Abstract
A fluorescence resonance energy transfer assay has been developed for monitoring Bacillus anthracis lethal factor (LF) protease activity. A fluorogenic 16-mer peptide based on the known LF protease substrate MEK1 was synthesized and found to be cleaved by the enzyme at the anticipated site. Extension of this work to a fluorogenic 19-mer peptide, derived, in part, from a consensus sequence of known LF protease targets, produced a much better substrate, cleaving approximately 100 times more efficiently. This peptide sequence was modified further on resin to incorporate donor/quencher pairs to generate substrates for use in fluorescence resonance energy transfer-based appearance assays. All peptides cleaved at similar rates with signal/background ranging from 9–16 at 100% turnover. One of these substrates, denoted (Cou)Consensus(K(QSY-35)GG)-NH2, was selected for additional assay optimization. A plate-based assay requiring only low nanomolar levels of enzyme was developed for screening and inhibitor characterization.
Fatalities resulting from the recent bioterrorist attacks in the U.S. have highlighted the inadequacy of available medical treatments for anthrax infection. Although Bacillus anthracis, the causative agent of anthrax, can be cleared from the host with antibiotics, the ongoing action of the secreted anthrax toxin limits the clinical efficacy of these drugs (1). A pharmacological agent that directly targets the toxin might thus prove to have valuable synergy with more traditional antibacterial treatments.
The anthrax toxin consists of three proteins, a receptor-binding component designated protective antigen, and two enzymatic components termed edema factor and lethal factor (LF) (2). Edema factor is a calmodulin-dependent adenylate cyclase, whereas LF is a zinc-dependent metalloprotease that has been shown to cleave near the N termini of several MAP kinase kinases (MKKs). Although the complete mechanism of pathogenesis is unclear, the disruption of key signaling pathways mediated by MKKs seems to lead first to the lysis of macrophages and later to the death of the host. The pivotal role of LF in the virulence of the toxin suggests that inhibitors of the enzyme may provide protection against cytotoxicity.
The only known inhibitors of LF are weak hydroxamates (IC50s >300 μM) and chelating agents such as EDTA and ortho-phenanthroline (3). Existing assays for LF activity, such as SDS/PAGE (4, 5) or HPLC (3), are impractical for high-throughput screening of compound collections. Identification of potent and selective LF inhibitors requires an assay that is less labor-intensive, has faster turnaround, and utilizes low levels of purified enzyme. In this article we describe the development of a peptide-based fluorescence resonance energy transfer (FRET) assay for LF protease that meets these criteria.
Materials and Methods
Substrate Synthesis.
Peptide synthesis was carried out on an ABI Model 433A peptide synthesizer using FastMoc chemistry with increased acylation times on a 250-μmol scale. All reagents, including amino acids, were from PE Applied Biosystems except for (Fmoc)-l-Nle (Bachem), (Fmoc)-l-Lys(Mtt) (Nova Biochem), and PL-Rink resin (Polymer Laboratories, Amherst, MA). All fluorophores and quenchers were from Molecular Probes.
Peptide labeling with reporters was carried out on resin subsequent to peptide synthesis. Incorporation of materials at the N terminus was accomplished by labeling with the commercially available N-hydroxysuccinimidyl esters. Typical reactions were carried out on a 20–100-μmol resin scale with a 1–10-fold excess of the label in a minimal volume (1–3 ml) of N-methylpyrrolidinone overnight. Incorporation of the two reporters for the FRET substrates was accomplished by first labeling the N terminus. The second reporter was then introduced by selectively removing the Lys(Mtt)-protecting group (CH2Cl2 with 2% trifluoroacetic acid and 3% triisopropylsilane, room temperature, 45 min), resin washing, and reaction with the label as described above.
After incorporation of appropriate reporters, the resin was washed with NMP, acetic acid, CH2Cl2, and methanol (3 times each), dried briefly in vacuo, and the peptides were cleaved with 95% trifluoroacetic acid/2.5% H2O/2.5% triisopropylsilane for 90 min. After precipitation from cold diethyl ether, the crude peptides were purified on a Waters PrepLC 4000 system with a 25 × 400 mm 300 Å DeltaPak C18 column and a CH3CN/H2O gradient (both with 0.1% trifluoroacetic acid). Purified peptides were lyophilized and their molecular weight confirmed by mass spectral analysis. All peptides were >95% pure by RP-HPLC (A214).
