ABSTRACT
The knowledge on hazards and risks connected to human exposure to engineered nanomaterials (ENMs) is still very limited, despite several decades of research and regulatory efforts at the international level. In particular, sex/gender‐related responses to such exposure have not been clearly articulated so far in any of the existing guidance documents or regulatory relevant opinions provided to the parties involved in the risk assessment and risk management of ENMs. We aimed to demonstrate the relevance of the sex/gender dimension for the characterization of the risks and hazards associated with ENMs by analyzing existing scientific data on sex‐related differences in response to ENMs exposure. This was achieved by performing an extensive review of in vivo mammalian toxicity studies published in PubMed and Web of Science databases. Further analysis was performed only for data reported in publications that satisfied scientific quality criteria assessed using the GUIDEnano approach. Finally, we demonstrated the importance of the sex/gender dimension for safety testing of ENMs in the future and provided recommendations on how to include the sex/gender dimension in toxicity testing of ENMs to ensure precise, transparent, and reliable conclusions in the process of hazard and risk assessments.
This article is categorized under:
Toxicology and Regulatory Issues in Nanomedicine > Toxicology of Nanomaterials
Toxicology and Regulatory Issues in Nanomedicine > Regulatory and Policy Issues in Nanomedicine
Sex differences are important in the context of nanomaterials risk and hazard assessment, and all observed differences in response in animals/humans of each sex/gender should be reported.

1. Introduction
1.1. Regulatory Policies and Guidelines on Inclusion of Sex‐Related Human Risk Assessment
Development and implementation of the sex/gender dimension in Research and Innovation (R&I) activities started in the 1960s with the Women's Health Movement in the US and the UK. In the US, the Equal Pay Act was established in 1963, the Civil Rights Act in 1964, Executive Order 11,246 including affirmative action applied to women in 1967, and the Equal Employment Opportunity Act was implemented in 1972. These documents prohibited discrimination by employers and labor unions on the basis of sex. Regulatory activities of the EU started more than a decade later with the European Council Equal Pay Directive in 1975, giving rise to legal, regulatory, and administrative means of ensuring equal pay for equal work, and with the European Council Equal Treatment Directive in 1976 to establish the equal treatment of women and men. Due to these changes, over the next decades, regulation on sex/gender equity evolved in many sectors, including R&I (Duffy et al. 2020; White et al. 2021).
Government‐based funding agencies such as the European Commission (EC), the US National Institutes of Health (NIH), the Food and Drug Administration (FDA), and the Canadian Institutes of Health Research (CIHR) played a crucial role in the implementation of policies and mechanisms to improve sex and gender integration into research (Duffy et al. 2020; White et al. 2021). In 1985, the US Public Health Service Task Force (US PHTSF) issued a report that discussed the detrimental effects of excluding women from/as subjects of clinical research (Women's Health. Report of the Public Health Service Task Force on Women's Health Issues 1985), and the year after, the NIH released a policy that recommended inclusion of women as the subjects of clinical research (NIH 1986). The FDA also provided two guidance documents, in 1987 and 1988, which endorsed the inclusion of animals of both sexes in preclinical studies (FDA 1987) and recommended sex‐specific safety and efficacy studies during drug clinical trials (FDA 1988). However, two reports from the 1990s issued by the US Government Accountability Office (GAO) concluded that NIH guidelines were not being followed, such that women were still severely underrepresented in clinical research and that sex‐specific analyses was provided in less than 50% of drug trials (U.S. Government Accountability Office 1990, 1992). This resulted in regulations that required inclusion of females as subjects in all NIH‐funded clinical research (NIH 1993, 1994), as well as an FDA notice in 1993 (FDA 1993a) and an FDA regulation in 1994 (FDA 1998) with a requirement for reporting sex‐specific safety and efficacy results in New Drug Applications. The implementation of these regulations was slow and between 1997 and 2000 eight out of ten withdrawals of already registered drugs by the FDA were due to unexpected side‐effects or health risks in women (Loddo et al. 2014). In the early 2000s, some new reports were published by the GAO on the quality of implementation of previously issued guidelines (U.S. Government Accountability Office 2000, 2001, 2015). which concluded that there were some improvements in the inclusion of both sexes in clinical studies design, but the analysis of sex differences remained inadequate. This is contradicting the fact that many diseases in humans show clear sex differences (Westergaard et al. 2019). The sex of a person has been shown to be of highly impacting the outcome of animal experiments such as liver metabolism (Chella Krishnan et al. 2018) or cardiovascular diseases (Lawton 2011) to proteomic effects (I. Miller et al. 2016). In responses, the NIH issued in 2015 the Sex as Biological Variable (SABV) guidelines that require inclusion of a representative sample of both sexes in all biomedical research to be able to evaluate sex differences in the human hazard and risk assessment (Clayton and Collins 2014; Collins and Tabak 2014; NIH 2015), and in January 2025 the FDA updated its Guidance for Industry on “Study of Sex Differences in the Clinical Evaluation of Medical Products” noting that it was a recommendation and is currently out for commenting (as of January 2025) (FDA 1993b).
In Europe, significant regulatory changes for science, engineering, and technology occurred in 1998 when the Council of Europe defined gender mainstreaming as “the (re)organisation, improvement, development and evaluation of policy processes, so that a gender equality perspective is incorporated in all policies, at all levels, and at all stages, by the actors normally involved in policymaking.” In the same year, the European Technology Assessment Network was founded as a working group of female scientists aiming to collect national‐level data on women in science, and proposing recommendations to address gender in research and to balance scientific careers and family life. The same aim led to the establishment of the Advancement of Women and Minorities in Science, Engineering, and Technology Development Act in the US. From that time, policy and regulatory activities were constantly reinforced. During the EU's Framework Programme 5 (FP5), Gender Impact Assessment Studies were conducted, while the EU FP6 funding programme included the requirement to integrate both sex and gender analysis into research design (when appropriate) and this requirement was integrated into the EC Gender Equality Strategy (European Commission 2020a). Furthermore, the EC is committed to promoting gender equality in R&I and has developed the Gender Equality Strategy 2020–2025 (European Commission 2020a). Apart from the EC, the European Institute for Gender Equality has introduced the Gender Equality in Academia and Research (GEAR) toolbox to ensure incorporation of sex and gender analysis throughout the entire R&I cycle. In 2012, the European Association of Science Editors established a Gender Policy Committee to develop a set of guidelines for reporting of Sex and Gender Equity in Research (SAGER) (Van Epps et al. 2022). The SAGER guidelines were designed to serve the scientific community by providing a tool for standardization of sex and gender reporting in scientific publications (Heidari et al. 2016).
One of the most significant projects launched to provide scientists and engineers with practical methods for sex and gender analysis is the Gendered Innovations project (Stanford University, n.d.) involving experts from across the U.S. and the EU 27 Member States. Gendered Innovations was initiated at Stanford University in 2009, while collaborations with the EU started in 2012 after the EC set up an expert group “Innovation through Gender” in 2011. Presentation of the Gendered Innovations project to the European Parliament in 2013 resulted in the publication “Gendered Innovations: How Gender Analysis Contributes to Research” (European Commission 2013). In the period of 2018–2020, Gendered Innovations (G12), as the Horizon 2020 Expert Group, updated and expanded the Gendered Innovations methods and case studies. These activities resulted in the report “Gendered Innovations 2: How Inclusive Analysis Contributes to Research and Innovation” (European Commission 2020b), published by the Publications Office of the European Union in 2020. In 2021, the Organization for Economic Cooperation and Development (OECD) published a report on the role of gender in achievement of the United Nations Sustainable Development Goals (SDGs) “Gender and the Environment: Building Evidence and Policies to Achieve the SDGs”(OECD 2021).
1.2. Definitions of Sex and Gender Dimensions
Sex and gender are important variables when it comes to hazard and risk assessment of chemicals and materials. In order to provide the most rigorous, accurate, and valid research, sex and gender should be strictly defined, and there should be a clear distinction between them (Kraus et al. 2023). Guidelines should provide instructions on the use of both terms in research design and reporting of the results of research studies (McGregor et al. 2016). No implementation of both sex and gender dimensions during research design is detrimental to the extraction of reproducible and relevant conclusions (Tannenbaum et al. 2016, 2019). Definitions of these variables as provided by different authorities (Table 1) are broadly similar. In general, sex refers to biological attributes in humans and animals and is therefore associated with physical and physiological features, while gender is defined through socially constructed roles and behaviors and identifies humans as male, female, or gender‐diverse (Canadian Institutes of Health Research—Institute of Gender and Health 2012).
TABLE 1.
Sex and gender as defined by different authorities and agencies.
| Authority | Sex refers to… | Gender refers to… |
|---|---|---|
| European Commission (EC) | “the biological attributes that distinguish male, female, and/or intersex in humans; biological attributes that distinguish male, female, and/or hermaphrodite in animals; anatomical and physiological characteristics that may impact the design of products, systems, and processes in engineering & product design research” | “sociocultural norms, identities, and relations that: (1) structure societies and organizations; and (2) shape behaviors, products, technologies, environment and knowledge (Schiebinger 2000); gender attitudes and behaviors are complex and change across time and place; gender is multidimensional(Hyde et al. 2019) and intersects with other social categories, such as sex, age, socioeconomic status, sexual orientation and ethnicity; gender is distinct from sex (Fausto‐Sterling 2012)” |
| World Health Organization (WHO) | “different biological and physiological characteristics of females, males and intersex persons, such as chromosomes, hormones and reproductive organs” | “characteristics of women, men, girls and boys that are socially constructed, which includes norms, behaviors and roles associated with being a woman, man, girl or boy, as well as relationships with each other. Gender varies from society to society and can change over time” |
| US National Institutes of Health (US NIH) | “biological differences between females and males, including chromosomes, sex organs, and endogenous hormonal profiles” | “socially constructed and enacted roles and behaviors which occur in a historical and cultural context and vary across societies and over time; with continuous interaction between sex and gender, health is determined by both biology and the expression of gender” |
| European Institute for Gender Equality (EIGE) | “biological characteristics which define humans as female or male. These sets of biological characteristics are not mutually exclusive as there are individuals who possess both, but these characteristics tend to differentiate humans as males and females” | “social attributes and opportunities associated with being male and female and the relationships between women and men and girls and boys, as well as the relations between women and those between men. These attributes, opportunities and relationships are socially constructed and are learned through socialization processes. They are context/time‐specific and changeable. In most societies there are differences and inequalities between women and men in responsibilities assigned, activities undertaken, access to and control over resources, as well as decision‐making opportunities” |
| Canadian Institutes of Health Research (CIHR) | “set of biological attributes in humans and animals. It is primarily associated with physical and physiological features including chromosomes, gene expression, hormone levels and function, and reproductive/sexual anatomy. Sex is usually categorized as female or male but there is variation in the biological attributes that comprise sex and how those attributes are expressed” | “socially constructed roles, behaviors, expressions and identities of girls, women, boys, men, and gender diverse people. It influences how people perceive themselves and each other, how they act and interact, and the distribution of power and resources in society. Gender identity is not confined to a binary (girl/woman, boy/man) nor is it static; it exists along a continuum and can change over time. There is considerable diversity in how individuals and groups understand, experience and express gender through the roles they take on, the expectations placed on them, relations with others and the complex ways that gender is institutionalized in society” |
| Office for National Statistics (UK government) | “biological aspects of an individual as determined by their anatomy, which is produced by their chromosomes, hormones and their interactions; generally male or female; something that is assigned at birth” | “a social construction relating to behaviors and attributes based on labels of masculinity and femininity; gender identity is a personal, internal perception of oneself and so the gender category someone identifies with may not match the sex they were assigned at birth; where an individual may see themselves as a man, a woman, as having no gender, or as having a nonbinary gender—where people identify as somewhere on a spectrum between man and woman” |
Both sex and gender are multidimensional concepts. The variability of gender is more commonly recognized and acknowledged than that of sex (Table 1). All the definitions describe gender as “changeable”, “complex” and “varying across societies”. In contrast, some of definitions of sex include “intersex”, and “hermaphrodites” or acknowledge that there are deviations from the concept of males exclusively having XY chromosomes and females having XX chromosomes. Combinations of chromosomes such as X, XXX, XXY, XYY, and others exist, which result in different anatomical and physiological phenotypes (Ainsworth 2015). Progress in incorporating sex and gender as regular variables in the R&I process has been slow when it comes to the usual dichotomous classification of biological sex. Inclusion of gender is far less represented in research, despite the multidimensionality of gender being acknowledged to a greater extent. Transgender/transex/intersex inclusion in toxicological research relevant for human health effects is still non‐existent, however, it could become the area of growing importance in the future (Freeman et al. 2017).
1.3. Main Aspects of Sex‐Related Differences for Human Health Risk and Hazard Assessments
Health‐related hazard assessment aims to estimate the probability of adverse outcomes after exposure to certain chemicals and materials and to recommend acceptable values for such exposure, considering the frequency of exposure and severity of resulting effects. Owing to sex/gender‐related differences in constitutive and physiological parameters, this dimension has to be included in all approaches and methods for hazard assessment; yet, as summarized in the introduction, it is not yet routinely achieved. Differences between sexes can occur due to various parameters: physiological (e.g., females have lower body weight and a higher percentage of body fat, also lower glomerular filtration rates in their kidneys), biochemical (e.g., lower gastric pH in females, hormonal variations during menstrual cycle and general hormonal differences between males and females, different levels and activity of various enzymes) and even epigenetics (e.g., expression of cytochrome P450, a group of enzymes that play a key role in the body's metabolism of drugs, chemicals, and other substances; imprinting of the X chromosome) (Davies 2010; Vahter et al. 2007; Zhong and Leeder 2013). Moreover, even the use of contraceptives by women of childbearing age enrolled in safety/toxicity studies may interfere with the results of these studies, and it is questionable whether such results can be extrapolated to females not taking contraceptives (Gochfeld 2017). On average, males and females differ in several physiological and biochemical parameters that can influence hazard and risk assessment; however, these are population‐level tendencies with broad overlap between the sexes and notable species/strain variability. Accordingly, sex‐related differences in toxicokinetics (TK) and toxicodynamics (TD) can occur, depending on the substance, dose, and model, but are not universal.
Despite many efforts and clear guidelines provided by regulatory agencies and policy makers as described in the introduction, many researchers still omit to adequately include the sex/gender dimension in biomedical and toxicological research activities (Weiss 2011). For example, about 70% of randomized clinical trials published in high‐impact journals funded by the NIH in the 1990s did not include a sex/gender dimension in their analysis (statistical) and did not provide adequate reasoning for this lack (Geller et al. 2018). Freeman et al. (Freeman et al. 2017) reviewed 165 protocols and found only 24 (14.5%) that provided reasoning for the choice of sex/gender of the studied population. The same authors concluded that only 2% of papers considered sex or gender effects on the primary outcome when it comes to topics in which sex/gender differences might be present. Most of the currently available information on this topic comes from TK studies of drugs, pesticides, biocides, and other chemicals (Gochfeld 2007), although results from those studies often refer to a specific drug and do not provide general information on sex differences in absorption, distribution, metabolism, and excretion (ADME) processes or their underlying mechanisms. This descriptive aggregation flags possible sex‐related differences but does not establish causation.
The internal dose of xenobiotics depends on TK, which can be defined as the study of the kinetics of ADME processes. The TK may depend on some of the most prominent sex‐related differences, such as body size, blood and organ volumes, and differences in activities of plasma proteins and enzymes involved in the metabolism of xenobiotics (Gochfeld 2007, 2017; Soldin and Mattison 2009; Vahter et al. 2007). Besides the intrinsic parameters of the substance (e.g., solubility), its absorption/bioavailability can also be affected by the administration route, thus by sex‐specific factors, for example, different activities of gastric alcohol dehydrogenase or different transport proteins in kidneys between males and females. Dermal absorption can depend on the condition of skin, hair follicle number, perspiration, skin thickness, presence of adipose tissue (larger in females), and use of cosmetic products (Arbuckle 2006) which was traditionally associated primarily with females, but in recent years, male grooming has become increasingly (re‐)normalized (British Beauty Council 2025). Oral administration implies absorption via the gastrointestinal system, and the bioavailability may be determined by many factors, such as gut motility, pH, gastric and intestinal enzymes, microbiome diversity, and expression of transporters (Arbuckle 2006; Nicolas et al. 2009). In males, the gastric fluid tends to be more acidic, the gastric emptying time is longer, while the transit times are shorter than in females (Nicolas et al. 2009; Soldin and Mattison 2009). Given that total body water volume, blood volume, and plasma volume are higher in males, the volume of distribution (Vd) is generally considered to be higher in males, which implies that the concentration of exogenous substances in the blood may be lower in males if the substance does not bind to plasma proteins in significant amounts (Soldin et al. 2011). Binding to plasma proteins is an extremely important parameter for the distribution of drugs and xenobiotics. Sex‐related differences have been demonstrated in the blood concentrations of α1‐acid glycoprotein, a transport protein in the bloodstream that binds various synthetic drugs and influences their distribution and availability in the body, which depends on estrogen level (Soldin and Mattison 2009). However, females have a larger percentage of body fat; therefore, the Vd of lipophilic substances may be higher in females (Nicolas et al. 2009). Higher Vd implies lower elimination time, tissue accumulations, and possible toxic reactions (e.g., related to sudden weight loss and release of substances stored in adipose tissue back into the bloodstream).
Biotransformation or metabolism of xenobiotics can also be considered sex‐related variabilities (Arbuckle 2006; Czerniak 2001), which have been extensively reported (Gochfeld 2007, 2017; Soldin and Mattison 2009; Vahter et al. 2007). For example, many studies on the expression and activity of cytochrome P450 enzymes confirmed clear sex differences (Scandlyn et al. 2008; Waxman and Holloway 2009; Wolbold et al. 2003; L. Yang and Li 2012). Variabilities between males and females in humans have also been noticed in phase II enzymes such as sulfotransferases, methyl transferases, and UDP‐glucuronosyl transferases (Krishna et al. 1995; Mitchell et al. 1997; Soldin et al. 2011; Soldin and Mattison 2009), that catalyze conjugation reactions which make substances more hydrophilic and therefore more prone to excretion. Additionally, activities of enzymes may be susceptible to hormonal changes during hormone replacement therapy (L. Yang and Toriola 2024), pregnancy, or menopause (Tsiokou et al. 2020). While evidence on TK changes during menopause is conflicting (Tsiokou et al. 2020), physiological changes during pregnancy are known to affect the TK of xenobiotics including Vd, increased plasma volume, extracellular fluid space, total body water, and regional blood flow changes (e.g., increased uterine, renal, skin and mammary blood flow, decreased skeletal blood flow), increased stroke volume (early pregnancy), increased heart rate (later in pregnancy), respiratory changes (compensated respiratory alkalosis, pH −7.44), decreased plasma albumin, and absorption changes such as prolonged gastric evacuation time, liver CYP450 enzyme, and uridine diphosphate glucuronosyltransferase isoenzyme changes, as well as increased renal blood flow (Costantine 2014; Feghali et al. 2015). Therefore, health hazard assessment of pregnancy requires special attention. It is also worth mentioning that males have higher basal metabolic rates which can be attributed to a higher percentage of muscle tissue and a lower percentage of adipose tissue in males compared to females.
Elimination of xenobiotics can also be sex‐dependent. Beyond study design, sex‐related physiology (e.g., renal blood flow, GFR, and renal transporter expression) may influence concentration–time profiles, but these differences are substance‐ and model‐dependent. Exogenous substances are eliminated from organisms (including humans) through different routes, for example, via urine, feces, sweat, and lung. For renal elimination, some studies showed lower renal blood flow in females compared to males with consequent lower glomerular filtration rates and longer retention in the organism. Another factor affecting dissimilarities in male and female renal excretion is the difference in expression of membrane transporters present in kidneys (Cheng and Klaassen 2009; S. Joseph et al. 2015; Kwekel et al. 2013; Trevisan et al. 2012), for example, sex‐specific expression of organic anion transporters in proximal renal tubule cells that leads to faster elimination of perfluorooctanoic acid in female versus male rats (Kudo et al. 2002).
TD describes the dynamic interactions between a xenobiotic and its biological target, leading ultimately to biological (and possibly adverse) effects. A biological target, also known as the site of action, can vary between binding proteins, ion channels, nucleic acids, or a variety of receptors. Differences in xenobiotic interaction with its targets in an organism are mainly affected by the effectiveness of expression of receptors in target cells or by hormonal effects on receptor sensitivity/actions (Franconi and Campesi 2014; M. A. Miller 2001). Males and females are characterized by two distinct reproductive organ systems and specific regulations and receptors for dedicated hormones depending on the sex. The interference of chemicals and materials with this kind of system can lead to the production or increase of production of certain proteins as well as the modification of hormone levels. These descriptive patterns may point to sex‐related differences in some contexts, but evidence is insufficient to ascribe greater overall susceptibility to one sex to adverse effects caused by exogenous substances (xenobiotics) such as alteration in receptor number and binding, and in signal transduction pathways following receptor binding (Soldin and Mattison 2009). Unfortunately, the exact mechanisms of sex‐related TD differences remain uncertain, and the more frequent limitation is the failure to analyze sex differences rigorously even when both sexes are included, rather than outright under‐representation.
1.4. Gender‐Related Differences in Human Risk Assessment
Differences in exposure patterns represent the first point to be considered during health human risk assessment. When accounting for the gender dimension, this is reflected by different exposure durations to certain agents as men and women spent different time at home, in the community or in the workplace environment. Factors such as dietary habits, drug consumption, occupational parameters and lifestyle significantly affect exposure to diverse stressors and xenobiotics (Biswas et al. 2021; Fiala and Brázdová 2000). Men and women perform different activities and gender‐related behavior is apparent already from the very early stages of infancy (Bando et al. 2024; McIntyre and Edwards 2009). Moreover, disparities between women and men may significantly induce gender‐related risk of adverse health effects (Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes 2016; Heise et al. 2019; S.‐E. Kim 2015; Klein et al. 2015). For example, gender roles often have an impact on occupational choices, leading to different interactions with environmental and chemical hazards (C. T. Lee et al. 2021; Narendran et al. 2019; Quinn and Smith 2018).
Despite the importance of these factors, the term gender is rarely used in toxicological research and most studies that use the term gender actually discuss sex‐related differences rather than differences caused by determinants of gender such as socio‐economic, psychological, and exposure parameters (Box 1).
BOX 1. Main study aims.
Considering all important aspects of the sex/gender dimensions relevant for exposure to xenobiotics and consequent health effects as discussed in the introduction section, we aimed to perform an extensive analysis of scientific data available for sex/gender‐related differences in response to ENMs. As our main focus was human health effects of ENMs, we restricted our analysis to in vivo mammalian studies due to the lack of reliable data on human response to ENMs exposure. Non‐mammalian studies were beyond the scope of this study due to the more considerable challenges in translation from in vivo to in human context as invertebrates display a great variety of different sex‐determining mechanisms. In many cases, even vertebrate animals poorly replicate male–female differences in human physiology and pathology. In addition, in vitro studies were omitted due to scarcity of published data and challenges to evaluate sex‐related effects under in vitro settings. Most in vitro studies are based on use of commercial cell lines derived either from male or female donors, and cell sex is not a standard parameter that is reported (Sharifi et al. 2021). Moreover, it is difficult to maintain the sex‐related differences during in vitro experiments due to their dedifferentiation and loss of the chromosome Y after the loss of contact with some external mediators. Under in vitro settings, it is challenging to define to what extent the physiological circumstances need to be replicated (i.e., using specific systems such as microfluidic systems) in order to maintain the difference of cell capacities between males and females (Veser et al. 2024).
2. Literature Search and Data Analysis Methodology
The comprehensive literature review was carried out in the PubMed and Web of Science (WoS) databases using the keywords presented in Table 2. The search was performed for studies published in the period from January 1st, 2002 to August 14th, 2024. Data published before 2002 are not included in this study, as a clear recommendation to include both sex and gender in research was first given by the European Commission (EC) through the Framework Programme 6 (2002–2006). Moreover, data on the physico‐chemical characterization of nanomaterials prior to 2002 were also very limited.
TABLE 2.
Number of papers (No.) found in PubMed and Web of Science (WoS) databases for selected keywords.
| Keywords | No. in PubMed | No. in WoS |
|---|---|---|
| ((“in vivo”) AND (“nano*”)) AND (“sex”) | 174 | 238 |
| ((“in vivo”) AND (“nano*”)) AND (“gender”) | 173 | 88 |
| ((“rat”) OR (“mice”) OR (“animal”)) AND (“nano*”) AND (“sex”) | 773 | 846 |
| ((“rat”) OR (“mice”) OR (“animal”)) AND (“nano*”) AND (“exposure”) AND (“sex”) | 209 | 227 |
| ((“in vivo”) OR (“animal”)) AND (“exposure”) AND (“nano*”) AND (“sex”) | 198 | 138 |
| TOTAL | 1527 | 1537 |
Total of 3064 papers were identified in PubMed and WoS databases according to the selected keywords. These were subjected to further analysis as presented in Figure 1. First step was merging all papers in Excel file and removing duplicates using the “Remove duplicates” option in Microsoft Excel. Then, papers were analyzed by title and abstracts, to exclude all papers that did not report in vivo testing on mammalian animal models or that did not test ENMs.
FIGURE 1.

