: 10.1016/S2468-1253(23)00197-8. [DOI] [PubMed] [Google Scholar]
- 15.Blach S., Terrault N.A., Tacke F., et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 16.United Nations Department of Economic and Social Affairs PD . International Migrant Stock 2024. United Nations; New York, USA: 2025. [Google Scholar]
- 17.United Nations Department of Economic and Social Affairs PD . International Migrant Stock 2020. United Nations; New York, USA: 2020. [Google Scholar]
- 18.Sun J., Kelly M., Tsheten T., Pourmarzi D. Prevalence of hepatitis C among migrants: a systematic review and meta-analysis. J Viral Hepat. 2025;32(4) doi: 10.1111/jvh.70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppola N., Alessio L., Pisaturo M., et al. Hepatitis B virus infection in immigrant populations. World J Hepatol. 2015;7(30):2955–2961. doi: 10.4254/wjh.v7.i30.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bivegete S., McNaughton A.L., Trickey A., et al. Estimates of hepatitis B virus prevalence among general population and key risk groups in EU/EEA/UK countries: a systematic review. Euro Surveill. 2023;28(30) doi: 10.2807/1560-7917.ES.2023.28.30.2200738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppola N., Alessio L., Gualdieri L., et al. Hepatitis B virus, hepatitis C virus and human immunodeficiency virus infection in undocumented migrants and refugees in southern Italy, January 2012 to June 2013. Euro Surveill. 2015;20(35) doi: 10.2807/1560-7917.ES.2015.20.35.30009. [DOI] [PubMed] [Google Scholar]
- 22.Center for Disease Analysis Foundation . European Union Regional Dashboard. Center for Disease Analysis Foundation; Lafayette, CO, USA: 2025. [Google Scholar]
- 23.Lebano A., Hamed S., Bradby H., et al. Migrants' and refugees' health status and healthcare in Europe: a scoping literature review. BMC Public Health. 2020;20(1):1039. doi: 10.1186/s12889-020-08749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moonen C.P.B., den Heijer C.D.J., Dukers-Muijrers N., van Dreumel R., Steins S.C.J., Hoebe C. A systematic review of barriers and facilitators for hepatitis B and C screening among migrants in the EU/EEA region. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heider B., Stroms P., Koch J., Siedentop S. Where do immigrants move in Germany? The role of international migration in regional disparities in population development. Popul Space Place. 2020;26(e2363):1–19. [Google Scholar]
- 26.Hobart C., Pescarini J.M., Evans L., et al. Hepatitis B infection and immunity in migrant children and pregnant persons in Europe: a systematic review and meta-analysis. J Travel Med. 2024;31(6) doi: 10.1093/jtm/taae094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palm W., Webb E., Hernandez-Quevedo C., et al. Gaps in coverage and access in the European Union. Health Policy. 2021;125(3):341–350. doi: 10.1016/j.healthpol.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 28.EASL EASL policy statement addressing the liver health needs of migrant populations in Europe. 2024. https://easl.eu/wp-content/uploads/2024/06/EASL-Policy-Statement-Migrant-Health-Policy.pdf
- 29.Lebrun L.A. Effects of length of stay and language proficiency on health care experiences among immigrants in Canada and the United States. Soc Sci Med. 2012;74(7):1062–1072. doi: 10.1016/j.socscimed.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Yasseen A.S., Kwong J.C., Feld J.J., et al. The viral hepatitis B care cascade: a population-based comparison of immigrant groups. Hepatology. 2022;75(3):673–689. doi: 10.1002/hep.32162. [DOI] [PubMed] [Google Scholar]
- 31.Picchio C.A., Lens S., Hernandez-Guerra M., et al. Late presentation of chronic HBV and HCV patients seeking first time specialist care in Spain: a 2-year registry review. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-01885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myran D.T., Morton R., Biggs B.A., et al. The effectiveness and cost-effectiveness of screening for and vaccination against hepatitis B virus among migrants in the EU/EEA: a systematic review. Int J Environ Res Public Health. 2018;15(9) doi: 10.3390/ijerph15091898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenaway C., Makarenko I., Chakra C.N.A., et al. The effectiveness and cost-effectiveness of hepatitis C screening for migrants in the EU/EEA: a systematic review. Int J Environ Res Public Health. 2018;15(9) doi: 10.3390/ijerph15092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suijkerbuijk A.W.M., van Hoek A.J., Koopsen J., et al. Cost-effectiveness of screening for chronic hepatitis B and C among migrant populations in a low endemic country. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2018. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA. [Google Scholar]
- 36.Pew Research Center . Pew Research Center; Washington D.C.: 2019. Europe’s Unauthorized Immigrant Population Peaks in 2016, Then Levels Off. [Google Scholar]
- 37.Database on Irregular Migration. CLANDESTINO. www.irregular-migration.net Brussels, Belgium.
- 38.Parente P., Melnyk A., Lombardo P., et al. Demographic and epidemiological characteristics of Ukrainian refugees in an Italian Local Health Authority. Eur J Public Health. 2023;33(5):815–820. doi: 10.1093/eurpub/ckad130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.UNHCR Ukraine Refugee Situation. https://data.unhcr.org/en/situations/ukraine Geneva: UNHRC Europe.
- 40.Negro F., Lok A.S. Hepatitis D: a review. JAMA. 2023;330(24):2376–2387. doi: 10.1001/jama.2023.23242. [DOI] [PubMed] [Google Scholar]
- 41.Naveira M. In: Addressing Viral Hepatitis Among Europe’s Migrant and Refugee Population: Lessons Learnt and the Way Forward. Viral Hepatitis Prevention Board, editor. Viral Hepatitis Prevention Board; Antwerp, Belgium: 2024. Access to viral hepatitis services by refugees from Ukraine in the WHO European Region. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.