LF HPLC-Based Assay.
LF was obtained from S. Leppla (National Institutes of Health) or purified in J. Collier's laboratory at Harvard Medical School. Peptides were prepared as 1-mM stock solutions in doubly distilled water. As long-term solution stability has not been evaluated, these materials were protected from incident light and used within a week of preparation. Test peptides were incubated at 100 μM with varying levels of LF in 50 mM Hepes, pH 7.0/20 mM NaCl/10 mM MgCl2/100 μM CaCl2/100 μM ZnCl2/1 mg/ml BSA/1 mM DTT for various times. Before HPLC injection, samples were treated by precipitation of the protein materials by addition of a 10-fold excess volume of 60% CH3CN in H2O. The samples were then vortexed, allowed to sit on ice for at least 15 min, centrifuged (2,000 × g for 60 sec), and the supernatant was removed from the protein pellet. HPLC samples typically contained 150–300 ng (75–150 pmol) of labeled peptide. Peptide substrates and products were separated on a C18 column [HAIPEEK Targa C18 5 μm, 20 × 2.1 mm; Higgins Analytical (Mountain View, CA)] with a CH3CN/H2O (both with 0.1% trifluoroacetic acid) gradient on a Waters 625 LC system with in-line UV-visible (Waters 996) and fluorescence [Hitachi (Tokyo) F-1050] detection. Fluorescence signals (excitation 445 nm/emission 520 nm) were integrated with Water's millennium software.
LF Plate-Based Assay.
To each well of a 96-well black flat-bottomed plate (Packard) was added 25 μl of a 6 μM solution of the peptide substrate (Cou)Consensus(K(QSY-35)GG)-NH2 in assay buffer (20 mM Hepes, pH 7.0/1 mM CaCl2/0.1 mg/ml BSA/0.01% Tween-20). Test compounds in DMSO (1.5 μl) were added with additional assay buffer (18.5 μl) to the plate with a CyBi well dispenser. The enzymatic reaction was initiated with 30 μl of 10 nM of LF in assay buffer and terminated after 15 min at room temperature by the addition of 25 μl of 4 mM ortho-phenanthroline/40 mM EDTA (both steps were executed on a Tecan Genesis workstation). The fluorescence was read on a Victor2 V plate reader with the umbelliferone protocol (excitation 355 nm/emission 460 nm).
Results and Discussion
Identification of a Peptide Substrate.
To evaluate peptide substrates for measuring LF activity we chose as a starting point the only known cellular substrates, the MKK protein family (4–6). Vitale et al. (4) have used microsequencing to identify the specific cleavage sites in several MKKs (Table 1). As noted by Vitale et al., there is a moderate level of homology between the substrates with a small stretch of basic residues N-terminal to the cleavage site and two hydrophobic residues at the P2 and P1′ positions. A strongly acidic region of the protein surface seen in the recent x-ray crystal structure of LF rationalizes the preference for basic residues (7). There are no residues, however, that are absolutely conserved among the sequences, and it has been suggested that there may be other more remote substrate determinants (4).
Table 1.