Workflow of the process of selection and analysis of papers found using keywords listed in Table 1.
Resulted number of papers was further analyzed by the quality criteria developed as part of the GUIDEnano approach (Fernández‐Cruz et al. 2018) which was established to guide authors in the preparation of high‐quality nanotoxicity publications. This approach follows the principles of the Klimisch score (Klimisch et al. 1997) related to the quality of the study and risk of bias evaluation by considering study design and reporting considerations. Due to the purpose of this review, papers were first evaluated for K‐score by employing a slightly modified K‐score questionnaire (see Table 3) due to our specific aim to extract data relevant for sex/gender differences in response to ENMs. According to the K‐score, papers were considered as: K1—study is reliable; K2—study is reliable with restrictions; and K3—study is not reliable.
TABLE 3.
Comparison of questions proposed by the GUIDEnano approach to analyze quality of papers according to the K‐score (left column) and additional questions introduced in this work to assess sex/gender dimension in reported data (right column).
| Questions from GUIDEnano K‐score approach | Questions used in this literature research |
|---|---|
| Is the test model given?* | Is the test model given and appropriately described?* |
| Is information given on the source/origin of the cell line? | Is information given on the source/origin of the animal model? |
| Are necessary information on test system properties, and on conditions of cultivation and maintenance given? | Are necessary information on test system properties, and on conditions of cultivation and maintenance given? |
| Is the method of administration given?* | Is the method of administration given? |
| Are duration of exposure as well as time‐points of observations explained?* | Are duration of exposure as well as time‐points of observations explained? |
| Were negative and positive controls included (where and when needed)?* | Were negative and positive controls included (where and when needed)? |
| Is the number of replicates (or complete repetitions of experiment) given?* | Is the number of replicates (or complete repetitions of experiment) given? |
| Are the study endpoint(s) and their method(s) of determination clearly described?* | Are the study endpoint(s) and their method(s) of determination clearly described? |
| Have the results been analyzed using statistical methods?* | Have the results been analyzed using statistical methods? |
| — | Were both female and male animals used?* |
| — | Were results from females and males analyzed separately?* |
| — | Were differences between females and males statistically analyzed? |
Note: Questions marked with an asterisk (*) had to be answered positively.
The left column in Table 3 reproduces original GUIDEnano K‐score questions (which reference ‘cell lines’); the right column shows in vivo adaptations (animal models). We excluded in vitro studies from screening and analysis.
The papers scored by K1 and K2 were then subjected to the evaluation according to the S‐score to obtain the information on an overall quality following the questionnaire given in Table 4. S‐scoring results are numerically classified as follows: S1—very high quality of the physicochemical characterization, S2—physicochemical characterization is acceptable, and S3—physicochemical characterization is not acceptable. Only papers scored by S3 were excluded from further analysis. It should be highlighted that our meta‐analysis was descriptive, and no formal statistical meta‐analysis was performed testing causation.
TABLE 4.
S‐score questions used to analyze quality of papers according to the GUIDEnano approach.
| S‐score questions from GUIDENano |
| Was the test ENM identified?* |
| Is information on the source of the ENM given? |
| Is purity (concentration) of the ENM given? |
| Is endotoxin content of the ENM given? |
| Were impurities stated? |
| Was the type of test medium or vehicle used stated?* |
| Were protocols of dispersion and characterization in the exposure medium identified? Were protocols of preparation of exposure medium stated? |
| Was the ENM concentration measured in the exposure medium? |
| Was the stability of the ENM concentration measured during the exposure period? |
| Are doses administered or concentrations in exposure media given?* |
| Primary particle size* |
| Particle size in exposure medium |
| Size at the start or at the end of the exposure period |
| Surface area |
| Surface charge |
| Surface charge in media |
| Shape |
| Other relevant information (i.e., crystal structure, solubility, magnetic properties, acidity/basicity, redox potential, catalysis, photosensitivity, hydrophobicity, radical production capacity, etc.) |
Note: Questions marked with * had to be answered positively.
Studies from the primary literature research that met both the K‐ and S‐score criteria were reviewed, all information were collected in Excel file (Supporting Information File 1) and coded according to the legend outlined in Table 5.
TABLE 5.
Scoring legend for toxicity parameters in analyzed papers.
| Scoring for males/females | Scoring for sex‐related differences |
|---|---|
| /—endpoint not analyzed in the paper | /—endpoint not analyzed in the paper |
| 0—no effect | 0—no sex‐related differences |
| 1—lower than vehicle/negative control | 1—higher in males than in females |
| 2—higher than vehicle/negative control | 2—lower in males than in females |
3. Results and Discussion
3.1. Literature Review Results
After the literature search, a total of 3064 papers from both databases were merged, while removing duplicates resulted in a total of 1485 papers (see Figure 1). These papers were then evaluated based on the information from the title and abstract. Papers that were in vivo or ex vivo studies on mammalian models were included, while in vitro studies and those that used non‐mammalian models, as well as reviews, were excluded. This resulted in 122 papers (Aftab et al. 2018; Ajdary et al. 2015; Akram et al. 2020; Al‐Radadi and Adam 2020; Ammendolia et al. 2017; An, Hong, et al. 2014; An, Kim, Lee, et al. 2014; An, Kim, et al. 2014; An, Lee, et al. 2014; Atia and Alghriany 2021; Badkoobeh et al. 2013; Baki et al. 2014; Barbir et al. 2019; Bautista‐Pérez et al. 2024; Beck et al. 2012; Boudreau et al. 2016; Brand et al. 2020; Chen et al. 2006, 2019, 2020, 2021; Copeland and Stabenfeldt 2020; Ćurlin et al. 2021; Dam et al. 2015; DeLorme et al. 2012; El‐Ela et al. 2019; Fonseca‐Gomes et al. 2020; Ghaderi et al. 2015; Gokulan et al. 2020; Grodzicki et al. 2024; Guilloteau et al. 2022; Guo et al. 2020; Haghani et al. 2020; Han et al. 2020; Hong et al. 2014; Hussain et al. 2020; Jackson et al. 2012; A. Joseph et al. 2018; Kang et al. 2024; Kasai, Umeda, Ohnishi, Kondo, et al. 2015; Kasai, Umeda, Ohnishi, Mine, et al. 2015; Kawamura et al. 2002; Khairy Elkady et al. 2024; J.‐C. Kim et al. 2016; M. Kim et al. 2021; W.‐Y. Kim et al. 2009; Y. S. Kim et al. 2008, 2018; Kiratipaiboon et al. 2020; Ko et al. 2015; Kobyliak et al. 2015; Kong et al. 2014; Kotb et al. 2016; Lai et al. 2024; J. Lee et al. 2019; J. H. Lee et al. 2020; Lelovas et al. 2018; Q. Li, Jiang, Feng, et al. 2024; X. Li, Xu, Zhao, et al. 2024; Y. Li, Xu, Wang, et al. 2024; C. Liang, Lin, et al. 2024; Y. Liang, Yang, et al. 2024; Lim et al. 2011; Liu et al. 2013; Lu et al. 2024; Mahmoud et al. 2021; Matsumoto et al. 2012; Milivojević et al. 2016; Mioc et al. 2019; Mohammadpour et al. 2020; Moore et al. 2016; Morris‐Schaffer et al. 2019; Mortensen et al. 2022; Narciso et al. 2020; Naz et al. 2024; Notter et al. 2018; Oh et al. 2019; Ong et al. 2016; Paek et al. 2013, 2014; E.‐J. Park et al. 2016, 2017, 2020, 2021; H.‐S. Park et al. 2014; Ray and Holian 2019; Riaz et al. 2020; Seok et al. 2013; Shende et al. 2015; Singh et al. 2013; Smith et al. 2019; Sofranko et al. 2021; Soltaninejad et al. 2021; Sulaiman et al. 2015; Sung et al. 2011; Tada et al. 2013; Tariba Lovaković et al. 2021; Tarlan et al. 2020; Tassinari, Cordelli, et al. 2021; Tassinari, Martinelli, et al. 2021; Tassinari et al. 2014, 2020, 2023; Tubesha et al. 2013; Vakilzadeh et al. 2018; Villani et al. 2022; Vlaanderen et al. 2017; Wang et al. 2012; Warheit et al. 2015; Wąsowicz et al. 2017; Wen et al. 2024; M. Wu et al. 2018; X. Wu et al. 2024; J. Xu et al. 2011; S. Xu et al. 2015; Xue et al. 2012; J.‐L. J. Yang et al. 2021; L. Yang et al. 2018; You et al. 2020; Yousef et al. 2021; Yu et al. 2014; Zhang et al. 2013) that reported the toxicity of ENMs in mammals under in vivo or ex vivo settings, which were then scored according to the GUIDEnano approach (as explained above). Out of these 122 papers, 87 of them (71.3%) reported data on animals of both sexes (see Figure 2). The papers that included just one sex in the study, but did not provide a proper reasoning for the use of only one sex, were also excluded from this analysis.
FIGURE 2.

Number of papers reporting in vivo response to ENMs selected for quality analysis according to the GUIDEnano approach. The papers were classified as including both male and female animals (or not), and for those that included both male and female animals whether the results were analyzed separately or not, and if analyzed separately whether the analysis was statistical or not.
Besides inclusion of both sexes in the study design, another important consideration was how the obtained data were analyzed and presented, and whether this included statistical analysis of differences between the responses of male and female animals. Results from males and females were analyzed separately in 64 (73.6%) out of 87 papers that included animals of both sexes, while the remaining studies included animals of both sexes only for randomization of test groups, but did not consider sex‐related response to ENMs. Sex‐related response was statistically analyzed separately in only 21 (32.8%) out of these 64 papers (Figure 2). This shows that adequate analysis of differences in response to ENMs exposure or treatment between males and females was done in only 17.2% of all initially found papers reporting in vivo mammalian data. This finding was quite surprising, especially considering all policy and regulatory requirements and recommendations (see Table 1) about the importance of implementation of the sex/gender dimension in research study design and data analysis. Quality criteria scoring was performed for all 122 papers that reported findings for both sexes revealing that only 64 papers met the K1 and K2 scoring criteria (Table 3). Out of these 64, S‐scoring resulted finally in 53 papers as 9 papers did not meet quality criteria based on S scores, while 2 papers were excluded because they focused primarily on reproductive and developmental toxicity without reporting on general toxicity on both sexes. Therefore, a total of 53 papers from the primary literature were finally analyzed in detail and coded according to the guidelines outlined in Table 5 (see Supporting Information Files 1 and 2 for detailed information).
Results from scoring were used to check for correlation between ENMs properties, dosing and/or treatment duration and sex‐related biological and toxicological response to ENMs. All identified biological effects were categorized, and endpoints were listed in the following categories: “General Toxicity” including food intake, water consumption and body weight gain; “Organ Weights” for heart, brain, liver, kidney, adrenal gland; “Hematology” including hemoglobin (HGB), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocytes (RET or RETA), platelets (PLT), leukocytes (LEU), neutrophils (NEU), lymphocytes (LYM), eosinophils (EOS), monocytes (MON), basophiles (BAS), white blood cells (WBC) and red blood cells (RBC); “Blood Biochemistry” including albumin (ALB), glucose (GLU), blood urea nitrogen (BUN), creatinine (CR), total proteins, creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (BIL), γ‐glutamyl aminotransferase (GGT), alkali phosphatase (ALP), cholesterol, triglycerides (TG), phospholipids (PL), Ca, K, Na, Cl, inorganic phosphorus (IP); “Biodistribution” by means of accumulation in blood, liver, kidney, spleen, adrenal gland, testis, ovaries, brain, stomach, intestine, lung and excretion by feces and urine; “Behavior” by means of depressive behavior (forced swim test), transcriptomic changes in hippocampus and spatial cognition abilities; “Diabetes Related Parameters” including HbA1c, glycated serum proteins, insulin, glucagon and C peptide; “Oxidative Stress Parameters” including activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and levels of malondialdehydes (MDA), reduced glutathione (GSH), and peroxyl radicals (DCFH); “Inflammatory Response”; “Thyroid Gland Function” by means of levels of T3 and TSH hormones; and “Genotoxicity” including DNA damage in blood, liver and kidneys (see Supporting Information File 1). Other endpoints were not reported in any of analyzed papers. Endpoints were first noted as either decreased or increased in observed effect compared to non‐treated animals. This was done separately for females and males. Additionally, the information about statistical analysis associated with sex‐related differences for each endpoint was extracted from the original papers. This information was crucial because many studies did not specifically analyze sex‐related responses, even though separate studies for each sex were performed. Therefore, in Figures 3, 4, 5, 6, 7, 8, sex‐related effects were coded relative to sex‐matched concurrent controls at each reported dose; when multiple doses were available, each dose was coded and visualized in the aggregated counts This analysis could offer insights into possible sex‐related differences that were not highlighted in the original publications, where statistical comparisons between males and females were not conducted.
FIGURE 3.

Effects of ENMs on general toxicity parameters including food intake, water consumption, and body weight gain in males and females, based on data acquired from a meta‐analysis of literature review. The lower sets of bars (F, M) indicate data for F and M test subjects compared and analyzed statistically only in relation to control, untreated animals.
FIGURE 4.

Results for hematological parameters including red blood cells count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) in males (M) and females (F) based on data acquired from meta‐analysis of literature review. The lower sets of bars (F, M) indicate data for F and M test subjects compared and analyzed statistically only in relation to control, untreated animals.
FIGURE 5.

Results for hematological parameters including white blood cells (WBC); monocytes (MON), basophiles (BAS), lymphocytes (LYM), and eosinophils (EOS) in males (M) and females (F) based on data acquired from meta‐analysis of literature review. The lower sets of bars (F, M) indicate data for F and M test subjects compared and analyzed statistically only in relation to control, untreated animals.
FIGURE 6.

Results for inflammatory response and blood biochemistry parameters including albumin (ALB), glucose (GLU), blood urea nitrogen (BUN) and creatinine (CREA) in males (M) and females (F) based on data acquired from a meta‐analysis of literature search results. The “sex‐related” set of bars shows whether the results were checked for sex‐related differences (M and F compared statistically).
FIGURE 7.
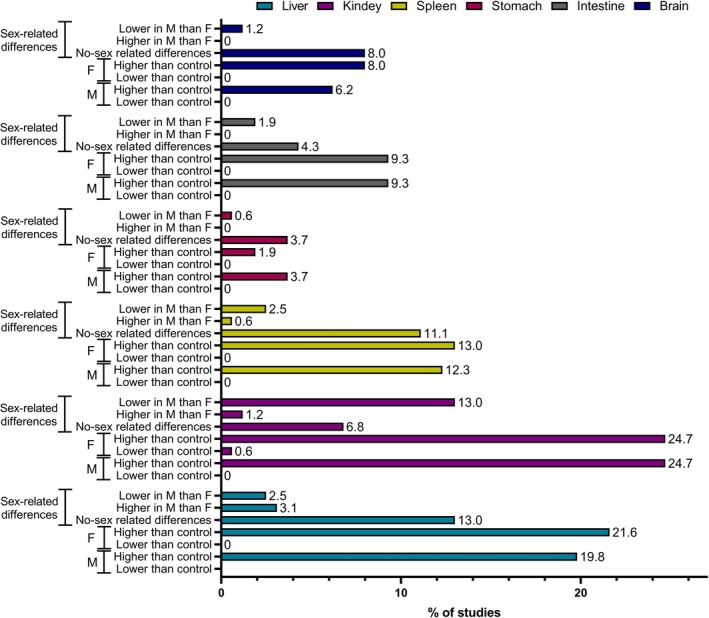
Reported ENMs accumulation in various tissues (liver, kidney, spleen, stomach, intestine and brain) for males (M) and females (F) based on data acquired from a meta‐analysis of literature review. The sex‐related set of bars shows whether the results were checked for sex‐related differences (M and F compared statistically).
FIGURE 8.

Data on oxidative stress biomarkers including levels of reduced glutathione (GSH), malondialdehyde (MDA), superoxide radicals (DHE) and peroxyl radicals (DCFH), and activities of superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) in males (M) and females (F) treated with ENMs acquired from meta‐analysis of literature review. The sex‐related set of bars shows whether the results were checked for sex‐related differences (M and F compared statistically).
Additionally, data on the physico‐chemical properties of the tested ENMs, treatment protocols, dosage information, and administration routes were recorded as well. Each variation in ENMs characterization and/or treatment (i.e., core, surface functionalization, different dose, different treatment duration) was considered a separate study, and data for that specific treatment was marked and scored in separate Excel table rows (see Supporting Information File 1). A comprehensive database was developed to compile information on endpoints affected by ENMs exposure, emphasizing the differences in observed outcomes between sexes.
3.2. Computational Meta‐Analysis of Literature Data
Meta‐analysis of gathered literature data (Supporting Information File 1), which was performed as descriptive but not statistical, revealed that sex‐related differences were unfortunately not considered across most endpoints, despite regulatory guidelines recommending this approach. In fact, over 50% of the analyzed endpoints lacked a proper examination of sex‐related effects, and most studies included in this analysis considered oral and inhalation routes of exposure. All analyzed studies used rodent models (mice or rats) except for the two that reported ex vivo results on human terminal ileum tissue and an in vivo study on cynomolgus monkeys (Supporting Information File 2).
While substantial gaps remain in accounting for sex‐related differences in induced response following ENMs exposure, the data were further analyzed to identify those endpoints that at least showed differences between males and females, even if they were not compared statistically. Additionally, this analysis was used to gain the relation between the physico‐chemical properties of ENMs or parameters of study design with outcomes that express sex‐related differences. The primary approach used in our analysis involved comparing papers that reported sex‐related differences in induced response following the ENMs exposure. Specifically, the focus was on differences observed between treated males and females and their respective controls (non‐treated animals). This allowed for the identification of treatment effects within each sex group, as reported by the study authors. However, it is important to note that most studies did not present statistically significant differences between sexes. This limitation highlights a gap in the existing literature, where sex‐specific analyses often lack the statistical rigor or power to draw definitive conclusions about intersex differences.
For endpoints classified under “General Toxicity” in Figure 3, we observed that sex‐related differences were found for food intake, water consumption, and body weight gain. Studies reporting “lower than control” effects in males were predominant for food intake and body weight gain. In contrast, for females, the number of studies showing “lower than control” effects were comparable to those reporting “higher‐than‐control” effects. In terms of body weight gain, both males and females experienced weight loss, with males being more affected. While water consumption increased in both males and females, a slightly higher number of papers reported an increase in females compared to males. Notably, body weight gain was the only parameter for which sex‐related differences were analyzed in the original studies.
Another set of parameters that showed sex‐related differences was “Hematology” (Figures 4 and 5) which revealed that a larger number of papers reported a decrease of HGB, MCH, and MCV levels in animals treated with ENMs compared to controls. In addition, the decrease of HGB and MCH levels was reported more often for females compared to males, while this was the opposite for a decrease of MCV. Data for HCT showed interesting differences because a predominant number of studies reported decreased HCT in males and increased HCT in females treated vs. non‐treated animals (Figure 4). Similar effects were noticed with blood cell counts, as counts for WBC, MON, and EOS were oppositely affected in males and females exposed to ENMs according to the extracted data (Figure 5). ENMs effect on RBC counts was more prominent in females (Figure 4), while LYM counts were more affected in males treated with ENMs compared to control animals (Figure 5). Statistically significant differences between males and females were reported in only 0.6% of papers, and this concerned lower WBC and albumin in ENM‐treated males compared to ENM‐treated females.
Analysis of “Blood Biochemistry” parameters resulted in more visible differences between males and females (Figure 6) with ALB and CR levels being much more decreased in ENM‐treated females than in ENM‐treated males. Levels of GLU were reported more often as lower in ENM‐treated males compared to control animals, while the opposite was observed for females whose blood GLU levels were more often reported as higher compared to non‐treated animals. Changes in BUN were similar for males and females. Thus, the higher impact of ENMs on females for most of the analyzed biochemical parameters should be considered as descriptive patterns, which may point to sex‐related differences in some contexts, but evidence is insufficient to ascribe greater overall susceptibility to one sex to adverse effects of ENMs exposure.
Figure 7 shows the analysis of results for ENMs accumulation in various organs of females and males. The highest number of papers reported differences in ENMs accumulation between males and females for kidneys, which was significantly lower in males. Lower ENMs accumulation in males compared to females was also reported for spleen, stomach, intestine, and brain by several papers, while higher ENMs accumulation by males was reported more often only in the case of liver (Supporting Information File 1).
Reported data for inflammatory response (Figure 6) and oxidative stress (Figure 8) also showed quite perceptible sex‐related differences. A slightly higher number of papers showed statistically higher values for inflammatory response, MDA level, and activities of SOD and GPX in males compared to females following exposure to ENMs, while more papers reported lower values only for superoxide radical level (DHE) in ENM‐treated males compared to ENM‐treated females (Figure 8). This may indicate that males are more susceptible to oxidative damage and inflammation when exposed to ENMs.
However, data analysis showed in Figures 3, 4, 5, 6, 7, 8 does not distinguish either organ‐ or tissue‐specific data, nor does it categorize findings based on the type of ENMs involved and route of exposure. This lack of specificity limits the ability to draw precise conclusions on how sex‐related differences manifest in particular biological contexts or in response to various types of ENMs. Thus, the next stage of analysis focused on examining the relationship between the ENMs properties and toxicity endpoints with noticeable sex‐related differences. Given the available data, ENM core composition, zeta potential, and surface modification were included in the analysis. Table 6 illustrates information about each toxicity endpoint where sex‐related differences were observed, with various ENM types represented by different colored circles. Endpoints are listed in the left columns, while the right columns indicate observed patterns in sex‐related differences: higher value of particular endpoint in males than females or lower value for specific endpoint in males than females.
TABLE 6.
Correlation between nanomaterial cores and endpoints that were affected in a sex‐related manner.
| Endpoint | ↑ in ♂ than in ♀ | ↓ in ♂ than in ♀ | |
|---|---|---|---|
| Body weight gain |