Alignment of known LF protease cleavage sites within the MKK family (4), the substrate motif (where H = hydrophobic residue and B = basic residue), the resulting consensus sequence, and substrates synthesized for this article
| Substrate | Peptide sequence | |
|---|---|---|
| MEK11–16 | MPKKKPTP | IQLNPAPD |
| MEK23–18 | ARRKPVLP | ALTINPTI |
| MKK3b19–34 | SKRKKDVR | ISCMSKPP |
| MKK6b7–22 | KKRNPGLK | IPKEAFEQ |
| MKK438–53 | QGKRKALK | LNFANPPF |
| MKK451–66 | PPFKSTAR | FTLNPNPT |
| MKK7β37–52 | QRPRPTLQ | LPLANDGG |
| MKK7β69–84 | ARPRHMLG | LPSTLFTP |
| MKK substrate motif | -BBBB-H- | H------- |
| Derived Consensus peptide* | NleKKKKVLP | IQLNAATD |
| (Flu)MEK11–16-NH2 | (Flu)MPKKKPTP | IQLNPAPD-NH2 |
| (Flu)[Nle1]MEK11–16(KGG)-NH2 | (Flu)NlePKKKPTP | IQLNPAPDKGG-NH2 |
| (Flu)Consensus(KGG)-NH2 | (Flu)NleKKKKVLP | IQLNAATDKGG-NH2 |
| (Cou)Consensus(K(QSY-35)GG)-NH2 | (Cou)NleKKKKVLP | IQLNAATDK(QSY-35)GG-NH2 |
| (Cou)Consensus(K(DAB)GG)-NH2 | (Cou)NleKKKKVLP | IQLNAATDK(DAB)GG-NH2 |
| (QSY-35)Consensus(K(Cou)GG)-NH2 | (QSY-35)NleKKKKVLP | IQLNAATDK(Cou)GG-NH2 |
| (DAB)Consensus(K(Cou)GG)-NH2 | (DAB)NleKKKKVLP | IQLNAATDK(Cou)GG-NH2 |
Flu, 5,6-carboxyfluoresceinyl; Cou, 7-hydroxy-4-methyl-3-acetylcoumarinyl; DAB, 4-dimethylaminoazobenzene-4′-carboxyl; QSY-35, N-({4-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]phenyl}acetyl.
Denoted “Consensus.”
Because the literature provided no guidance on the relative turnover rates of the various MKKs with LF, we arbitrarily chose the MEK1 substrate as our starting point and synthesized the fluoresceinated amide analog (Flu)MPKKKPTPIQLNPAPD-NH2 (cleavage site underlined). The N-terminal fluoresceinyl group was incorporated with two goals in mind. First, it provided a sensitive spectroscopic handle for HPLC analysis of the anticipated cleavage reaction. Second, it allowed us to probe the suitability of large chromophores on the N terminus of the peptide, which, if tolerated by the enzyme, would facilitate development of a doubly labeled substrate for a FRET-based assay.
In initial experiments this peptide was incubated at 100 μM with various amounts of LF in a metal ion-supplemented buffer (see Materials and Methods) similar to that found in the literature (3). Good levels of turnover could be observed (15–30%) but only after incubation with high levels of enzyme (≈1 μM) for extended periods (2–4 h at 30°C). The anticipated products, those resulting from cleavage of the Pro-8-Ile-9 peptide bond, were detected by mass spectral analyses (data not shown). However, HPLC analyses were complicated by an unanticipated side reaction: a fluorescein-mediated oxidation of the side chain of the methionine residue to the corresponding sulfoxide. The product of this reaction [the Met(O) substrate] nearly coeluted with the cleavage product, (Flu)MPKKKPTP(OH), making analysis difficult. This oxidation occurred with peptide both as a solid and in solution, and was particularly rapid in solution in the presence of light with up to 50% conversion within 1 h (data not shown).
To circumvent this oxidation issue and to potentially extend the utility of the peptide approach, a new substrate was synthesized. This peptide (Flu)NlePKKKPTPIQLNPAPDKGG-NH2 [“[Nle1]Mek11–16(KGG)-NH2”] differed from the original peptide in two ways. First, the methionine was replaced by its isostere norleucine. Second, an additional three residues, -KGG-, were incorporated at the C terminus with the lysyl residue being added as an Mtt-protected amino acid. Although not used in this synthesis, this differentially protected lysine provided a site for introduction of specific reporters by means of its selective deprotection while still on the resin. The glycyl residues were added to facilitate labeling by providing a spacer between the lysyl side chain and the resin. As anticipated, the substrate was stable and showed no propensity toward oxidation. It was cleaved by LF between the Pro-8-Ile-9 bond (identified by mass spectral analysis) at a rate similar to the original peptide, indicating that the substitution of norleucine for methionine and the addition of three amino acids had no effect.