|
||
| Heamatology | WBC |

|
|
| Biochemistry | Total protein |

|
|
| ALB |

|
||
| ALT |

|
||
| AST |

|
||
| BIL |

|
||
| Excrection | Urine |

|
|
| Accumulation | Blood |

|

|
| Kidney |

|

|
|
| Liver |

|

|
|
| Lung |

|

|
|
| Spleen |

|
||
| Brain |

|
||
| Intestine |

|
||
| Stomach |

|
||
| Adrenal gland |

|
||
| Behavior | Depressive |

|
|
| Spatial cognition |

|
||
| Hippocapmal changes |

|
||
| Inflammatory response |

|

|
|
| Oxidative stress | DCFH |

|

|
| DHE |

|

|
|
| MDA |

|

|
|
| CAT |

|
||
| GPX |

|
||
| SOD |

|

|
|
| GSH |

|
||
| Genotoxicity | DNA damage (blood) |

|
|
| DNA damage (kidney) |

|
||
| DNA damage (liver) |

|
||
Note: One point on the plot indicates one datapoint in the analyzed paper (i.e., one ENM type/size/coating, one dose, one timepoint). The symbols ↓, ↑, ♂, and ♀ correspond to lower, higher, males, and females, respectively.  .
.
This analysis revealed that the majority of ENMs exhibited stronger effects on females than on males. Notably, most of the evidences on sex‐related response pertains to Ag‐based ENMs (AgNMs), although this may be due to the greater volume of scientific data available for AgNMs in general as Ag is one of the most frequently studied ENMs (Wheeler et al. 2021). AgNMs show similar accumulation patterns in males and females with liver, spleen, and kidneys identified as the most prominent organs for the accumulation of this ENM type, while the brain is described as the least probable organ for their accumulation (Gan et al. 2020; J. H. Lee et al. 2013). Despite the similar general accumulation patterns in males and females, sex‐related difference in TK of AgNMs was confirmed in several papers with the highest accumulation rate of AgNMs in kidneys of females (Boudreau et al. 2016; J. H. Lee et al. 2020). Females also show generally higher distribution volumes and lower elimination rates for AgNMs (Supporting Information File 2). Lower elimination rates result in higher elimination half‐lives and therefore longer retention of AgNMs in females. However, data do not provide clear evidence that sex differences are specific for nanoparticulate form of Ag and not for Ag in general, but such analysis was beyond the scope of this study. Liver and spleen were the most frequently described as the organs with the highest accumulation and longest retention of ENMs by more than 10 studies which could indicate that the reticulo‐endothelial system is responsible for ENMs elimination (Supporting Information File 2). For other ENMs, a significant lack of data limits the drawing of any conclusions. Apart from AgNMs, there were also several papers discussing TiO2‐based ENMs, but the TK data acquired are not detailed enough to conclude on any trend.
Correlation of sex‐related response to ENMs with study design parameters such as administration route, exposure durations, and administered doses was also examined, but it was not possible to gain a clear outcome due to a significant lack of data for most ENMs except for AgNMs (Supporting Information File 2). While AgNMs provide some insight into these dynamics, the limited scope and variability in study designs prevent the establishment of clear trends or patterns. Similar problems were encountered in trying to correlate ENMs properties and sex‐related response. For example, modified AgNMs with similar size and zeta potential have been reported to induce contrasting results; in one case, higher accumulation of ENMs was observed in the blood of males compared to females (Barbir et al. 2019), while another study indicated the opposite trend (Narciso et al. 2020).
These inconsistencies highlight the need for more systematic and standardized study design and statistical evaluation of results. Studies must include uniform methodologies for characterizing ENM properties and rigorously assess their biological interactions across both sexes. Such efforts are essential to advance our understanding of how ENM properties influence sex‐specific responses, paving the way for more reliable and generalizable findings.
4. Conclusion
Despite regulatory agencies and policy makers recommending inclusion of the sex/gender dimension in biomedical and toxicological research activities, this has still not been systematically and comprehensively studied in nanotoxicology. The importance of considering also the sex of the donor of cells used in developing in vitro models has been described (Gutleb and Gutleb 2023). Significant imbalances in the reporting of participant sex and gender in the European Genome‐phenome Archive (EGA) and the database of genotypes and phenotypes (dbGaP) were very recently highlighted by the study led by the Barcelona Supercomputing Center with the collaboration of the EC Joint Research Center (JRC) and other research partner organizations (Ruiz‐Serra et al. 2024). A similar problem of overlooking the significance of sex differences at various physiological levels has been recognized also in the field of nanomedicine (Hajipour et al. 2021). Another problem is that current risk assessment committees such as those at EFSA do not consider an effect positive if it appears only in one sex, at least for genotoxicity (Younes et al. 2024). However, the reasoning behind this is also due to the low evidence of effect or due to the irrelevant route of exposure considered in existing studies. Researchers analyzed sex classification in human data from EGA and dbGaP databases and concluded that this is a critical lack as it is well known that women and men might be differently affected by some conditions, exposures, or diseases. Without properly including the sex and gender dimension in designing and reporting research data, these differences cannot be completely identified. Therefore, future risk assessments should recognize sex differences.
Our analysis confirmed that sex differences are important in the context of ENMs risk and hazard assessment; they are not artifacts and can have a large impact on our health. Proper study design and detailed statistical analysis of the results are crucial to enable recognition of sex‐related differences in response to ENMs exposure. Our review and analysis revealed a significant lack of consistent reporting, insufficient ENMs characterization, and statistical analysis of sex‐related responses in existing literature, preventing unequivocal conclusions based on scientifically valid data.
When studies included male and female subjects, they analyzed data for each sex separately without analyzing or reporting sex‐based differences in the responses to the end‐points being assessed. Statistical comparison of results for males and females was provided in less than 20% of all the papers extracted from the literature search. The most detailed analyses available pertain to AgNMs, but these remain insufficient to draw definitive conclusions about the relationships between ENMs properties and sex differences in the observed responses (Box 2).
BOX 2. Future considerations within a regulatory‐relevant context.
Study design should intentionally include animals/humans of both sexes and all genders relevant to the subject of research, and the results acquired from animals/humans of each sex and/or gender should be analyzed separately and then compared to the control group of the same sex and/or gender. This would enable a detailed report on differences in response in animals/humans of each sex/gender for all of the tested parameters. Apart from results being analyzed separately, comparison between all included sexes and/or genders should be provided to see whether there are any significant sex/gender‐related differences. This kind of analysis and reporting of the results will not only elevate the quality of research but also possibly enable new scientific discoveries when it comes to sex/gender‐related differences between animals/humans.
Another important consideration for future hazard and risk assessment of ENMs is that many studies that discuss physiological differences of male and female test subjects in response to ENMs use the term gender instead of sex. There should be a consensus for scientific research and regulatory communities that would enable strict differentiation of these two terms and provide a set of rules for when each of these terms should be used. Since hazard and risk assessment of ENMs is still in its early stages, these kinds of rules and regulations should be discussed as soon as possible and implemented in all future research on this matter. The term sex should be used when discussing physiological and physical adverse effects of ENMs, while the term gender should be applied in exposure studies and other risk assessment studies related to behavioral and socio‐economic factors.
Author Contributions
Lucija Božičević: conceptualization (supporting), data curation (lead), formal analysis (equal), investigation (lead), methodology (equal), writing – original draft (lead), writing – review and editing (supporting). Karolina Jagiello: formal analysis (lead), investigation (equal), methodology (equal), writing – review and editing (supporting). Anita Sosnowska: formal analysis (equal), investigation (equal), methodology (equal), visualization (equal), writing – review and editing (supporting). Maciej Stepnik: investigation (equal), methodology (equal), software (supporting), supervision (supporting), validation (supporting), writing – review and editing (supporting). Maria Dusinska: conceptualization (supporting), funding acquisition (equal), investigation (supporting), project administration (lead), supervision (supporting), writing – review and editing (supporting). Iseult Lynch: funding acquisition (equal), investigation (supporting), supervision (supporting), validation (supporting), writing – review and editing (supporting). Nikolina Peranić: data curation (supporting), formal analysis (supporting), investigation (supporting), writing – review and editing (supporting). Ivona Capjak: formal analysis (supporting), investigation (supporting), validation (supporting). Valérie Fessard: investigation (supporting), methodology (supporting), validation (supporting), writing – review and editing (supporting). Mihaela R. Cimpan: investigation (supporting), validation (supporting), writing – original draft (supporting), writing – review and editing (supporting). Arno C. Gutleb: investigation (supporting), validation (supporting), writing – review and editing (supporting). Elise Runden Pran: investigation (supporting), validation (supporting), writing – review and editing (supporting). Tomasz Puzyn: methodology (supporting), resources (equal), software (lead), supervision (supporting), writing – review and editing (supporting). Ivana Vinković Vrček: conceptualization (lead), formal analysis (supporting), funding acquisition (equal), methodology (supporting), supervision (lead), validation (supporting), writing – original draft (supporting), writing – review and editing (lead).
Conflicts of Interest
The authors declare no conflicts of interest.
Related WIREs Articles
Nanomaterial exposure, toxicity, and impact on human health
Supporting information
Data S1: Supporting Information on the literature survey and keywords used (Table S1), quality scoring of extracted publication according to K score (Table S2) and S score (Table S3), data extracted (Table S4) and se‐xrelated analysis report (Table S5).
Table S6: In vivo studies on ADME properties and toxic effects of various nanomaterials. Physico‐chemical characteristics (shape, size, surface coating) are given for each NM type. For each animal model, details about treatment (test protocol. duration, doses in mg/kg body weight), differences in distribution and toxicity parameters (↑ or ↓ in males (♂) vs. females (♀)) are briefly listed.
Acknowledgments
Open access publishing facilitated by Institut za medicinska istrazivanja i medicinu rada, as part of the Wiley ‐ National and University Library in Zagreb Consortium Croatian Academic and Research Libraries Consortium agreement.
Božičević, L. , Jagiello K., Sosnowska A., et al. 2025. “Sex and Gender Dimensions in Hazard and Risk Assessment of Engineered Nanomaterials.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 17, no. 5: e70034. 10.1002/wnan.70034.
Editor‐in‐Chief: Fabiana Quaglia
Executive Editor: Nancy Ann Monteiro‐Riviere
Funding: Supported by the Horizon 2020 project RiskGONE (Grant Agreement no. 8144259), the Horizon Europe funded Partnership for Assessment of the Risks of Chemicals (PARC) via Grant Agreement no. 101057014, with the UK's contribution to PARC funded via the UKRI Innovate UK Horizon Guarantee Fund (project number 10045979), the European Regional Development Fund project KK.01.1.1.02.0007 “Research and Education Centre of Environmental Health and Radiation Protection—Reconstruction and Expansion of the Institute for Medical Research and Occupational Health” and the European Union—Next Generation EU (Program Contract of 8 December 2023, Class: 643‐02/23‐01/00016, Reg. no. 533‐03‐23‐0006).
Data Availability Statement
Data are available in article Supporting Information or openly available in a public repository that issues datasets with DOIs.
References
- Aftab, M. N. , Akram I. N., Khosa T., et al. 2018. “Oral Supplementation of Lanthanum Zirconate Nanoparticles Moderately Affected Behavior but Drastically Disturbed Leukocyte Count, Serum Cholesterol Levels and Antioxidant Parameters From Vital Organs of Albino Mice in a Gender Specific Manner.” Metabolic Brain Disease 33, no. 5: 1421–1429. 10.1007/s11011-018-0248-9. [DOI] [PubMed] [Google Scholar]
- Ainsworth, C. 2015. “Sex Redefined.” Nature 518, no. 7539: 288–291. 10.1038/518288a. [DOI] [PubMed] [Google Scholar]
- Ajdary, M. , Negahdary M., Arefian Z., and Dastjerdi H.. 2015. “Toxic Effects of Mn2O3 Nanoparticles on Rat Testis and Sex Hormone.” Journal of Natural Science, Biology and Medicine 6, no. 2: 335–339. 10.4103/0976-9668.159998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram, I. N. , Akhtar S., Khadija G., et al. 2020. “Synthesis, Characterization, and Biocompatibility of Lanthanum Titanate Nanoparticles in Albino Mice in a Sex‐Specific Manner.” Naunyn‐Schmiedeberg's Archives of Pharmacology 393, no. 6: 1089–1101. 10.1007/s00210-020-01819-z. [DOI] [PubMed] [Google Scholar]
- Al‐Radadi, N. S. , and Adam S. I. Y.. 2020. “Green Biosynthesis of Pt‐Nanoparticles From Anbara Fruits: Toxic and Protective Effects on CCl4 Induced Hepatotoxicity in Wister Rats.” Arabian Journal of Chemistry 13, no. 2: 4386–4403. 10.1016/j.arabjc.2019.08.008. [DOI] [Google Scholar]
- Ammendolia, M. G. , Iosi F., Maranghi F., et al. 2017. “Short‐Term Oral Exposure to Low Doses of Nano‐Sized TiO 2 and Potential Modulatory Effects on Intestinal Cells.” Food and Chemical Toxicology 102: 63–75. 10.1016/j.fct.2017.01.031. [DOI] [PubMed] [Google Scholar]
- An, S. S. A. , Hong J.‐S., Park M.‐K., et al. 2014a. “Prenatal Development Toxicity Study of Zinc Oxide Nanoparticles in Rats.” International Journal of Nanomedicine 9, no. Supplement 2: 159–171. 10.2147/IJN.S57932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S. S. A. , Kim Y.‐R., Lee S.‐Y., et al. 2014b. “Toxicity of Colloidal Silica Nanoparticles Administered Orally for 90 Days in Rats.” International Journal of Nanomedicine 9, no. Supplement 2: 67–78. 10.2147/IJN.S57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S. S. A. , Kim Y.‐R., Park J.‐I., et al. 2014c. “Toxicity of 100 Nm Zinc Oxide Nanoparticles: A Report of 90‐Day Repeated Oral Administration in Sprague Dawley Rats.” International Journal of Nanomedicine 9, no. Supplement 2: 109–126. 10.2147/IJN.S57928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S. S. A. , Lee J.‐A., Kim M.‐K., et al. 2014. “Tissue Distribution and Excretion Kinetics of Orally Administered Silica Nanoparticles in Rats.” International Journal of Nanomedicine 9: 251. 10.2147/IJN.S57939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle, T. E. 2006. “Are There Sex and Gender Differences in Acute Exposure to Chemicals in the Same Setting?” Environmental Research 101, no. 2: 195–204. 10.1016/j.envres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Atia, M. M. , and Alghriany A. A. I.. 2021. “Adipose‐Derived Mesenchymal Stem Cells Rescue Rat Hippocampal Cells From Aluminum Oxide Nanoparticle‐Induced Apoptosis via Regulation of P53, Aβ, SOX2, OCT4, and CYP2E1.” Toxicology Reports 8: 1156–1168. 10.1016/j.toxrep.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badkoobeh, P. , Parivar K., Kalantar S. M., Hosseini S. D., and Salabat A.. 2013. “Effect of Nano‐Zinc Oxide on Doxorubicin‐ Induced Oxidative Stress and Sperm Disorders in Adult Male Wistar Rats.” Iranian Journal of Reproductive Medicine 11, no. 5: 355–364. [PMC free article] [PubMed] [Google Scholar]
- Baki, M. E. , Miresmaili S. M., Pourentezari M., et al. 2014. “Effects of Silver Nano‐Particles on Sperm Parameters, Number of Leydig Cells and Sex Hormones in Rats.” Iranian Journal of Reproductive Medicine 12, no. 2: 139–144. [PMC free article] [PubMed] [Google Scholar]
- Bando, R. , Lopez‐Boo F., Fernald L., Gertler P., and Reynolds S.. 2024. “Gender Differences in Early Child Development: Evidence From Large‐Scale Studies of Very Young Children in Nine Countries.” Journal of Economics, Race, and Policy 7, no. 2: 82–92. 10.1007/s41996-023-00131-1. [DOI] [Google Scholar]
- Barbir, R. , Goessler W., Ćurlin M., et al. 2019. “Protein Corona Modulates Distribution and Toxicological Effects of Silver Nanoparticles in Vivo.” Particle & Particle Systems Characterization 36, no. 8: 1900174. 10.1002/ppsc.201900174. [DOI] [Google Scholar]
- Bautista‐Pérez, R. , Cano‐Martínez A., Herrera‐Rodríguez M. A., et al. 2024. “Oral Exposure to Titanium Dioxide E171 and Zinc Oxide Nanoparticles Induces Multi‐Organ Damage in Rats: Role of Ceramide.” International Journal of Molecular Sciences 25, no. 11: 5881. 10.3390/ijms25115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, G. R. , Ha S.‐W., Camalier C. E., et al. 2012. “Bioactive Silica‐Based Nanoparticles Stimulate Bone‐Forming Osteoblasts, Suppress Bone‐Resorbing Osteoclasts, and Enhance Bone Mineral Density in Vivo.” Nanomedicine: Nanotechnology, Biology, and Medicine 8, no. 6: 793–803. 10.1016/j.nano.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, A. , Harbin S., Irvin E., et al. 2021. “Sex and Gender Differences in Occupational Hazard Exposures: A Scoping Review of the Recent Literature.” Current Environmental Health Reports 8, no. 4: 267–280. 10.1007/s40572-021-00330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau, M. D. , Imam M. S., Paredes A. M., et al. 2016. “Differential Effects of Silver Nanoparticles and Silver Ions on Tissue Accumulation, Distribution, and Toxicity in the Sprague Dawley Rat Following Daily Oral Gavage Administration for 13 Weeks.” Toxicological Sciences 150, no. 1: 131–160. 10.1093/toxsci/kfv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A.‐F. , Hynes J., Walker L. A., et al. 2020. “Biological and Anthropogenic Predictors of Metal Concentration in the Eurasian Otter, a Sentinel of Freshwater Ecosystems.” Environmental Pollution 266: 115280. 10.1016/j.envpol.2020.115280. [DOI] [PubMed] [Google Scholar]
- British Beauty Council . 2025. “British Beauty Council 2025 Foundation”.
- Canadian Institutes of Health Research – Institute of Gender and Health . 2012. “What a Difference Sex and Gender Make: A Gender, Sex and Health Research Casebook. In What a Difference Sex and Gender Make: A Gender, Sex and Health Research Casebook”.
- Chella Krishnan, K. , Mehrabian M., and Lusis A. J.. 2018. “Sex Differences in Metabolism and Cardiometabolic Disorders.” Current Opinion in Lipidology 29, no. 5: 404–410. 10.1097/MOL.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Han S., Zhang J., et al. 2021. “Exploring Urine Biomarkers of Early Health Effects for Occupational Exposure to Titanium Dioxide Nanoparticles Using Metabolomics.” Nanoscale 13, no. 7: 4122–4132. 10.1039/D0NR08792K. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Han S., Zheng P., Zhou S., and Jia G.. 2020. “Combined Effect of Titanium Dioxide Nanoparticles and Glucose on the Blood Glucose Homeostasis in Young Rats After Oral Administration.” Journal of Applied Toxicology 40, no. 9: 1284–1296. 10.1002/jat.3985. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Meng H., Xing G., et al. 2006. “Acute Toxicological Effects of Copper Nanoparticles In Vivo.” Toxicology Letters 163, no. 2: 109–120. 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Zhou D., Zhou S., and Jia G.. 2019. “Gender Difference in Hepatic Toxicity of Titanium Dioxide Nanoparticles After Subchronic Oral Exposure in Sprague‐Dawley Rats.” Journal of Applied Toxicology 39, no. 5: 807–819. 10.1002/jat.3769. [DOI] [PubMed] [Google Scholar]
- Cheng, X. , and Klaassen C. D.. 2009. “Tissue Distribution, Ontogeny, and Hormonal Regulation of Xenobiotic Transporters in Mouse Kidneys.” Drug Metabolism and Disposition 37, no. 11: 2178–2185. 10.1124/dmd.109.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, J. A. , and Collins F. S.. 2014. “Policy: NIH to Balance Sex in Cell and Animal Studies.” Nature 509, no. 7500: 282–283. 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, F. S. , and Tabak L. A.. 2014. “Policy: NIH Plans to Enhance Reproducibility.” Nature 505, no. 7485: 612–613. 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, C. , and Stabenfeldt S. E.. 2020. “Leveraging the Dynamic Blood–Brain Barrier for Central Nervous System Nanoparticle‐Based Drug Delivery Applications.” Current Opinion in Biomedical Engineering 14: 1–8. 10.1016/j.cobme.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantine, M. M. 2014. “Physiologic and Pharmacokinetic Changes in Pregnancy.” Frontiers in Pharmacology 5: 65. 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćurlin, M. , Barbir R., Dabelić S., et al. 2021. “Sex Affects the Response of Wistar Rats to Polyvinyl Pyrrolidone (PVP)‐coated Silver Nanoparticles in an Oral 28 Days Repeated Dose Toxicity Study.” Particle and Fibre Toxicology 18, no. 1: 38. 10.1186/s12989-021-00425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak, R. 2001. “Gender‐Based Differences in Pharmacokinetics in Laboratory Animal Models.” International Journal of Toxicology 20, no. 3: 161–163. 10.1080/109158101317097746. [DOI] [PubMed] [Google Scholar]
- Dam, D. H. M. , Culver K. S. B., Kandela I., et al. 2015. “Biodistribution and in Vivo Toxicity of Aptamer‐Loaded Gold Nanostars.” Nanomedicine: Nanotechnology, Biology and Medicine 11, no. 3: 671–679. 10.1016/j.nano.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, W. 2010. “Genomic Imprinting on the X Chromosome: Implications for Brain and Behavioral Phenotypes.” Annals of the New York Academy of Sciences 1204, no. s1: 14–19. 10.1111/j.1749-6632.2010.05567.x. [DOI] [PubMed] [Google Scholar]
- DeLorme, M. P. , Muro Y., Arai T., et al. 2012. “Ninety‐Day Inhalation Toxicity Study With A Vapor Grown Carbon Nanofiber in Rats.” Toxicological Sciences 128, no. 2: 449–460. 10.1093/toxsci/kfs172. [DOI] [PubMed] [Google Scholar]
- Duffy, K. A. , Ziolek T. A., and Epperson C. N.. 2020. “Filling the Regulatory Gap: Potential Role of Institutional Review Boards in Promoting Consideration of Sex as a Biological Variable.” Journal of Women's Health 29, no. 6: 868–875. 10.1089/jwh.2019.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Ela, F. I. A. , Farghali A. A., Mahmoud R. K., Mohamed N. A., and Moaty S. A. A.. 2019. “New Approach in Ulcer Prevention and Wound Healing Treatment Using Doxycycline and Amoxicillin/LDH Nanocomposites.” Scientific Reports 9, no. 1: 6418. 10.1038/s41598-019-42842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . 2020a. “A Union of Equality: Gender Equality Strategy 2020‐2025. COM/2020/152 Final, 4”.
- European Commission . 2013. “Gendered Innovations—How Gender Analysis Contributes to Research: Report of the Expert Group Innovation Through Gender. Publications Office”.
- European Commission . 2020b. “Gendered Innovations—How Inclusive Analysis Contributes to Research and Innovation. Publications Office”.
- 2016. “Gender in Cardiovascular Diseases: Impact on Clinical Manifestations, Management, and Outcomes.” European Heart Journal 37, no. 1: 24–34. 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- Fausto‐Sterling, A. 2012. “The Dynamic Development of Gender Variability.” Journal of Homosexuality 59, no. 3: 398–421. 10.1080/00918369.2012.653310. [DOI] [PubMed] [Google Scholar]
- FDA . 1987. “Guideline for the Format and Content of the Nonclinical Pharmacology/Toxicology Section of an Application”.
- FDA . 1988. “Guideline for the Format of and Content of the Clinical and Statistical Sections of an Application”.
- FDA . 1993a. “Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs; Notice. In Federal Register (Vol. 58, Issue 139, pp. 39406–39416)”. [PubMed]
- FDA . 1993b. “Study and Evaluation of Sex Differences in the Clinical Evaluation of Drugs”.
- FDA . 1998. “Investigational New Drug Applications and New Drug Applications.” Federal Register 63: 6854–6862. [PubMed] [Google Scholar]
- Feghali, M. , Venkataramanan R., and Caritis S.. 2015. “Pharmacokinetics of Drugs in Pregnancy.” Seminars in Perinatology 39, no. 7: 512–519. 10.1053/j.semperi.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Cruz, M. L. , Hernández‐Moreno D., Catalán J., et al. 2018. “Quality Evaluation of Human and Environmental Toxicity Studies Performed With Nanomaterials – The GUIDEnano Approach.” Environmental Science: Nano 5, no. 2: 381–397. 10.1039/C7EN00716G. [DOI] [Google Scholar]
- Fiala, J. , and Brázdová Z.. 2000. “A Comparison Between the Lifestyles of Men and Women—Parents of School Age Children.” Central European Journal of Public Health 8, no. 2: 94–100. [PubMed] [Google Scholar]
- Fonseca‐Gomes, J. , Loureiro J. A., Tanqueiro S. R., et al. 2020. “In Vivo Bio‐Distribution and Toxicity Evaluation of Polymeric and Lipid‐Based Nanoparticles: A Potential Approach for Chronic Diseases Treatment.” International Journal of Nanomedicine 15: 8609–8621. 10.2147/IJN.S267007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi, F. , and Campesi I.. 2014. “Pharmacogenomics, Pharmacokinetics and Pharmacodynamics: Interaction With Biological Differences Between Men and Women.” British Journal of Pharmacology 171, no. 3: 580–594. 10.1111/bph.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, A. , Stanko P., Berkowitz L. N., et al. 2017. “Inclusion of Sex and Gender in Biomedical Research: Survey of Clinical Research Proposed at the University of Pennsylvania.” Biology of Sex Differences 8, no. 1: 22. 10.1186/s13293-017-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, J. , Sun J., Chang X., et al. 2020. “Biodistribution and Organ Oxidative Damage Following 28 Days Oral Administration of Nanosilver With/Without Coating in Mice.” Journal of Applied Toxicology 40, no. 6: 815–831. 10.1002/jat.3946. [DOI] [PubMed] [Google Scholar]
- Geller, S. E. , Koch A. R., Roesch P., Filut A., Hallgren E., and Carnes M.. 2018. “The More Things Change, the More They Stay the Same.” Academic Medicine 93, no. 4: 630–635. 10.1097/ACM.0000000000002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi, S. , Tabatabaei S. R. F., Varzi H. N., and Rashno M.. 2015. “Induced Adverse Effects of Prenatal Exposure to Silver Nanoparticles on Neurobehavioral Development of Offspring of Mice.” Journal of Toxicological Sciences 40, no. 2: 263–275. 10.2131/jts.40.263. [DOI] [PubMed] [Google Scholar]