Although these studies demonstrated that an MEK1-derived peptide could be used as a substrate in an assay of LF proteolytic activity, the high concentration of enzyme required, even with long reaction times, rendered the assay impractical for screening. We thus sought a substrate with a higher turnover number by synthesizing a more general peptide based on the shared elements between the reported natural substrates (Table 1). This “consensus peptide” was designed to reflect these common elements and to allow incorporation of reporters in an orthogonal manner. In keeping with our HPLC work we synthesized the following derivative: (Flu)NleKKKKVLPIQLNAATDKGG-NH2. This peptide differs from the [Nle1]Mek11–16(KGG)-NH2 substrate described above at five positions. The three proline residues at positions 6, 13, and 15 were converted to residues found in the other MKK substrates to reduce the possibility of alternate cleavages adjacent to prolyl residues and to minimize potential secondary structure. The proline at the second position was converted to lysine for these reasons and to reflect the tetrabasic component of the consensus sequence. The threonine at position 7 was converted to leucine, a residue found in 5 of the 8 known sequences. Again, the -KGG- functionality was retained at the C terminus.
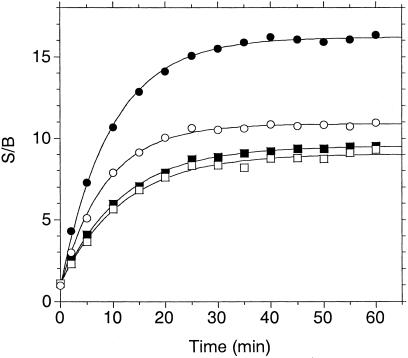
By using the HPLC assay, the fluoresceinated consensus peptide was compared head-to-head with the [Nle1]Mek11–16(KGG)-NH2 peptide. As shown in Fig. 1, the consensus peptide was a much better substrate, with an improvement in turnover number of approximately 100-fold. Again, mass spectral analysis confirmed that the appropriate cleavage was taking place. By reducing the amount of enzyme required per experiment as well as shortening reaction times, this breakthrough discovery enabled an accelerated pace of assay optimization experiments. We next embarked on the identification of a suitable fluorophore/quencher pair of FRET peptide labels. Several known fluorophores and quenchers were chosen and examined for their ease of synthesis, degree of quenching, and substrate suitability. Of those materials examined, we found the best pairs to be those of a coumarin fluorophore (7-hydroxy-4-methyl-3-acetylcoumarinyl; λex 386 nm, λem 448 nm) paired with either 4-dimethylaminoazobenzene-4′-carboxyl (DABCYL; λmax 454 nm) or QSY-35 [(N-({4-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]phenyl}acetyl), λmax 475 nm] as the quencher. We made analogs of the consensus peptide with the coumarin fluorophore at either the C or N terminus and one of the two quenchers at the distal end of the peptide (four peptides total; Table 1). Their reactivity with LF was then analyzed by means of the increase in fluorescence after separation of fluorophore and quencher. As shown in Fig. 2, all four peptides cleaved at approximately the same rate with similar signal/background (S/B) varying between 9 and 16 at 100% cleavage. The progress curves were first-order, indicating that the Kms of the four substrates are ≫10 μM. The peptide with the largest S/B, denoted (Cou)Consensus(K(QSY-35)GG)-NH2, was chosen for further studies. Mass spectral analysis of the enzyme reaction again ascertained that cleavage occurred at the Pro-Ile bond, and extended digestion (>4 substrate half-lives) did not show formation of additional products (data not shown).
Figure 1.
Enzymatic cleavage of the substrates (Flu)[Nle1]Mek11–16(KGG)-NH2 (closed circles) and (Flu)Consensus(KGG)-NH2 (open circles) as assessed by the HPLC cleavage assay. [Substrate] = 100 μM, [LF] = 1 μM, T = 37°C with time points as indicated. First-order rate constants were derived from curve fitting each set of data; comparison of these rate constants indicated that the turnover of the consensus sequence was ≈100-fold faster than the MEK1 sequence.
Figure 2.