- Gochfeld, M. 2007. “Framework for Gender Differences in Human and Animal Toxicology.” Environmental Research 104, no. 1: 4–21. 10.1016/j.envres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gochfeld, M. 2017. “Sex Differences in Human and Animal Toxicology: Toxicokinetics.” Toxicologic Pathology 45, no. 1: 172–189. 10.1177/0192623316677327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokulan, K. , Williams K., Orr S., and Khare S.. 2020. “Human Intestinal Tissue Explant Exposure to Silver Nanoparticles Reveals Sex Dependent Alterations in Inflammatory Responses and Epithelial Cell Permeability.” International Journal of Molecular Sciences 22, no. 1: 9. 10.3390/ijms22010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki, W. , Dziendzikowska K., Gromadzka‐Ostrowska J., et al. 2024. “In Vivo pro‐Inflammatory Effects of Silver Nanoparticles on the Colon Depend on Time and Route of Exposure.” International Journal of Molecular Sciences 25, no. 9: 4879. 10.3390/ijms25094879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau, E. , Djouina M., Caboche S., et al. 2022. “Exposure to Atmospheric ag, TiO2, Ti and SiO2 Engineered Nanoparticles Modulates Gut Inflammatory Response and Microbiota in Mice.” Ecotoxicology and Environmental Safety 236: 113442. 10.1016/j.ecoenv.2022.113442. [DOI] [PubMed] [Google Scholar]
- Guo, Y. , Jiang N., Zhang L., and Yin M.. 2020. “Green Synthesis of Gold Nanoparticles From Fritillaria Cirrhosa and Its Anti‐Diabetic Activity on Streptozotocin Induced Rats.” Arabian Journal of Chemistry 13, no. 4: 5096–5106. 10.1016/j.arabjc.2020.02.009. [DOI] [Google Scholar]
- Gutleb, H. N. R. , and Gutleb A. C.. 2023. “A Short History of the Consideration of Sex Differences in Biomedical Research — Lessons for the in Vitro Community From Animal Models and Human Clinical Trials.” Alternatives to Laboratory Animals 51, no. 2: 144–150. 10.1177/02611929231156720. [DOI] [PubMed] [Google Scholar]
- Haghani, A. , Johnson R. G., Woodward N. C., et al. 2020. “Adult Mouse Hippocampal Transcriptome Changes Associated With Long‐Term Behavioral and Metabolic Effects of Gestational Air Pollution Toxicity.” Translational Psychiatry 10, no. 1: 218. 10.1038/s41398-020-00907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajipour, M. J. , Aghaverdi H., Serpooshan V., Vali H., Sheibani S., and Mahmoudi M.. 2021. “Sex as an Important Factor in Nanomedicine.” Nature Communications 12, no. 1: 2984. 10.1038/s41467-021-23230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H.‐Y. , Cho J.‐W., Seong E., et al. 2020. “Amorphous Silica Nanoparticle‐Induced Pulmonary Inflammatory Response Depends on Particle Size and Is Sex‐Specific in Rats.” Toxicology and Applied Pharmacology 390: 114890. 10.1016/j.taap.2020.114890. [DOI] [PubMed] [Google Scholar]
- Heidari, S. , Babor T. F., De Castro P., Tort S., and Curno M.. 2016. “Sex and Gender Equity in Research: Rationale for the SAGER Guidelines and Recommended Use.” Research Integrity and Peer Review 1, no. 1: 2. 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise, L. , Greene M. E., Opper N., et al. 2019. “Gender Inequality and Restrictive Gender Norms: Framing the Challenges to Health.” Lancet 393, no. 10189: 2440–2454. 10.1016/S0140-6736(19)30652-X. [DOI] [PubMed] [Google Scholar]
- Hong, J.‐S. , Park M.‐K., Kim M.‐S., et al. 2014. “Effect of Zinc Oxide Nanoparticles on Dams and Embryo‐Fetal Development in Rats.” International Journal of Nanomedicine 9, no. Suppl 2: 145–157. 10.2147/IJN.S57931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, M. F. , Naeem Ashiq M., Gulsher M., Akbar A., and Iqbal F.. 2020. “Exposure to Variable Doses of Nickel Oxide Nanoparticles Disturbs Serum Biochemical Parameters and Oxidative Stress Biomarkers From Vital Organs of Albino Mice in a Sex‐Specific Manner.” Biomarkers 25, no. 8: 719–724. 10.1080/1354750X.2020.1841829. [DOI] [PubMed] [Google Scholar]
- Hyde, J. S. , Bigler R. S., Joel D., Tate C. C., and van Anders S. M.. 2019. “The Future of Sex and Gender in Psychology: Five Challenges to the Gender Binary.” American Psychologist 74, no. 2: 171–193. 10.1037/amp0000307. [DOI] [PubMed] [Google Scholar]
- Jackson, P. , Hougaard K. S., Vogel U., et al. 2012. “Exposure of Pregnant Mice to Carbon Black by Intratracheal Instillation: Toxicogenomic Effects in Dams and Offspring.” Mutation Research, Genetic Toxicology and Environmental Mutagenesis 745, no. 1–2: 73–83. 10.1016/j.mrgentox.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Joseph, A. , Wood T., Chen C.‐C., et al. 2018. “Curcumin‐Loaded Polymeric Nanoparticles for Neuroprotection in Neonatal Rats With Hypoxic‐Ischemic Encephalopathy.” Nano Research 11, no. 10: 5670–5688. 10.1007/s12274-018-2104-y. [DOI] [Google Scholar]
- Joseph, S. , Nicolson T. J., Hammons G., Word B., Green‐Knox B., and Lyn‐Cook B.. 2015. “Expression of Drug Transporters in Human Kidney: Impact of Sex, Age, and Ethnicity.” Biology of Sex Differences 6, no. 1: 1–15. 10.1186/s13293-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H. , Huang D., Jing J., et al. 2024. “Fasn Involved in the Nephrotoxicity Induced by Polystyrene Nanoplastics and the Intervention of Melatonin Through Intestinal Microbiota‐Mediated Lipid Metabolism Disorder.” Nano Research 17, no. 8: 7365–7375. 10.1007/s12274-024-6610-9. [DOI] [Google Scholar]
- Kasai, T. , Umeda Y., Ohnishi M., et al. 2015. “Thirteen‐Week Study of Toxicity of Fiber‐Like Multi‐Walled Carbon Nanotubes With Whole‐Body Inhalation Exposure in Rats.” Nanotoxicology 9, no. 4: 413–422. 10.3109/17435390.2014.933903. [DOI] [PubMed] [Google Scholar]
- Kasai, T. , Umeda Y., Ohnishi M., et al. 2015. “Lung Carcinogenicity of Inhaled Multi‐Walled Carbon Nanotube in Rats.” Particle and Fibre Toxicology 13, no. 1: 53. 10.1186/s12989-016-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, M. , Naito T., Ueno M., et al. 2002. “Induction of Mucosal IgA Following Intravaginal Administration of Inactivated HIV‐1‐Capturing Nanospheres in Mice.” Journal of Medical Virology 66, no. 3: 291–298. 10.1002/jmv.2144. [DOI] [PubMed] [Google Scholar]
- Khairy Elkady, N. , Roshdy Abouelkheir A., Ghaleb S., et al. 2024. “Evaluating the Possible Genotoxicity of Nanoaluminum Incorporated in Human Vaccines and the Potential Protective Role of Nanocurcumin: An in Vivo Study.” Toxicology Mechanisms and Methods 34, no. 7: 813–820. 10.1080/15376516.2024.2352736. [DOI] [PubMed] [Google Scholar]
- Kim, J.‐C. , Lee I.‐C., Ko J.‐W., et al. 2016. “Comparative Toxicity and Biodistribution of Copper Nanoparticles and Cupric Ions in Rats.” International Journal of Nanomedicine 11: 2883–2900. 10.2147/IJN.S106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. , Hosseindoust A., Choi Y., et al. 2021. “Effects of Hot‐Melt Extruded Nano‐Copper as an Alternative for the Pharmacological Dose of Copper Sulfate in Weanling Pigs.” Biological Trace Element Research 199, no. 8: 2925–2935. 10.1007/s12011-020-02426-y. [DOI] [PubMed] [Google Scholar]
- Kim, S.‐E. 2015. “Sex‐ and Gender‐Specific Disparities in Colorectal Cancer Risk.” World Journal of Gastroenterology 21, no. 17: 5167. 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W.‐Y. , Kim J., Park J. D., Ryu H. Y., and Yu I. J.. 2009. “Histological Study of Gender Differences in Accumulation of Silver Nanoparticles in Kidneys of Fischer 344 Rats.” Journal of Toxicology and Environmental Health, Part A 72, no. 21–22: 1279–1284. 10.1080/15287390903212287. [DOI] [PubMed] [Google Scholar]
- Kim, Y. S. , Kim J. S., Cho H. S., et al. 2008. “Twenty‐Eight‐Day Oral Toxicity, Genotoxicity, and Gender‐Related Tissue Distribution of Silver Nanoparticles in Sprague‐Dawley Rats.” Inhalation Toxicology 20, no. 6: 575–583. 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kim, Y. S. , Park J. S., Park M., et al. 2018. “PLGA Nanoparticles With Multiple Modes Are a Biologically Safe Nanocarrier for Mammalian Development and Their Offspring.” Biomaterials 183: 43–53. 10.1016/j.biomaterials.2018.08.042. [DOI] [PubMed] [Google Scholar]
- Kiratipaiboon, C. , Voronkova M., Ghosh R., et al. 2020. “SOX2Mediates Carbon Nanotube‐Induced Fibrogenesis and Fibroblast Stem Cell Acquisition.” ACS Biomaterials Science & Engineering 6, no. 9: 5290–5304. 10.1021/acsbiomaterials.0c00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S. L. , Marriott I., and Fish E. N.. 2015. “Sex‐Based Differences in Immune Function and Responses to Vaccination.” Transactions of the Royal Society of Tropical Medicine and Hygiene 109, no. 1: 9–15. 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimisch, H.‐J. , Andreae M., and Tillmann U.. 1997. “A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data.” Regulatory Toxicology and Pharmacology 25, no. 1: 1–5. 10.1006/rtph.1996.1076. [DOI] [PubMed] [Google Scholar]
- Ko, J.‐W. , Hong E.‐T., Lee I.‐C., et al. 2015. “Evaluation of 2‐Week Repeated Oral Dose Toxicity of 100 Nm Zinc Oxide Nanoparticles in Rats.” Laboratory Animal Research 31, no. 3: 139. 10.5625/lar.2015.31.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobyliak, N. M. , Falalyeyeva T. M., Kuryk O. G., et al. 2015. “Antioxidative Effects of Cerium Dioxide Nanoparticles Ameliorate Age‐Related Male Infertility: Optimistic Results in Rats and the Review of Clinical Clues for Integrative Concept of Men Health and Fertility.” EPMA Journal 6, no. 1: 12. 10.1186/s13167-015-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L. , Tang M., Zhang T., et al. 2014. “Nickel Nanoparticles Exposure and Reproductive Toxicity in Healthy Adult Rats.” International Journal of Molecular Sciences 15, no. 11: 21253–21269. 10.3390/ijms151121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb, S. , Piraquive J., Lamberton F., et al. 2016. “Safety Evaluation and Imaging Properties of Gadolinium‐Based Nanoparticles in Nonhuman Primates.” Scientific Reports 6, no. 1: 35053. 10.1038/srep35053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, U. , Jacke K., Dandolo L., et al. 2023. “Operationalization of a Multidimensional Sex/Gender Concept for Quantitative Environmental Health Research and Implementation in the KORA Study: Results of the Collaborative Research Project INGER.” Frontiers in Public Health 11: 1128918. 10.3389/fpubh.2023.1128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, N. S. R. , Getchell T. V., Awasthi Y. C., Dhooper N., and Getchell M. L.. 1995. “Age‐ and Gender‐Related Trends in the Expression of Glutathione S ‐Transferases in Human Nasal Mucosa.” Annals of Otology, Rhinology and Laryngology 104, no. 10: 812–822. 10.1177/000348949510401012. [DOI] [PubMed] [Google Scholar]
- Kudo, N. , Katakura M., Sato Y., and Kawashima Y.. 2002. “Sex Hormone‐Regulated Renal Transport of Perfluorooctanoic Acid.” Chemico‐Biological Interactions 139, no. 3: 301–316. 10.1016/S0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Kwekel, J. C. , Desai V. G., Moland C. L., Vijay V., and Fuscoe J. C.. 2013. “Sex Differences in Kidney Gene Expression During the Life Cycle of F344 Rats.” Biology of Sex Differences 4, no. 1: 14. 10.1186/2042-6410-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, X. , Wang M., Zhang Z., et al. 2024. “ZNPs Reduce Epidermal Mechanical Strain Resistance by Promoting Desmosomal Cadherin Endocytosis via mTORC1‐TFEB‐BLOC1S3 Axis.” Journal of Nanobiotechnology 22, no. 1: 312. 10.1186/s12951-024-02519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton, J. S. 2011. “Sex and Gender Differences in Coronary Artery Disease.” Seminars in Thoracic and Cardiovascular Surgery 23, no. 2: 126–130. 10.1053/j.semtcvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Lee, C. T. , Adegunsoye A., Chung J. H., et al. 2021. “Characteristics and Prevalence of Domestic and Occupational Inhalational Exposures Across Interstitial Lung Diseases.” Chest 160, no. 1: 209–218. 10.1016/j.chest.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Hosseindoust A., Kim M., et al. 2019. “Effects of Hot Melt Extrusion Processed Nano‐Iron on Growth Performance, Blood Composition, and Iron Bioavailability in Weanling Pigs.” Journal of Animal Science and Technology 61, no. 4: 216–224. 10.5187/jast.2019.61.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Kim Y. S., Song K. S., et al. 2013. “Biopersistence of Silver Nanoparticles in Tissues From Sprague‐Dawley Rats.” Particle and Fibre Toxicology 10, no. 1: 36. 10.1186/1743-8977-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Kim Y. S., Sung J. H., et al. 2020. “Sex‐Specific Accumulation of Silver Nanoparticles in Rat Kidneys Is Not Ovarian Hormone Regulated but Elimination Limited.” NanoImpact 20: 100255. 10.1016/j.impact.2020.100255. [DOI] [Google Scholar]
- Lelovas, P. , Efthimiadou E. K., Mantziaras G., Siskos N., Kordas G., and Kostomitsopoulos N.. 2018. “In Vivo Toxicity Study of Quatro Stimuli Nanocontainers in Pregnant Rats: Gestation, Parturition and Offspring Evaluation.” Regulatory Toxicology and Pharmacology 98: 161–167. 10.1016/j.yrtph.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Jiang L., Feng J., et al. 2024. “Aged Polystyrene Microplastics Exacerbate Alopecia Associated With Tight Junction Injuries and Apoptosis via Oxidative Stress Pathway in Skin.” Environment International 186: 108638. 10.1016/j.envint.2024.108638. [DOI] [PubMed] [Google Scholar]
- Li, X. , Xu H., Zhao X., et al. 2024. “Ferroptosis Contributing to Cardiomyocyte Injury Induced by Silica Nanoparticles via miR‐125b‐2‐3p/HO‐1 Signaling.” Particle and Fibre Toxicology 21, no. 1: 17. 10.1186/s12989-024-00579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Xu H., Wang Y., et al. 2024. “Epithelium‐Derived Exosomes Promote Silica Nanoparticles‐Induced Pulmonary Fibroblast Activation and Collagen Deposition via Modulating Fibrotic Signaling Pathways and Their Epigenetic Regulations.” Journal of Nanobiotechnology 22, no. 1: 331. 10.1186/s12951-024-02609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, C. , Lin L., Hu J., Ma Y., Li Y., and Sun Z.. 2024. “Comprehensive Pulmonary Metabolic Responses to Silica Nanoparticles Exposure in Fisher 344 Rats.” Ecotoxicology and Environmental Safety 275: 116256. 10.1016/j.ecoenv.2024.116256. [DOI] [PubMed] [Google Scholar]
- Liang, Y. , Yang Y., Lu C., et al. 2024. “Polystyrene Nanoplastics Exposure Triggers Spermatogenic Cell Senescence via the Sirt1/ROS Axis.” Ecotoxicology and Environmental Safety 279: 116461. 10.1016/j.ecoenv.2024.116461. [DOI] [PubMed] [Google Scholar]
- Lim, J. , Kim S., Shin I., et al. 2011. “Maternal Exposure to Multi‐Wall Carbon Nanotubes Does Not Induce Embryo–Fetal Developmental Toxicity in Rats.” Birth Defects Research Part B: Developmental and Reproductive Toxicology 92, no. 1: 69–76. 10.1002/bdrb.20283. [DOI] [PubMed] [Google Scholar]
- Liu, P. , Huang Z., and Gu N.. 2013. “Exposure to Silver Nanoparticles Does Not Affect Cognitive Outcome or Hippocampal Neurogenesis in Adult Mice.” Ecotoxicology and Environmental Safety 87: 124–130. 10.1016/j.ecoenv.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Loddo, G. , Cottonaro S., Daga F., and Bellini P.. 2014. “Gender Medicine A New Approach for Healthcare”.
- Lu, Y.‐Y. , Hua W., Lu L., Tian M., and Huang Q.. 2024. “The Size‐Dependence and Reversibility of Polystyrene Nanoplastics‐Induced Hepatic Pyroptosis in Mice Through TXNIP/NLRP3/GSDMD Pathway.” Toxicology Research 13, no. 4: tfae106. 10.1093/toxres/tfae106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, N. S. , Mohamed M. R., Ali M. A. M., Aglan H. A., Amr K. S., and Ahmed H. H.. 2021. “Nanomaterial‐Induced Mesenchymal Stem Cell Differentiation Into Osteoblast for Counteracting Bone Resorption in the Osteoporotic Rats.” Tissue and Cell 73: 101645. 10.1016/j.tice.2021.101645. [DOI] [PubMed] [Google Scholar]
- Matsumoto, M. , Serizawa H., Sunaga M., et al. 2012. “No Toxicological Effects on Acute and Repeated Oral Gavage Doses of Single‐Wall or Multi‐Wall Carbon Nanotube in Rats.” Journal of Toxicological Sciences 37, no. 3: 463–474. 10.2131/jts.37.463. [DOI] [PubMed] [Google Scholar]
- McGregor, A. J. , Hasnain M., Sandberg K., Morrison M. F., Berlin M., and Trott J.. 2016. “How to Study the Impact of Sex and Gender in Medical Research: A Review of Resources.” Biology of Sex Differences 7, no. Suppl 1: 46. 10.1186/s13293-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, M. H. , and Edwards C. P.. 2009. “The Early Development of Gender Differences.” Annual Review of Anthropology 38, no. 1: 83–97. 10.1146/annurev-anthro-091908-164338. [DOI] [Google Scholar]
- Milivojević, T. , Drobne D., Romih T., et al. 2016. “Chronic Exposure to Zinc Oxide Nanoparticles Increases Ischemic‐Reperfusion Injuries in Isolated Rat Hearts.” Journal of Nanoparticle Research 18, no. 10: 309. 10.1007/s11051-016-3573-0. [DOI] [Google Scholar]
- Miller, I. , Serchi T., Cambier S., et al. 2016. “Hexabromocyclododecane (HBCD) Induced Changes in the Liver Proteome of Eu‐ and Hypothyroid Female Rats.” Toxicology Letters 245: 40–51. 10.1016/j.toxlet.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Miller, M. A. 2001. “Gender‐Based Differences in the Toxicity of Pharmaceuticals—The Food and Drug Administration's Perspective.” International Journal of Toxicology 20, no. 3: 149–152. 10.1080/109158101317097728. [DOI] [PubMed] [Google Scholar]
- Mioc, A. , Mioc M., Ghiulai R., et al. 2019. “Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy.” Current Medicinal Chemistry 26, no. 35: 6493–6513. 10.2174/0929867326666190506123721. [DOI] [PubMed] [Google Scholar]
- Mitchell, A. E. , Morin D., Lakritz J., and Jones A. D.. 1997. “Quantitative Profiling of Tissue‐ and Gender‐Related Expression of Glutathione S‐Transferase Isoenzymes in the Mouse.” Biochemical Journal 325, no. 1: 207–216. 10.1042/bj3250207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour, R. , Cheney D. L., Grunberger J. W., et al. 2020. “One‐Year Chronic Toxicity Evaluation of Single Dose Intravenously Administered Silica Nanoparticles in Mice and Their Ex Vivo Human Hemocompatibility.” Journal of Controlled Release 324: 471–481. 10.1016/j.jconrel.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. , Yang J., Lan T. T. H., et al. 2016. “Biocompatibility Assessment of Detonation Nanodiamond in Non‐Human Primates and Rats Using Histological, Hematologic, and Urine Analysis.” ACS Nano 10, no. 8: 7385–7400. 10.1021/acsnano.6b00839. [DOI] [PubMed] [Google Scholar]
- Morris‐Schaffer, K. , Merrill A., Jew K., et al. 2019. “Effects of Neonatal Inhalation Exposure to Ultrafine Carbon Particles on Pathology and Behavioral Outcomes in C57BL/6J Mice.” Particle and Fibre Toxicology 16, no. 1: 10. 10.1186/s12989-019-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen, N. P. , Pathmasiri W., Snyder R. W., et al. 2022. “Oral Administration of TiO2 Nanoparticles During Early Life Impacts Cardiac and Neurobehavioral Performance and Metabolite Profile in an Age‐ and Sex‐Related Manner.” Particle and Fibre Toxicology 19, no. 1: 3. 10.1186/s12989-021-00444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narciso, L. , Coppola L., Lori G., et al. 2020. “Genotoxicity, Biodistribution and Toxic Effects of Silver Nanoparticles After in Vivo Acute Oral Administration.” NanoImpact 18: 100221. 10.1016/j.impact.2020.100221. [DOI] [Google Scholar]
- Narendran, N. , Luzhna L., and Kovalchuk O.. 2019. “Sex Difference of Radiation Response in Occupational and Accidental Exposure.” Frontiers in Genetics 10: 260. 10.3389/fgene.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . 1993. “Inclusion of Women and Minorities in Clinical Research. 292(1), 1–6”.
- Naz, S. , Ali J. S., Zia M., et al. 2024. “Green and Chemical Synthesis of CuO Nanoparticles: Comparative Reproductive Toxicity Evaluation at Various Phases and in Offsprings.” Materials Research Express 11, no. 7: 075401. 10.1088/2053-1591/ad5f0d. [DOI] [Google Scholar]
- Nicolas, J. M. , Espie P., and Molimard M.. 2009. “Gender and Interindividual Variability in Pharmacokinetics.” Drug Metabolism Reviews 41, no. 3: 408–421. 10.1080/10837450902891485. [DOI] [PubMed] [Google Scholar]
- NIH . 1986. “Inclusion of Women in Study Populations. NIH Guide for Grants and Contracts, 15(22), 1”.
- NIH . 1994. “NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. Federal Register, 59, 1408–1413”.
- NIH . 2015. “Consideration of Sex as a Biological Variable in NIH‐Funded Research.” https://grants.nih.gov/grants/guide/notice‐files/not‐od‐15‐102.html.
- Notter, T. , Aengenheister L., Weber‐Stadlbauer U., et al. 2018. “Prenatal Exposure to TiO2 Nanoparticles in Mice Causes Behavioral Deficits With Relevance to Autism Spectrum Disorder and Beyond.” Translational Psychiatry 8, no. 1: 193. 10.1038/s41398-018-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD . 2021. Gender and the Environment. OECD. 10.1787/3d32ca39-en. [DOI] [Google Scholar]
- Oh, S. M. , Kim M. J., Hosseindoust A., et al. 2019. “Hot Melt Extruded‐Based Nano Zinc as an Alternative to the Pharmacological Dose of ZnO in Weanling Piglets.” Asian‐Australasian Journal of Animal Sciences 33, no. 6: 992–1001. 10.5713/ajas.19.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, Y. S. , Saiful Yazan L., Ng W. K., et al. 2016. “Acute and Subacute Toxicity Profiles of Thymoquinone‐Loaded Nanostructured Lipid Carrier in BALB/c Mice.” International Journal of Nanomedicine 11: 5905–5915. 10.2147/IJN.S114205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek, H.‐J. , Chung H.‐E., Lee J.‐A., et al. 2014. “Quantitative Determination of Silica Nanoparticles in Biological Matrices and Their Pharmacokinetics and Toxicokinetics in Rats.” Science of Advanced Materials 6, no. 7: 1605–1610. 10.1166/sam.2014.1817. [DOI] [Google Scholar]
- Paek, H.‐J. , Lee Y.‐J., Chung H.‐E., et al. 2013. “Modulation of the Pharmacokinetics of Zinc Oxide Nanoparticles and Their Fates in Vivo.” Nanoscale 5, no. 23: 11416. 10.1039/c3nr02140h. [DOI] [PubMed] [Google Scholar]
- Park, E.‐J. , Choi J., Kim J.‐H., et al. 2016. “Subchronic Immunotoxicity and Screening of Reproductive Toxicity and Developmental Immunotoxicity Following Single Instillation of HIPCO‐Single‐Walled Carbon Nanotubes: Purity‐Based Comparison.” Nanotoxicology 10, no. 8: 1188–1202. 10.1080/17435390.2016.1202348. [DOI] [PubMed] [Google Scholar]
- Park, E.‐J. , Han J.‐S., Park E.‐J., et al. 2020. “Repeated‐Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the Next Generation.” Toxicology Letters 324: 75–85. 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Park, E.‐J. , Jeong U., Kim Y., Lee B.‐S., Cho M.‐H., and Go Y.‐S.. 2017. “Deleterious Effects in Reproduction and Developmental Immunity Elicited by Pulmonary Iron Oxide Nanoparticles.” Environmental Research 152: 503–513. 10.1016/j.envres.2016.08.025. [DOI] [PubMed] [Google Scholar]
- Park, E.‐J. , Yoon C., Han J.‐S., et al. 2021. “Effect of PM10 on Pulmonary Immune Response and Fetus Development.” Toxicology Letters 339: 1–11. 10.1016/j.toxlet.2020.11.024. [DOI] [PubMed] [Google Scholar]
- Park, H.‐S. , Kim S.‐J., Lee T.‐J., et al. 2014. “A 90‐Day Study of Sub‐Chronic Oral Toxicity of 20 Nm Positively Charged Zinc Oxide Nanoparticles in Sprague Dawley Rats.” International Journal of Nanomedicine 9: 3. 10.2147/IJN.S57927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Women's health . 1985. “Report of the Public Health Service Task Force on Women's Health Issues.” Public Health Rep 100, no. 1: 73–106. [PMC free article] [PubMed] [Google Scholar]
- Quinn, M. M. , and Smith P. M.. 2018. “Gender, Work, and Health.” Annals of Work Exposures and Health 62, no. 4: 389–392. 10.1093/annweh/wxy019. [DOI] [PubMed] [Google Scholar]
- Ray, J. L. , and Holian A.. 2019. “Sex Differences in the Inflammatory Immune Response to Multi‐Walled Carbon Nanotubes and Crystalline Silica.” Inhalation Toxicology 31, no. 7: 285–297. 10.1080/08958378.2019.1669743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz, H. , Hashmi R., Abid S., et al. 2020. “Intraperitoneal Injections of Copper Ferrite Nanoparticles Disturb Blood, Plasma, and Antioxidant Parameters of Wistar Rats in a Sex‐Specific Manner.” Naunyn‐Schmiedeberg's Archives of Pharmacology 393, no. 11: 2019–2028. 10.1007/s00210-020-01899-x. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Serra, V. , Buslón N., Philippe O. R., et al. 2024. “Analyzing Sex Imbalance in EGA and dbGaP Biological Databases: Recommendations for Better Practices.” IScience 27, no. 10: 110831. 10.1016/j.isci.2024.110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandlyn, M. J. , Stuart E. C., and Rosengren R. J.. 2008. “Sex‐Specific Differences in CYP450 Isoforms in Humans.” Expert Opinion on Drug Metabolism & Toxicology 4, no. 4: 413–424. 10.1517/17425255.4.4.413. [DOI] [PubMed] [Google Scholar]
- Schiebinger, L. 2000. “Has Feminism Changed Science?” Signs: Journal of Women in Culture and Society 25, no. 4: 1171–1175. 10.1086/495540. [DOI] [PubMed] [Google Scholar]
- Seok, S. H. , Cho W., Park J. S., et al. 2013. “Rat Pancreatitis Produced by 13‐Week Administration of Zinc Oxide Nanoparticles: Biopersistence of Nanoparticles and Possible Solutions.” Journal of Applied Toxicology 33, no. 10: 1089–1096. 10.1002/jat.2862. [DOI] [PubMed] [Google Scholar]
- Sharifi, S. , Caracciolo G., Pozzi D., et al. 2021. “The Role of Sex as a Biological Variable in the Efficacy and Toxicity of Therapeutic Nanomedicine.” Advanced Drug Delivery Reviews 174: 337–347. 10.1016/j.addr.2021.04.028. [DOI] [PubMed] [Google Scholar]
- Shende, P. , Kulkarni Y. A., Gaud R. S., et al. 2015. “Acute and Repeated Dose Toxicity Studies of Different β‐Cyclodextrin‐Based Nanosponge Formulations.” Journal of Pharmaceutical Sciences 104, no. 5: 1856–1863. 10.1002/jps.24416. [DOI] [PubMed] [Google Scholar]
- Singh, S. P. , Kumari M., Kumari S. I., Rahman M. F., Mahboob M., and Grover P.. 2013. “Toxicity Assessment of Manganese Oxide Micro and Nanoparticles in Wistar Rats After 28 Days of Repeated Oral Exposure.” Journal of Applied Toxicology 33, no. 10: 1165–1179. 10.1002/jat.2887. [DOI] [PubMed] [Google Scholar]
- Smith, L. C. , Moreno S., Robinson S., et al. 2019. “Multi‐Walled Carbon Nanotubes Inhibit Estrogen Receptor Expression in Vivo and in Vitro Through Transforming Growth Factor beta1.” NanoImpact 14: 100152. 10.1016/j.impact.2019.100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofranko, A. , Wahle T., Heusinkveld H. J., et al. 2021. “Evaluation of the Neurotoxic Effects of Engineered Nanomaterials in C57BL/6J Mice in 28‐Day Oral Exposure Studies.” Neurotoxicology 84: 155–171. 10.1016/j.neuro.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Soldin, O. P. , Chung S. H., and Mattison D. R.. 2011. “Sex Differences in Drug Disposition.” Journal of Biomedicine and Biotechnology 2011: 7–9. 10.1155/2011/187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin, O. P. , and Mattison D. R.. 2009. “Sex Differences in Pharmacokinetics and Pharmacodynamics.” Clinical Pharmacokinetics 48, no. 3: 143–157. 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltaninejad, H. , Zare‐Zardini H., Amirkhani M. A., et al. 2021. “Effect of Nanoalumina on Sex Hormones and Fetuses in Pregnant Rats.” JBRA Assisted Reproduction 26: 241–246. 10.5935/1518-0557.20210045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford University . n.d. “Gendered Innovations.” Retrieved 10 June 2023. https://genderedinnovations.stanford.edu/.
- Sulaiman, F. A. , Adeyemi O. S., Akanji M. A., et al. 2015. “Biochemical and Morphological Alterations Caused by Silver Nanoparticles in Wistar Rats.” Journal of Acute Medicine 5, no. 4: 96–102. 10.1016/j.jacme.2015.09.005. [DOI] [Google Scholar]