Enzymatic cleavage of four FRET analogs of the MKK consensus sequence. Peptides at 10 μM in 50 mM Hepes, pH 7.0/20 mM NaCl/10 mM MgCl2/100 μM CaCl2/100 μM ZnCl2/1 mg/ml BSA/1 mM DTT were incubated with 100 nM LF in a volume of 75 μl in a black 96-well plate. The increase in fluorescence (excitation 355 nm/emission 460 nm) was read on a Victor2 V plate reader. (Cou)Consensus(K(QSY-35)GG)-NH2 (closed circles); (QSY-35)Consensus(K(Cou)GG)-NH2 (open circles); (Cou)Consensus(K(DAB)GG)-NH2 (closed squares); (DAB)Consensus(K(Cou)GG)-NH2 (open squares).
A series of optimization experiments indicated that a simplified buffer consisting of only 20 mM Hepes (pH 7.0) and 1 mM CaCl2 (S. Leppla, personal communication) improved the enzyme activity more than 10-fold relative to the more complex buffer used earlier. BSA and Tween-20 were added at low levels (0.1 mg/ml and 0.01%, respectively) to prevent adsorptive losses of enzyme and facilitate automated liquid handling. Conversion of the continuous assay into a fixed time assay for plate-based screening was achieved by terminating the reaction with 1 mM ortho-phenanthroline/10 mM EDTA. As described in Materials and Methods, the assay is configured to run in 96-well plates with a 75-μl reaction volume followed by a 25-μl quench. Although these volumes are convenient for both manual and automated liquid handling, there is clearly room for miniaturization to higher-density formats if desired. With this protocol the signal/background (S/B) is ≈9 at 100% cleavage of 2 μM substrate; 4 nM LF leads to 40–50% cleavage in 15 min at room temperature for a typical assay S/B of ≈4. Because the reaction is first-order, the degree of inhibition by a test compound is a linear function of the reduction in the rate constant, not the reduction in the amount of product formed; however, for turnover <50% the correction is small and the percent inhibition is well approximated by the percent decrease in the net fluorescent signal. As implemented on our semiautomated system, the Z′ factor (a measure of assay robustness) (8) is routinely >0.7. As with other assays using a fluorophore at this wavelength, artifactually low signal (because of compound absorption) or high signal (because of compound fluorescence) can be encountered during screening. Longer wavelength fluorophore-quencher pain should reduce these interferences.
Summary
We have developed a FRET-based assay for LF protease activity by using a doubly labeled peptide substrate that is readily accessible through solid-phase synthesis. This assay uses low nanomolar levels of enzyme with short incubation times and can be carried out in a multiwell plate-based format. It has been found to be suitable for general screening, inhibitor characterization (such as IC50 determinations), and enzyme kinetics. It is anticipated that this in vitro protease assay will facilitate screening for lead identification and subsequent support of medicinal chemistry.
Acknowledgments
We thank Rachel Legmann and Borden Lacy of John Collier's laboratory (Harvard Medical School) and Sheryl Goodart of Bill Deitrich's laboratory (Harvard Medical School) for assisting C.M.D. and J.D.H. in purification of recombinant LF. We also recognize Steve Bruner of Chris Walsh's laboratory (Harvard Medical School) for his help with initial HPLC and mass spectral analyses. We also thank Steve Leppla (National Institutes of Health) for providing purified LF from B. anthracis. Lastly, we thank Patrick Griffin and John Mehl (Merck Research Laboratories) for their support with mass spectral analyses.
Footnotes
Abbreviations LF, lethal factor; MKK, MAP kinase kinase; FRET, fluorescence resonance energy transfer.
See commentary on page 6527.
References
- 1.Dixon T C, Meselson M, Guillemin J, Hanna P C. N Engl J Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 2.Mock M, Fouet A. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 3.Hammond S E, Hanna P C. Infect Immun. 1998;66:2374–2378. doi: 10.1128/iai.66.5.2374-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Biochem J. 2000;352:739–745. [PMC free article] [PubMed] [Google Scholar]
- 5.Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel K R, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Vande Woude G F. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 6.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 7.Pannifer A D, Wong T Y, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, Lacy D B, Collier R J, Park S, Leppla S H, et al. Nature (London) 2001;414:229–233. doi: 10.1038/n35101998. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J H, Chung T D, Oldenburg K R. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]