- Sung, J. H. , Ji J. H., Park J. D., et al. 2011. “Subchronic Inhalation Toxicity of Gold Nanoparticles.” Particle and Fibre Toxicology 8, no. 1: 16. 10.1186/1743-8977-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, Y. , Yano N., Takahashi H., et al. 2013. “Long‐Term Pulmonary Responses to Quadweekly Intermittent Intratracheal Spray Instillations of Magnetite (Fe3O4) Nanoparticles for 52 Weeks in Fischer 344 Rats.” Journal of Toxicologic Pathology 26, no. 4: 393–403. 10.1293/tox.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum, C. , Ellis R. P., Eyssel F., Zou J., and Schiebinger L.. 2019. “Sex and Gender Analysis Improves Science and Engineering.” Nature 575, no. 7781: 137–146. 10.1038/s41586-019-1657-6. [DOI] [PubMed] [Google Scholar]
- Tannenbaum, C. , Greaves L., and Graham I. D.. 2016. “Why Sex and Gender Matter in Implementation Research.” BMC Medical Research Methodology 16, no. 1: 1–9. 10.1186/s12874-016-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariba Lovaković, B. , Barbir R., Pem B., et al. 2021. “Sex‐Related Response in Mice After Sub‐Acute Intraperitoneal Exposure to Silver Nanoparticles.” NanoImpact 23: 100340. 10.1016/j.impact.2021.100340. [DOI] [PubMed] [Google Scholar]
- Tarlan, M. , Sajedianfard J., and Fathi M.. 2020. “Effect of Titanium Dioxide Nanoparticles Administered During Pregnancy on Depression‐Like Behavior in Forced Swimming and Tail Suspension Tests in Offspring Mice.” Toxicology and Industrial Health 36, no. 4: 297–304. 10.1177/0748233720925707. [DOI] [PubMed] [Google Scholar]
- Tassinari, R. , Cordelli E., Eleuteri P., et al. 2021. “Effects of Sub‐Chronic Oral Exposure to Pyrogenic Synthetic Amorphous Silica (NM‐203) in Male and Female Sprague‐Dawley Rats: Focus on Reproductive Systems.” Reproductive Toxicology 105: 17–24. 10.1016/j.reprotox.2021.08.001. [DOI] [PubMed] [Google Scholar]
- Tassinari, R. , Cubadda F., Moracci G., et al. 2014. “Oral, Short‐Term Exposure to Titanium Dioxide Nanoparticles in Sprague‐Dawley Rat: Focus on Reproductive and Endocrine Systems and Spleen.” Nanotoxicology 8, no. 6: 654–662. 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- Tassinari, R. , Di Felice G., Butteroni C., et al. 2020. “Hazard Identification of Pyrogenic Synthetic Amorphous Silica (NM‐203) After Sub‐Chronic Oral Exposure in Rat: A Multitarget Approach.” Food and Chemical Toxicology 137: 111168. 10.1016/j.fct.2020.111168. [DOI] [PubMed] [Google Scholar]
- Tassinari, R. , Martinelli A., Valeri M., and Maranghi F.. 2021. “Amorphous Silica Nanoparticles Induced Spleen and Liver Toxicity After Acute Intravenous Exposure in Male and Female Rats.” Toxicology and Industrial Health 37, no. 6: 328–335. 10.1177/07482337211010579. [DOI] [PubMed] [Google Scholar]
- Tassinari, R. , Tammaro A., Martinelli A., Valeri M., and Maranghi F.. 2023. “Sex‐Specific Effects of Short‐Term Oral Administration of Food‐Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats.” Toxics 11, no. 9: 776. 10.3390/toxics11090776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan, A. , Chiara F., Mongillo M., Quintieri L., and Cristofori P.. 2012. “Sex‐Related Differences in Renal Toxicodynamics in Rodents.” Expert Opinion on Drug Metabolism & Toxicology 8, no. 9: 1173–1188. 10.1517/17425255.2012.698262. [DOI] [PubMed] [Google Scholar]
- Tsiokou, V. , Kilindris T., Begas E., Kouvaras E., Kouretas D., and Asprodini E. K.. 2020. “Altered Activity of Xenobiotic Detoxifying Enzymes at Menopause – A Cross‐Sectional Study.” Environmental Research 182: 109074. 10.1016/j.envres.2019.109074. [DOI] [PubMed] [Google Scholar]
- Tubesha, Z. , Imam M., Mahmud R., and Ismail M.. 2013. “Study on the Potential Toxicity of a Thymoquinone‐Rich Fraction Nanoemulsion in Sprague Dawley Rats.” Molecules 18, no. 7: 7460–7472. 10.3390/molecules18077460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Government Accountability Office . 1990. “NIH: Problems in Implementing Policy on Women in Study Populations. 160, 1–14”.
- U.S. Government Accountability Office . 1992. “FDA Needs to Ensure More Study of Gender Differences in Prescription Drug Testing (GAO/HRD‐93‐17)”.
- U.S. Government Accountability Office . 2000. “Report to Congressional Requesters: NIH Has Increased Its Efforts to Include Women in Research (GAO/HEHS‐00‐96). May, 1–37”.
- U.S. Government Accountability Office . 2001. “Women's Health: Women Sufficiently Represented in New Drug Testing, but FDA Oversight Needs Improvement: Gao‐01‐754, July, 1–36”.
- U.S. Government Accountability Office . 2015. “NIH: Better Oversight Needed to Help Ensure Continued Progress Including Women in Health Research. Gao‐16‐13, October, 1–52”.
- Vahter, M. , Gochfeld M., Casati B., et al. 2007. “Implications of Gender Differences for Human Health Risk Assessment and Toxicology.” Environmental Research 104, no. 1: 70–84. 10.1016/j.envres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Vakilzadeh, H. , Varshosaz J., and Minaiyan M.. 2018. “Pulmonary Delivery of Triptorelin Loaded in Pluronic Based Nanomicelles in Rat Model.” Current Drug Delivery 15, no. 5: 630–640. 10.2174/1567201815666180209113735. [DOI] [PubMed] [Google Scholar]
- Van Epps, H. , Astudillo O., Del Pozo Martin Y., and Marsh J.. 2022. “The Sex and Gender Equity in Research (SAGER) Guidelines: Implementation and Checklist Development.” European Science Editing 48: e86910. 10.3897/ese.2022.e86910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veser, C. , Carlier A., Dubois V., Mihăilă S. M., and Swapnasrita S.. 2024. “Embracing Sex‐Specific Differences in Engineered Kidney Models for Enhanced Biological Understanding of Kidney Function.” Biology of Sex Differences 15, no. 1: 99. 10.1186/s13293-024-00662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani, P. , Eleuteri P., Pacchierotti F., et al. 2022. “Pyrogenic Synthetic Amorphous Silica (NM‐203): Genotoxicity in Rats Following Sub‐Chronic Oral Exposure.” Mutation Research, Genetic Toxicology and Environmental Mutagenesis 876: 503458. 10.1016/j.mrgentox.2022.503458. [DOI] [PubMed] [Google Scholar]
- Vlaanderen, J. , Pronk A., Rothman N., et al. 2017. “A Cross‐Sectional Study of Changes in Markers of Immunological Effects and Lung Health due to Exposure to Multi‐Walled Carbon Nanotubes.” Nanotoxicology 11, no. 3: 395–404. 10.1080/17435390.2017.1308031. [DOI] [PubMed] [Google Scholar]
- Wang, M.‐Q. , Du Y.‐J., Wang C., Tao W.‐J., He Y.‐D., and Li H.. 2012. “Effects of Copper‐Loaded Chitosan Nanoparticles on Intestinal Microflora and Morphology in Weaned Piglets.” Biological Trace Element Research 149, no. 2: 184–189. 10.1007/s12011-012-9410-0. [DOI] [PubMed] [Google Scholar]
- Warheit, D. B. , Boatman R., and Brown S. C.. 2015. “Developmental Toxicity Studies With 6 Forms of Titanium Dioxide Test Materials (3 Pigment‐Different Grade & 3 Nanoscale) Demonstrate an Absence of Effects in Orally‐Exposed Rats.” Regulatory Toxicology and Pharmacology 73, no. 3: 887–896. 10.1016/j.yrtph.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Wąsowicz, M. , Marek K., Cićkiewicz M., and Cymerman M.. 2017. “Investigation of Nanodiamonds Interactions in Canine Blood. In A. N. Cartwright, D. V. Nicolau, & D. Fixler (Eds.), Proceedings (p. 100771C)”.
- Waxman, D. J. , and Holloway M. G.. 2009. “Sex Differences in the Expression of Hepatic Drug Metabolizing Enzymes.” Molecular Pharmacology 76, no. 2: 215–228. 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, B. 2011. “Same Sex, no Sex, and Unaware Sex in Neurotoxicology Bernard.” Neurotoxicology 32, no. 5: 509–517. 10.1016/j.neuro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. , Deng S., Wang B., et al. 2024. “Exposure to Polystyrene Nanoplastics Induces Hepatotoxicity Involving NRF2‐NLRP3 Signaling Pathway in Mice.” Ecotoxicology and Environmental Safety 278: 116439. 10.1016/j.ecoenv.2024.116439. [DOI] [PubMed] [Google Scholar]
- Westergaard, D. , Moseley P., Sørup F. K. H., Baldi P., and Brunak S.. 2019. “Population‐Wide Analysis of Differences in Disease Progression Patterns in Men and Women.” Nature Communications 10, no. 1: 666. 10.1038/s41467-019-08475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, K. E. , Chetwynd A. J., Fahy K. M., et al. 2021. “Environmental Dimensions of the Protein Corona.” Nature Nanotechnology 16, no. 6: 617–629. 10.1038/s41565-021-00924-1. [DOI] [PubMed] [Google Scholar]
- White, J. , Tannenbaum C., Klinge I., Schiebinger L., and Clayton J.. 2021. “The Integration of Sex and Gender Considerations Into Biomedical Research: Lessons From International Funding Agencies.” Journal of Clinical Endocrinology & Metabolism 106, no. 10: 3034–3048. 10.1210/clinem/dgab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbold, R. , Klein K., Burk O., et al. 2003. “Sex Is a Major Determinant of CYP3A4 Expression in Human Liver.” Hepatology 38, no. 4: 978–988. 10.1053/jhep.2003.50393. [DOI] [PubMed] [Google Scholar]
- Wu, M. , Chen L., Li R., et al. 2018. “Bio‐Distribution and Bio‐Availability of Silver and Gold in Rat Tissues With Silver/Gold Nanorod Administration.” RSC Advances 8, no. 22: 12260–12268. 10.1039/C8RA00044A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Shi J., Dai Y., Tang W., Cao H., and Chen J.. 2024. “Iron Indices and Hemogram in Renal Anemia and the Improvement With Tribulus Terrestris Green‐Formulated Silver Nanoparticles Applied on Rat Model.” Open Chemistry 22, no. 1: 20230212. 10.1515/chem-2023-0212. [DOI] [Google Scholar]
- Xu, J. , Sagawa Y., Futakuchi M., et al. 2011. “Lack of Promoting Effect of Titanium Dioxide Particles on Ultraviolet B‐Initiated Skin Carcinogenesis in Rats.” Food and Chemical Toxicology 49, no. 6: 1298–1302. 10.1016/j.fct.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Xu, S. , Zhang Z., and Chu M.. 2015. “Long‐Term Toxicity of Reduced Graphene Oxide Nanosheets: Effects on Female Mouse Reproductive Ability and Offspring Development.” Biomaterials 54: 188–200. 10.1016/j.biomaterials.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Xue, Y. , Zhang S., Huang Y., et al. 2012. “Acute Toxic Effects and Gender‐Related Biokinetics of Silver Nanoparticles Following an Intravenous Injection in Mice.” Journal of Applied Toxicology 32, no. 11: 890–899. 10.1002/jat.2742. [DOI] [PubMed] [Google Scholar]
- Yang, J.‐L. J. , Narayanamurthy R., Yager J. Y., and Unsworth L. D.. 2021. “How Does Biological Sex Affect the Physiological Response to Nanomaterials?” Nano Today 41: 101292. 10.1016/j.nantod.2021.101292. [DOI] [Google Scholar]
- Yang, L. , Kuang H., Zhang W., Wei H., and Xu H.. 2018. “Quantum Dots Cause Acute Systemic Toxicity in Lactating Rats and Growth Restriction of Offspring.” Nanoscale 10, no. 24: 11564–11577. 10.1039/C8NR01248B. [DOI] [PubMed] [Google Scholar]
- Yang, L. , and Li Y.. 2012. “Sex Differences in the Expression of Drug‐Metabolizing and Transporter Genes in Human Liver.” Journal of Drug Metabolism & Toxicology 3, no. 3: 1–20. 10.4172/2157-7609.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , and Toriola A. T.. 2024. “Menopausal Hormone Therapy Use Among Postmenopausal Women.” JAMA Health Forum 5, no. 9: e243128. 10.1001/jamahealthforum.2024.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, D. J. , Lee H. Y., Taylor‐Just A. J., Linder K. E., and Bonner J. C.. 2020. “Sex Differences in the Acute and Subchronic Lung Inflammatory Responses of Mice to Nickel Nanoparticles.” Nanotoxicology 14, no. 8: 1058–1081. 10.1080/17435390.2020.1808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes, M. , Aquilina G., Castle L., et al. 2024. “Re‐Evaluation of Silicon Dioxide (E 551) as a Food Additive in Foods for Infants Below 16 Weeks of Age and Follow‐Up of Its re‐Evaluation as a Food Additive for Uses in Foods for all Population Groups.” EFSA Journal 16, no. 1: e05088. 10.2903/j.efsa.2024.8880. [DOI] [Google Scholar]
- Yousef, M. I. , Abd H. H., Helmy Y. M., and Kamel M. A.‐N.. 2021. “Synergistic Effect of Curcumin and Chitosan Nanoparticles on Nano‐Hydroxyapatite‐Induced Reproductive Toxicity in Rats.” Environmental Science and Pollution Research 28, no. 8: 9362–9376. 10.1007/s11356-020-11395-7. [DOI] [PubMed] [Google Scholar]
- Yu, W.‐J. , Son J.‐M., Lee J., et al. 2014. “Effects of Silver Nanoparticles on Pregnant Dams and Embryo‐Fetal Development in Rats.” Nanotoxicology 8, no. sup1: 85–91. 10.3109/17435390.2013.857734. [DOI] [PubMed] [Google Scholar]
- Zhang, X.‐D. , Chen S., Wang S., et al. 2013. “Sex Differences in the Toxicity of Polyethylene Glycol‐Coated Gold Nanoparticles in Mice.” International Journal of Nanomedicine 2409: 2409–2419. 10.2147/IJN.S46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X. , and Leeder J. S.. 2013. “Epigenetic Regulation of ADME‐Related Genes: Focus on Drug Metabolism and Transport.” Drug Metabolism and Disposition 41, no. 10: 1721–1724. 10.1124/dmd.113.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information on the literature survey and keywords used (Table S1), quality scoring of extracted publication according to K score (Table S2) and S score (Table S3), data extracted (Table S4) and se‐xrelated analysis report (Table S5).
Table S6: In vivo studies on ADME properties and toxic effects of various nanomaterials. Physico‐chemical characteristics (shape, size, surface coating) are given for each NM type. For each animal model, details about treatment (test protocol. duration, doses in mg/kg body weight), differences in distribution and toxicity parameters (↑ or ↓ in males (♂) vs. females (♀)) are briefly listed.
Data Availability Statement
Data are available in article Supporting Information or openly available in a public repository that issues datasets with DOIs.


